
J. Cent. South Univ. (2018) 25: 2190-2198
DOI: https://doi.org/10.1007/s11771-018-3907-4
Benzohydroxamic acid to improve iron removal from potash feldspar ores
CAO Zhan-fang(曹占芳)1, 2, QIU Pei(邱培)1, 2, WANG Shuai(王帅)1, 2, ZHONG Hong(钟宏)1, 2
1. Hunan Provincial Key Laboratory of Efficient and Clean Utilization of Manganese Resources,Central South University, Changsha 410083, China;
2. College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
The technological mineralogy of the potash feldspar was investigated and a new collector named Yb105 was adopted to remove iron from potash feldspar ores. The technological mineralogy results indicate that the main components of the ore were feldspar, sericite, quartz and kaolinite, and iron mainly existed in limonite and hematite, most of which can be removed by beneficiation. The results show the benzohydroxamic acid can not only increase the recovery of iron and reduce the consumption of oleic acid collector, but also enhance the collecting performance of oleic acid at low temperature, which can realize the flotation of the ores at a low temperature and play an important role in saving energy to some extent. Compared with oleic oil, the benzohydroxamic acid had a great advantage in removing iron from potash feldspar, a potash feldspar concentrate with Fe grade of 0.23%, K2O grade of 12.59% and Na2O grade of 0.26% was obtained by flotation with Yb105 as collector, and the yield of the concentrate was 82.55%.
Key words:
potash feldspar; iron removal; reverse flotation; benzohydroxamic acid;
Cite this article as:
CAO Zhan-fang, QIU Pei, WANG Shuai, ZHONG Hong. Benzohydroxamic acid to improve iron removal from potash feldspar ores [J]. Journal of Central South University, 2018, 25(9): 2190–2198.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3907-41 Introduction
Feldspar is the most extensively distributed mineral in the world, and accounts for about 60% of the earth’s crust [1, 2], as one of the most important kind feldspars, potash feldspar is widely used in many fields. In agriculture, it can be a good resource to produce potash fertilizer, and considerable attention has been focused on how to transform the poorly-soluble sylvite in potash feldspar into the soluble form [3]. In industry, feldspar is the second important raw material after clay in ceramic industry, and it is often used as a fluxing agent in ceramic, ceramic glaze and glass industries to form glassy phase at low temperature for its excellent properties such as low melting point, long melting time interval and high melt viscosity [4].
However, the grade of potash feldspar is often impaired by impurities such as hematite, magnetite and white mica, among which, iron existing in the form of iron oxide is one of the biggest problems. The existence of iron oxide may result in dark spots on the production, which can affect the whiteness index, dielectric properties and chemical stability of the products, and therefore makes the products fail to meet the requirements in application [5–7].Consequently, there is a need to remove iron from the potash feldspar.
The way to remove the contaminated iron oxides from silicon aluminate ores not only includes chemical method, but also includes physical method, and chemical method was considered as the most cost-efficient method. There are three methods commonly used to remove iron from potash feldspar: magnetic separation process, acid leaching process and flotation process [8, 9]. The separation of iron from potash feldspar can be achieved by taking advantage of external magnetic field since some iron minerals in the potash feldspar possess magnetism [10]. A new high intensity wet permanent magnetic separator was adopted to separate iron from the potash feldspar, and the results indicate that an excellent separation could be obtained [7]. In the conventional acid leaching processes, the inorganic acid was employed to selectively dissolve iron mine in the silicon aluminate ores such as feldspar; however, the use of inorganic acid may give rise to the introduction of SO42– and Cl– which may contaminate the raw material. In recent years, many researchers have been engaged in employing organic acid to dissolve iron oxides in silicon aluminate ores [11–13]. Flotation is nearly the most widely used method to remove iron impurities and achieve the separation of potash feldspar and quartz [14]. Anionic collectors like oleic acid are usually used in the flotation to remove iron oxide minerals for its little floatability to feldspar [15]. Furthermore, there are other methods to remove iron from feldspar, for example, the selective flocculation of the starch and iron to change the particle settling rate has been performed by DOGU et al [16] to achieve the separation of the iron and potash feldspar.
In this work, the reverse flotation process has been investigated to reduce the iron content in the potash feldspar. And a new kind of anionic collector composed of benzohydroxamic acid and oleic acid was adopted, and the optimal operating conditions have been determined by investigating such parameters as the collector dosage and grinding fineness.
2 Experimental
2.1 Process mineralogy
The samples taken from Hunan Province, China, were prepared by crushing and screening to make the particle size smaller than 2 mm. The multi-element chemical composition analysis results of the ore sample are shown in Table 1, and the amounts of iron chemical phase are reported in Table 2.
Table 1 Multi-element chemical composition of ore (Mass fraction, %)
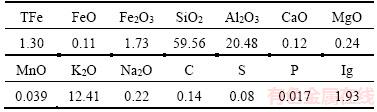
Table 2 Iron chemical phase analysis of ore (Mass fraction, %)
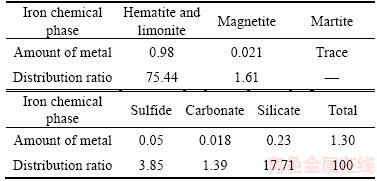
From Tables 1–2, it can be seen that the main components of the ore are quartz, alumina and potassium oxide. The iron content is 1.30% and mainly exists in the form of Fe2O3, hematite and limonite are the main phases of the iron, which accounts for 75.44%, followed by silicate comprising 17.71% of the iron, the remaining iron are distributed in magnetite, pyrite and other phases.
The characterization of the sample was performed by metallographic microscope, scanning electronic microscope and X-ray diffraction. The results illustrated in Figure 1 and Table 3 indicate that the dominant minerals of the ore are potassium feldspar, sericite, quartz and kaolinite. And the main metal minerals are limonite, hematite, and minor amounts of magnetite, ilmenite, pyrite and rutile.
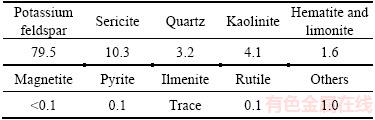
Based on the analyses of the metallographicmicroscope and scanning electron microscope, it can be seen that the mineral often generates the phenomenon of sericitization, kaolinization and montmorillonitization, and the kaolinization and montmorillonitization often occur on the surface, cleavage or fractures of the potash feldspar crystal grain. Besides, the mineral may inlaid with sericite in various forms (Figures 2–5) and encapsulate or attach to other minerals such as quartz, limonite, hematite and magnetite, ilmenite, zircon (Figures 4 and 5).
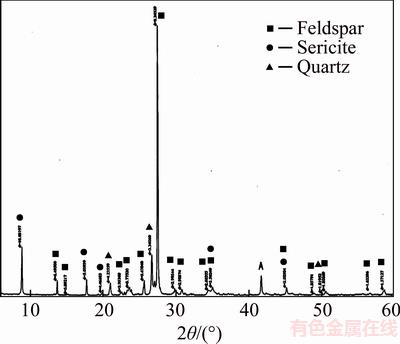
Figure 1 X-ray diffraction pattern of ore
Table 3 Content of main minerals in ore (Mass fraction, %)
For the potassium feldspar, iron mineral is the main harmful element that affects the feldspar product quality. It was observed from the metallographicmicroscope and scanning electron microscopy analyses that the iron mineral is mainly limonite, next comes hematite and a small amount of magnetite, ilmenite and pyrite. Limonite often occurs in three forms: firstly, it is formed by the oxidation alteration of pyrite for the similar granular morphology, with trace of reminded pyrite wrapped in it (Figure 6); secondly, the advanced limonite fills metasomatic along the edge or fissures of the early limonite and hematite in net veined (Figure 7); thirdly, the precipitation of the altered iron-containing silicate is mainly fine granular or attached by iron contaminants on the surface and fracture of the silicate, and partly mixes with feldspar to form clumpy assemblages (Figure 8). In addition, limonite also coexists with other metal minerals such as hematite and magnetite, zircon and rutile.

Figure 2 Potassium feldspar(F) adjacent disseminates in coarse white mica sheets (S) (orthogonal polarization)
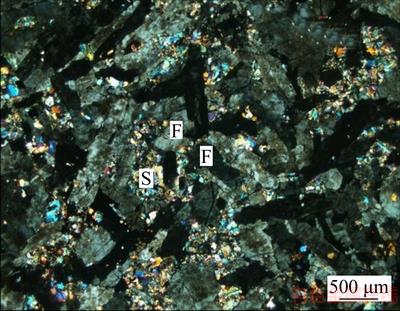
Figure 3 Spaces between grains of kaolinized potassium feldspar(F) filled with little sericite (S, color) (orthogonal polarization)

Figure 4 Scaly sericite (S) filling along gap of kaolinized potassium feldspar (F) as vein (Reddish brown showing iron contaminants (orthogonal polarization))
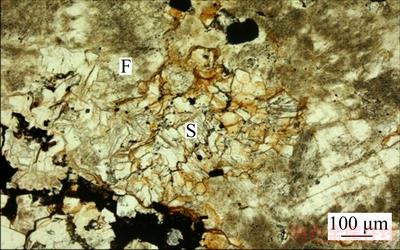
Figure 5 Kaolinization of potassium feldspar (F dark), with sheet sericite (S) embedded in it (Reddish brown showing iron contaminants (single polarization))

Figure 6 Limonite formed by oxidation of pyrite(L) disseminates in silicate minerals such as potassium feldspar (G, dark) (reflection)
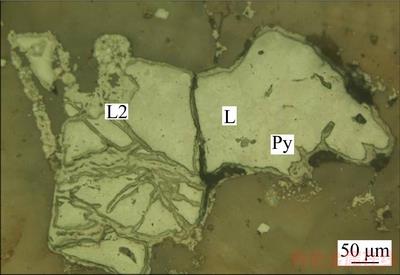
Figure 7 Advanced veinlike limonite filling along edge or fissure of early limonite(L) (Py indicating pyrite residual) (reflection)
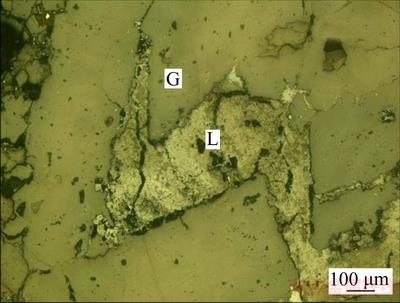
Figure 8 Crumb limonite (L) aggregation distributing in silicate minerals (G) (reflection)
2.2 Experiment methods
In the process of reverse flotation experiment, 300 g of ore sample was ground in a XMQ-f240×90 type conical ball mills each time with a grinding pulp concentration of 60%. And a 1 L XFD-63 single tank flotation machine was used for roughing and scavenging. Two kinds of collectors named oleic acid and Yb105 (oleic acid modified with 5% benzohydroxamic acid) were used in the flotation process. Since the oleic acid is sensitive to the temperature, the temperature was set at 25–26 °C in the process of flotation. (Other temperature conditions in this work will be noted particularly). Then, the concentrates and tailings were filtrated, dried and analyzed by ICP.
3 Results and discussion
3.1 Characteristic of oleic acid
Based on the characterization of the ore, reverse flotation was firstly carried out with oleic acid as collector. The flow-sheet of the flotation is shown in Figure 9.
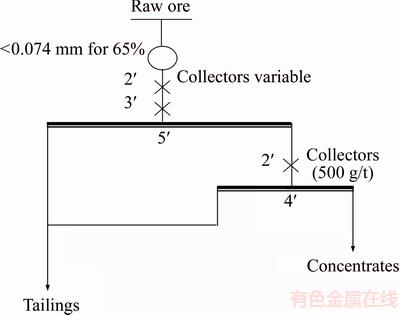
Figure 9 Flow-sheet of flotation
With the grinding fineness of <0.074 mm accounts for 65% (mass fraction), the oleic acid flotation experiments were conducted at room temperature by changing the amount of oleic acid, the results are shown in Figure 10.
It can be seen from Figure 10 that oleic acid did not work well in collecting the iron mineral from potash feldspar ore. With the increase of the dosage, the iron recovery in reverse flotation tailings increased significantly. With the dosage increasing from 700 to 1600 g/t, the iron recoveries in tailings rose significantly increased from 46.2% to 84.86%. However, the continuous increase of the dosage of oleic acid made little contribution to the declination of the Fe grade in concentrate, and meanwhile there was a distinct decrease in both the yield of flotation concentrate and the recoveries of K and Na in reverse flotation concentrate. Therefore, the dosage of the oleic acid should not be too large. Therefore, the dosage of the oleic acid is set as 1200 g/t.
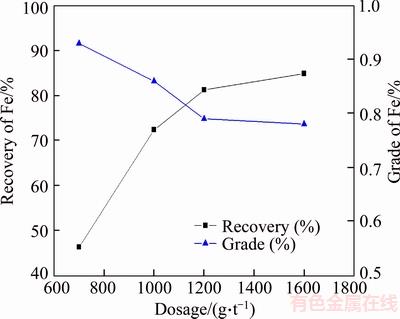
Figure 10 Results of oleic acid flotation experiments
Experiments aimed at investigating the effect of temperature on the oleic acid flotation was also carried out with 1200 g/t oleic acid used, the results are shown in Figure 11. It can be seen from Figure 11 that the Fe grade in reverse flotation concentrate went up from 0.67% to 0.93%, while the Fe recovery dropped down from 83.61% to 48.47% with the temperature decreasing from 45 to 15 °C for the poor solubility and collecting ability of oleic acid at low temperature, which will restrict the utilization of the oleic oil as a collector.
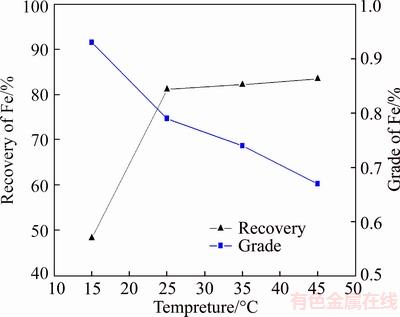
Figure 11 Results of temperature experiments with use of oleic acid
3.2 Characteristic of Yb105
For the merit of low cost and quite fine collecting performance, and to overcome demerit of the temperature sensitivity of the oleic acid, it is necessary to modify the oleic acid to obtain a new kind of collector. In this work, a new collector named Yb105 was obtained by modifying the oleic acid with 5% benzohydroxamic acid, and the collecting capacity for iron of the new collector in reverse flotation was investigated at different temperatures with 1400 g/t Yb105(1000 g/t for roughing, 400 g/t for scavenging), the results are shown in Figure 12.
The results illustrated in Figure 12 show that temperature exerted little influence on the collecting capacity of Yb105. Besides, compared with oleic acid, a concentrate with a higher iron recovery rate and a lower iron grade can be obtained with the same amount of Yb105 used at the same temperature.
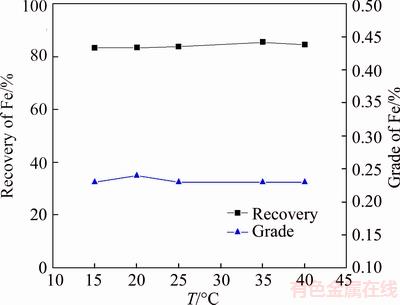
Figure 12 Results of temperature experiments with use of Yb105
It is known that the superior properties of high efficiency, low toxicity and selectivity make the benzohydroxamic acid a good chelating collector (Figure 13). Mulliken charges and NBO charges populations’ analysis of the benzohydroxamic acid are shown in Table 4.
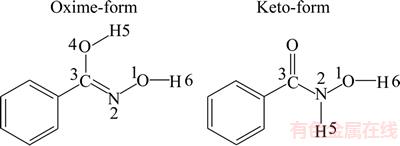
Figure 13 Two configurations of benzohydroxamic acid
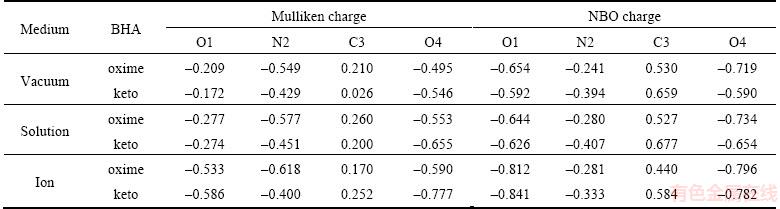
As shown in Table 4, charges of benzohydroxamic acid ion in aqueous solution are mainly distributed in the O1, O4 and N2 of hydroxamic groups, especially the keto configuration and charges distribution more concentrated, which is in favor of the effect of the metal ions on the surface of the metal ions and minerals. As the addition of benzohydroxamic acid in oleic acid, a more stable chelate will be formed by benzohydroxamic acid and iron for the existence of —C(O)NHOH and cyclobenzene in benzohydroxamic acid, which can effectively enhance the performance of the oleic acid with a low cost.
Table 4 Mulliken charges and NBO charges populations analysis of benzohydroxamic acid
According to the analysis above, the iron oxide ore is the main impurity ore in the potash feldspar, which will hydrolyse to hydroxyl compounds and iron ions. The new type collector combines the benzohydroxamic acid with oleic acid to make them work together. On one hand, the carboxyl in oleic acid fixates on the surface of oxidized ore by adsorption or complexation while the non-polar group in oleic acid facing out, which endows the grains of ore with a property of hydrophobic, on the other hand, the —C(O)NHOH group in the benzohydroxamic acid possess a strong chelating ability, which can chelate with metal ions to form stable chelate, and the л—л conjugated bond in the benzene ring can enhance the stability of chelate. A stable O—O chelate penta cyclic compound could be obtained through the interaction between the bidentate in benzohydroxamic acid and the iron ions in ores, and the hydrophobic hydroxyl in the benzohydroxamic acid will enable the iron ore rise. In this way, the iron is effectively removed from the potash feldspar by the new collector at a low cost and under a mild condition.
3.3 Effect of grinding fineness
With 1000 g/t Yb105 used in roughing and 400 g/t used in scavenging, the effect of grinding fineness on the reverse flotation of iron removal was investigated. The results are shown in Figure 14.
It is apparent from Figure 14 that as the proportion of the particles smaller than 0.074 mm went up from 55% to 65%, the iron recovery in tailings increased from 76.54% to 83.85%. However, the further increase of the proportion makes little contribution to the iron recovery, besides, the finer grain will result in a higher specific surface area, which will cause a waste of collector and a serious inclusion. Therefore, the proportion of the particles smaller than 0.074 mm was fixed at 65% in the following experiments.
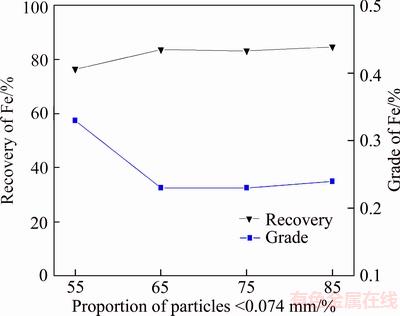
Figure 14 Results of grinding fineness experiments
3.4 Effect of reagent dosage
The proportion of the particles smaller than 0.074 mm was kept at 65%, and Yb105 was used as collectors, the effect of reagent dosage on the recovery of iron was investigated, the result is shown in Figure 15.
As shown in Figure 15, there was a distinct increase in the iron recovery in the tailing with the dosage of Yb105 increases from 1000 to 1400 g/t, but the increase of the dosage of Yb105 to 1700 g/t made little increase of iron recovery in tailing. As the surface activity of iron ions is different on the mineral surface, only part of them can chelate with benzohydroxamic acid or react with oleic acid to form a stable chelate, thus, once the iron ions with a certain activity is used up, further increasing amount of collector can make little different in iron remove. And a large reagent dosage can not only increase the amount of floating impurities in the process of flotation to reduce the flotation selectivity but also cause a waste of reagent, therefore, the dosage of Yb105 was set at 1400 g/t (1000 g/t for roughing, 400 g/t for scavenging), and a reverse flotation concentrate with Fe grade of 0.23% and yield of 82.55% could be obtained under this condition.
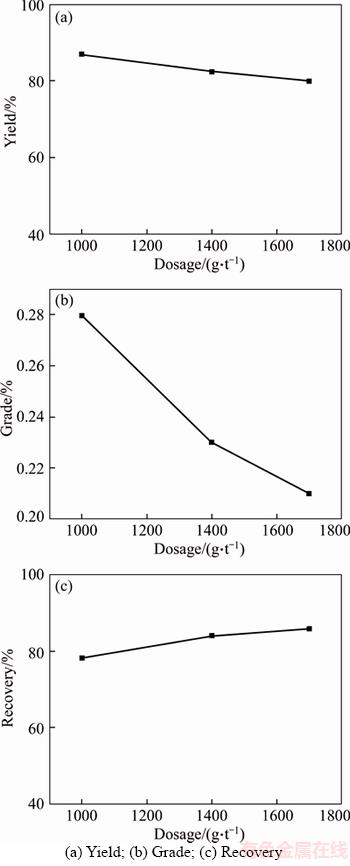
Figure 15 Results of reagent dosage experiments:
3.5 Analysis of concentrate and tailing
The XRD and chemical analysis results of the concentrate and the tailing obtained under the optimized conditions are shown in Table 5,Figures 16 and 17.
Table 5 Chemical analysis of concentrate and tailing (Mass fraction, %)
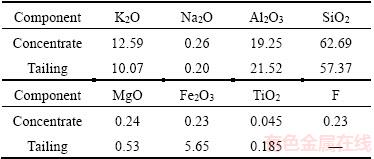
Figure 16 X-ray diffraction pattern of reverse flotation concentrate
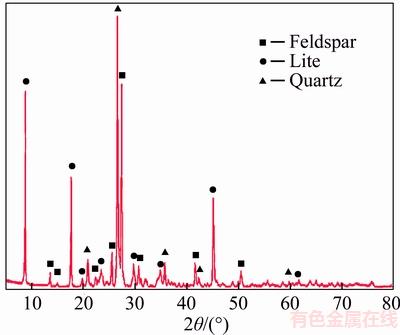
Figure 17 X-ray diffraction pattern of reverse flotation tailing
The results show that with Yb105 as collector in anionic reverse flotation technology, the impurity minerals such as sericite, kaolinite, limonite and hematite can be effectively removed. The main components in reverse flotation concentrate were quartz and potash feldspar despite a small amount of hydromuscovite which was caused by the alteration of potash feldspar and distributed in the feldspar particles. The content of Fe in reverse flotation concentrate was still 0.23%.
4 Conclusions
1) The main mineral in the ore is potassium feldspar, followed by sericite, quartz, kaolinite, and so on. The metal minerals are mainly limonite and hematite, and a small amount of magnetite, ilmenite, pyrite, rutile and so on. Iron is the main harmful elements affecting the quality of potash feldspar product, which mainly exists in hematite and limonite with content of 75.44%; followed by 17.71% in silicate. Part of the iron distributes on the surface and fracture of white mica and potassium feldspar, which is difficult to be removed by beneficiation.
2) A new combined collector named Yb105 was obtained by modifying oleic acid with benzohydroxamic acid, which can effectively remove iron from the potassium feldspar ore at a low cost. By using Yb105 as collector in reverse flotation, a concentrate can be obtained with Fe, K2O, Na2O grades of 0.23%, 12.59% and 0.26%, respectively, and the yield is 82.55%.
3) The addition of a small amount of benzohydroxamic acid into the oleic acid can not only increase the recovery of iron and reduce the consumption of oleic acid collector, but also enhance the collecting performance of oleic acid at low temperature, which can realize the flotation of the ores at a low temperature and play an important role in saving energy to some extent. In particular, the utilization of the new collector can greatly reduce the cost of flotation process in the industry.
References
[1] BAYAT O, ARSLAN V, CEBECI Y. Combined application of different collectors in the floatation concentration of Turkish feldspars [J]. Minerals Engineering, 2006, 19(1): 98–101.
[2] LIU J N, ZHAI Y C, WU Y, ZHANG J, SHEN X Y. Kinetics of roasting potash feldspar in presence of sodium carbonate [J]. Journal of Central South University, 2017, 24(7): 1544-1550.
[3] LI H, FAN X, WANG G, LIU D. Studies on conversion of K in potash feldspar into water-soluble form [J]. Chinese Journal of Chemical Engineering, 2001, 9(4): 447–450.
[4] BURAT F, KOKKILIC O, KANGAL O, GURKAN V, CELIK M S. Quartz-feldspar separation for the glass and ceramics industries [J]. Minerals & Metallurgical Processing, 2007, 24(2): 75–80.
[5] CHEN L Z, LIAO G P, QIAN Z H, CHEN J. Vibrating high gradient magnetic separation for purification of iron impurities under dry condition [J]. International Journal of Mineral Processing, 2012, 102–103: 136–140.
[6] LEI, S M, MA Q L, HUANG D D, LU Y. Experimental research on processing and iron removal of feldspar applied in the glass industry [J]. Mining Research and Development, 2014, 34(1): 48–51. (in Chinese)
[7] HU M Z. Removing iron by magnetic separation from a potash feldspar ore [J]. Journal of Wuhan University of Technology–Materials Science Edition, 2013, 28(2): 362–366.
[8] CAO Z F, QIU P, ZHONG H, WANG M M, YUE Y J, FAN F. A novel approach for flotation recovery of copper and molybdenite from a copper-arsenic ore [J]. Metall Res Technol, 2016, 113(1): 103–111.
[9] CAO Z F, YUE Y J, ZHONG H, QIU P, CHEN P, WEN X, WANG S, LIU G Y. A novel approach for flotation recovery of molybdenite, galena and pyrite from a complex molybdenum-lead ore [J]. Metall Res Technol, 2017, 114(2): 212–211.
[10] WANG H Y, LI X J. Magnetic separation technology and equipment research for super-pure feldspar [J]. Conservation and Utilization of Mineral Resources, 2004(3): 37–38. (in Chinese)
[11] LEE S O, TRAN T, PARK Y Y, KIM S J, MYONG J. Study on the kinetics of iron oxide leaching by oxalic acid [J]. International Journal of Mineral Processing, 2006, 80(2): 144–152.
[12] AMBIKADEVI V R, LALITHAMBIKA M. Effect of organic acids on ferric iron removal from iron-stained kaolinite [J]. Applied Clay Science, 2000, 16(3): 133–145.
[13] TAXIARCHOU M, PANIAS D, DOUNI I, PASPALIARIS I, KONTOPOULOS A. Dissolution of hematite in acidic oxalate solutions [J]. Hydrometallurgy, 1997, 44(3): 287–299.
[14] CELIK M S, CAN I, EREN R H. Removal of titanium impurities from feldspar ores by new flotation collectors [J]. Minerals Engineering, 1998, 11(12): 1201–1208.
[15] HEYES G W, ALLAN G C, BRUCKARD W J, SPARROW G J. Review of flotation of feldspar [J]. Mineral Processing and Extractive Metallurgy, 2012, 121(2): 72–78.
[16] DOGU I, AROLA I. Separation of dark-colored minerals from feldspar by selective flocculation using starch [J]. Powder Technology, 2004, 139(3): 258–263.
(Edited by FANG Jing-hua)
中文导读
采用苯甲羟肟酸强化钾长石矿物中铁的有效浮选分离
摘要:研究湖南某地钾长石矿石的工艺矿物学,并设计开发了新型捕收剂Yb105强化浮选分离钾长石矿石中的铁矿物。工艺矿物学研究表明,实验矿石的主要成分为长石、绢云母、石英和高岭石等,铁主要赋存于褐铁矿和赤铁矿中,其中大部分可通过选矿去除,但各矿物之间堪布紧密,难以高效地浮选分离含铁矿物。研究表明,苯并异羟肟酸不仅可以提高铁的去除率,减少油酸捕收剂的消耗,而且可以提高油酸在低温下的捕收性能,实现钾长石在低温下的浮选,这在一定程度上对节能是十分重要的;与油酸相比,苯甲羟肟酸对钾长石脱除铁有明显的强化,以Yb105为捕收剂,通过浮选可得到铁品位0.23%,K2O品位12.59%,Na2O品位0.26%的优质钾长石精矿,精矿产率为82.55%。
关键词:钾长石;除铁;反浮选;苯并异羟肟酸
Foundation item: Project(21776320) supported by the National Natural Science Foundation of China; Project(2016TP1007) supported by the Hunan Provincial Science and Technology Plan Project, China
Received date: 2017-06-06; Accepted date: 2018-01-11
Corresponding authors: WANG Shuai, PhD, Professor; Tel: +86–731–88836603, Fax: +86–731– 88879616; E-mail: wangshuai@ csu.edu.cn; ORCID: 0000-0002-1678-0211; ZHONG Hong, PhD, Professor; Tel: +86–731– 88836603, Fax: +86–731–88879616; E-mail: zhongh@csu.edu.cn; ORCID: 0000-0002-9536-2172
Abstract: The technological mineralogy of the potash feldspar was investigated and a new collector named Yb105 was adopted to remove iron from potash feldspar ores. The technological mineralogy results indicate that the main components of the ore were feldspar, sericite, quartz and kaolinite, and iron mainly existed in limonite and hematite, most of which can be removed by beneficiation. The results show the benzohydroxamic acid can not only increase the recovery of iron and reduce the consumption of oleic acid collector, but also enhance the collecting performance of oleic acid at low temperature, which can realize the flotation of the ores at a low temperature and play an important role in saving energy to some extent. Compared with oleic oil, the benzohydroxamic acid had a great advantage in removing iron from potash feldspar, a potash feldspar concentrate with Fe grade of 0.23%, K2O grade of 12.59% and Na2O grade of 0.26% was obtained by flotation with Yb105 as collector, and the yield of the concentrate was 82.55%.


