
Synthesis of rutile from high titania slag by pyrometallurgical route
ZHANG Li1, LI Guang-qiang2, ZHANG Wu1
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China;
2. School of Materials and Metallurgy, Wuhan University of Science and Technology, Wuhan 430081, China
Received 30 October 2010; accepted 27 May 2011
Abstract:
A new technique was developed for the conversion of high titania slag, containing 70%-75% TiO2 and with MgO, FeO, CaO, Al2O3 and SiO2 as main impurities, into a synthetic rutile, 90%-95% TiO2, which satisfies the requirements for fluidizing chlorination process with respect to impurity contents. After a pre-oxidation at around 1 042 °C and a heat-treatment above 1 510 °C, the Ti components in high titania slag can be enriched into the rutile phase which can precipitate and grow, and can be separated with dilute hydrochloric and sulfuric acid, respectively. The results show that the average crystal size of rutile phase is over 25 mm, and the synthetic rutile containing more than 95% TiO2 can be produced by selective leaching.
Key words:
high titania slag; oxidation; leaching; rutile;
1 Introduction
During the smelting of ilmenite by a suitable carbonaceous reductant, high titania slag containing 60%-80% TiO2 was produced. The slag represents a more valuable raw-material for the sulphate process, but it is not suitable as a raw material for a pigment production by the chlorination process because of the large amount of impurity, such as SiO2, Al2O3, CaO, MnO, V2O5 and MgO, especially, the high CaO and MgO contents. Therefore, it is necessary to develop a new technique for increasing the TiO2 content in the slag [1]. The main titanium-containing phase in the slag is M3O5 phase (anosovite) which is composed of Ti4+ and Ti3+, and contains some Al2O3 and other impurities. In addition to the M3O5 phase, the solidified slag contains a glassy silicate phase, in addition to SiO2 comprises MgO, CaO and TiO2 [2]. Although most of the Ti components in the slag could be enriched into M3O5 phase through a heat treatment, and M3O5 crystal could grow and coarsen, but it is difficult to separate M3O5 phase from the slag through an ore-dressing method due to its high fragility and low hardness [3].
On the basis of high level Ti3+ in the slag and thermodynamic considerations of the oxidation of Ti3+ to Ti4+ [4-12], after modifying by a pre-oxidation at low temperature (around 1042 °C) and a heat treatment at higher temperature(>1500 °C), Ti3+ in the slag was oxidized to Ti4+, and most of the Ti components in the slag were taken up into the rutile phase which is composed of Ti4+ due to the addition of P2O5 and CaO. In addition, the rutile phase could fully grow and coarsen during the heat treatment. Since the rutile cannot be dissolved in dilute hydrochloric acid and sulfuric acid, it is suitable for the rutile phase to be separated from modified slag by leaching the impurities with dilute acid. In order to obtain further information about the precipitation and separation of the rutile phase, it is necessary to study the conversion and separation of high titania slag.
The purpose of the present work is to study the selective separation of the Ti components in high titania slag through three aspects: 1) the selective enrichment of the Ti components into the rutile phase; 2) the selective precipitation and growth of the rutile phase; 3) the selective separation of the rutile phase from impurities.
2 Experimental
The slag from Panzhihua Iron and Steel Corporation was used in this study. The chemical composition of the slag is listed in Table 1. The additive agents of P2O5 and CaO were analytical grade. The slag for experiments was milled to 120 mm and dried in an oven at 100 °C for 3 h.
Table 1 Composition of high titania slag and dosages of CaO and P2O5 additives
The experiments with 200 g slag were carried out in a horizontal MoSi2 furnace fitted with a type thermocouple, which was controlled by a Shimaden SR-53 temperature programmed control instrument. The length of isothermal section was 60 mm, and the temperature accuracy was within ±3 °C. The pre-oxidation and the heat-treatment of the slag were carried out under static air.
The experiment consisted of three steps: 1) Ti2+ and Ti3+ were pre-oxidized to Ti4+ during the pre-oxidation process at 1 042 °C; 2) After the pre-oxidized slag was mixed with the additive agents of P2O5 and CaO, the enrichment and growth of the Ti components were carried out through the heat-treatment at higher temperature under static air (>1 500 °C); 3) The selective separation of the Ti components was carried out through leaching the treated slag with dilute hydrochloric and sulfuric acid, respectively.
The un-oxidized and oxidized slag samples were polished and then characterized by Quantime520 image analyzer, scanning electron microscope (SEM) equipped with energy dispersive spectrometer (EDX) and X-ray diffractometer (XRD). The volume fraction and average grain size of the rutile phase were measured on a Quantime520 image analyzer by the line intercept method (the average of 10 fields).
The TiO2 contents in the un-oxidized, oxidized slag and leaching product were determined by ammonium ferric sulfate titration.
3 Results and discussion
3.1 Selective enrichment of Ti components in slag
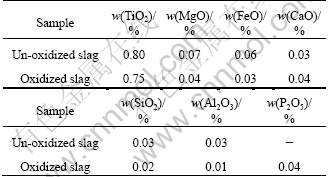
After cooling, the mineral phases in the un-oxidized and oxidized slags were examined by XRD, SEM and EDS. The XRD patterns of the un-oxidized slag, the slag pre-oxidized at 1 042 °C and the slag oxidized at 1 510 °C are shown in Fig. 1. The SEM images of the un-oxidized and oxidized slags are shown in Fig. 2 and Fig. 3. It can be seen that the main mineral phases in the un-oxidized slag are anosovite (M3O5), pseudobrookite and iron; when pre-oxidizing at 1 042 °C, the rutile phase gradually precipitates, and the phase changes from the anosovite (M3O5) and pseudobrookite to a mixture of the rutile, anosovite (M3O5) and pseudobrookite; but oxidizing at 1 510 °C favours the transport of oxygen due to the molten state and the increase oxidation reaction rate, which results in most of Ti2+ and Ti3+ in the slag being oxidized to Ti4+, so the anosovite phase (M3O5) in the slag almost vanishes. The oxidation at 1 510 °C together with the additive agents alters the structure of the slag to rutile phase in a phosphate matrix, most of the Ti components are enriched into the rutile phase and almost all the impurities partition to the phosphate glassy matrix. Consequently, there only is the rutile phase as a titanium-containing phase and a phosphate glassy phase.
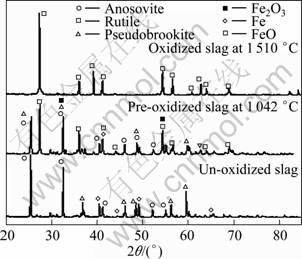
Fig. 1 XRD patterns of un-oxidized slag and oxidized slag at different temperatures

Fig. 2 Back-scattered electron image of un-oxidized slag (Sp1—Anosovite; Sp2—Silicate glass; Sp3—iron)
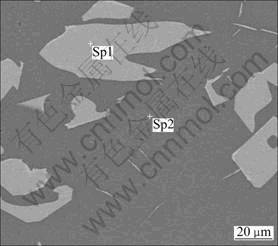
Fig. 3 Back-scattered electron image of slag oxidized at 1 510 °C (4% CaO, 4% P2O5) (Sp1—Rutile; Sp2—Phosphate glassy phase)
During the oxidation process at 1 510 °C, the majority of the impurities are transferred into the glassy phase in the form of phosphate. The reason is that the phosphorus oxide in the molten slag is an acid oxide, which reacts strongly with the basic oxide in the molten slag including FeO, MgO, CaO and so on. Accordingly, most of the impurities in the molten slag are selectively enriched into the phosphate glassy phase.
3.2 Selective precipitation and growth of Ti components in slag
Based on studies on the growth and coarsening of the Ti components in Ti-bearing slag [13-16], the effects of the melting temperature, the cooling rate and the holding time on the volume fraction and the grain size of the rutile phase were studied in order to optimize the heat-treatment conditions. The experimental results show that the melting temperature, the cooling rate and the holding time have significant effects on the grain size and a little effect on the volume fraction of the rutile phase (Figs. 4-6). This indicates that the precipitation rate of the rutile phase is very quick and it mainly occurs during the isothermal process, but the growth and coarsening of the rutile phase occur both in the isothermal and the cooling stages.
3.3 Selective separation of Ti components in slag
On the basis of the selective enrichment and precipitation, the effects of the stirring rate, the leaching temperature and the acid concentration on the leaching of the modified slag were studied. The leaching results in hydrochloric acid are shown in Figs. 7-9. The experimental results indicate that the stirring speed has little effects on the TiO2 content, and causes the modified slag to be suspended in the leaching solution. Compared with the stirring speed, the leaching temperature and the leaching time markedly affect the TiO2 content (Fig. 8). When the leaching temperature is 70 °C and leaching time is 10 min, the majority of the impurities are dissolved, and the TiO2 content in the product approaches 96%. When the leaching temperature is 35 °C and the leaching time is 10 min, a part of the impurities are dissolved in hydrochloric acid, and the TiO2 content in the product approaches 93%. But with a longer time, the TiO2 content change is negligible. The leaching rate markedly increases in the region of 35-55 °C, but very little in the region of 55-70 °C. The acid concentration also affects the leaching rate. The higher the acid concentration is,the higher the TiO2 content in the product is.
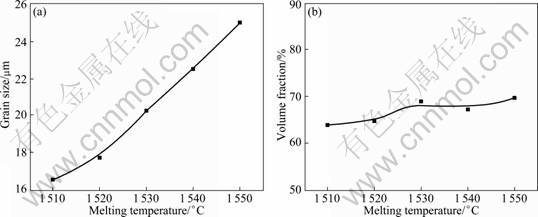
Fig. 4 Relationship between grain size (a) and volume fraction (b) of rutile phase with melting temperature
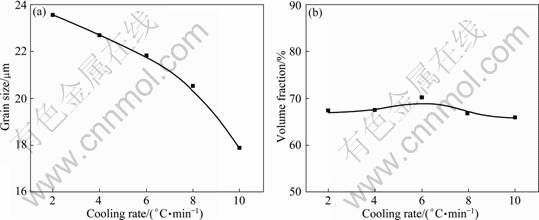
Fig. 5 Relationship between grain size (a) and volume fraction (b) of rutile phase with cooling rate
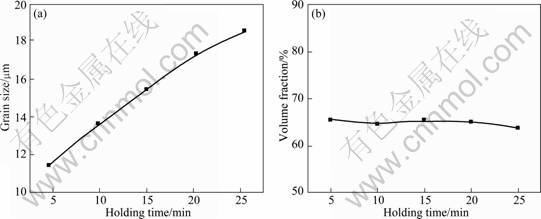
Fig. 6 Relationship between grain size (a) and volume fraction (b) of rutile phase with holding time
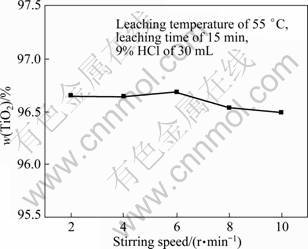
Fig. 7 Effect of stirring speed on TiO2 content in leaching product
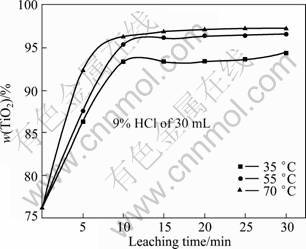
Fig. 8 Effects of leaching temperature and time on TiO2 content in leaching product
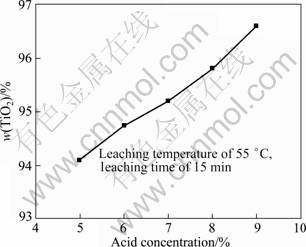
Fig. 9 Effect of acid concentration on TiO2 content in leaching product
X-ray diffraction analysis of the leaching product (un-dissolved phase) of the modified slag through a heat-treatment at high temperature (1 510 °C) is shown in Fig. 10. It can be seen from Fig. 10 that the main mineral phase in the leaching product is rutile, which contains a little metallic iron. This indicates that most of the impurities in the modified slag are dissolved in hydrochloric acid during the leaching process.
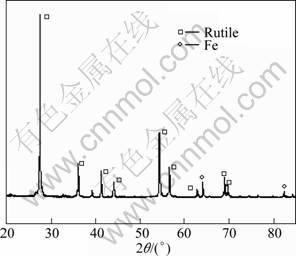
Fig. 10 XRD pattern of leaching product of modified slag at 1 510 °C
The TiO2 content in the leaching product of the slag pre-oxidized at low temperature (1 042 °C) is lower than that of the slag oxidized at high temperature (1 510 °C) (Fig. 11). X-ray diffraction analysis of the leaching product of the pre-oxidized slag is shown in Fig. 12. It indicates that the oxidation at higher temperature (1 510 °C) contributes to the leaching rate increase of the slag. The reason is that the enrichment of the Ti components in the pre-oxidized slag due to the oxidation of the solid state at low temperature (1 042 °C) is markedly lower than that in the modified slag due to the addition of P2O5 and the oxidation of the molten slag at high temperature (1 510 °C) (Fig. 1).
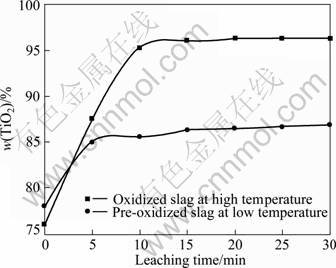
Fig. 11 Effect of pre-oxidizing at low temperature (around 1 042 °C) and oxidizing at high temperature (1 510 °C) for slag on TiO2 content in leaching product (leaching temperature 55 °C, 9% HCl 30 mL, stirring speed 4 r/min)
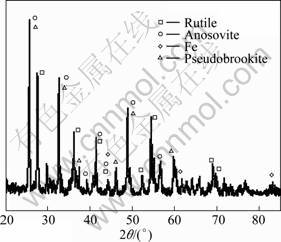
Fig. 12 XRD pattern of leaching product of slag pre-oxidized at 1 042 °C
The leaching experiments of the modified slag with sulfuric acid were carried out. Figure 13 shows the effect of leaching time on the TiO2 content in the leaching product. When the leaching temperature is 35 °C, the consumption of sulfuric acid is 140 mL and the leaching time is 250 min. The TiO2 content in the product approaches 89%, which is lower than that in the product leached in hydrochloric acid at 35 °C. Meanwhile, the leaching time of the modified slag with sulfuric acid is much greater than that in hydrochloric acid (Fig. 8). This indicates that the leaching result of the modified slag with hydrochloric acid is much better than that with sulfuric acid.
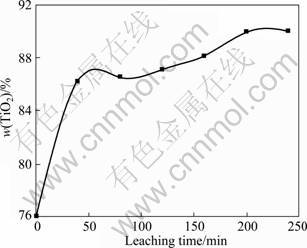
Fig. 13 Effect of sulfuric acid leaching time on TiO2 content in leaching product (leaching temperature 35 °C, stirring speed 4 r/min, 20% H2SO4 140 mL)
4 Conclusions
1) A modification of high titania slag through the additions of CaO and P2O5 together with a pre-oxidation at around 1 042 °C and a heat-treatment above 1 510 °C is in favor of the selective enrichment of the Ti components into the rutile phase, and promotes the precipitation and growth of the rutile phase.
2) The separation of the modified slag is carried out with dilute hydrochloric acid by a selective leaching. Stirring is necessary for the leaching. With a longer leaching time, by increasing leaching temperature and acid concentration, the TiO2 content in the leaching product increases. The leaching effect of the modified slag in dilute hydrochloric acid is much better than in dilute sulfuric acid.
References
[1] PISTOURIOUS P C, COETZEE C. Physicochemical aspects of titanium slag production and solidification [J]. Metals and Material Trans B, 2003, 34: 581-586.
[2] MO Wei. Titanium metallurgy [M]. Beijing: Metallurgical Industry Press, 1999: 182-192. (in Chinese)
[3] BESSINGER D, GELDENHURIS J M A, PISTOURIOUS P C. The decrepitation of solidified high titania slags [J]. Journal of Non-Crystalline Solids, 2001, 282: 132-142.
[4] ZHANG Li, LI Guang-qiang, LOU Tai-ping, SUI Zhi-tong. Selective enrichment and growth of Ti component in titaniferrous slag [J]. Acta Metallurgical Sinica, 2002, 38(4): 400-403. (in Chinese)
[5] ZHANG Li, ZHANG Lin-nan, WANG Ming-yu. Effect of perovskite phase precipitation on viscosity of Ti-bearing blast furnace slag under the dynamic oxidation condition [J]. Journal of Non-Crystal Solids, 2006, 352: 123-129.
[6] ZHANG Li, ZHANG Lin-nan, SUI Zhi-tong. Dynamic oxidation of the Ti-bearing blast furnace slag [J]. ISIJ International, 2006, 46: 458-465.
[7] ZHANG Li, ZHANG Lin-nan, WANG Ming-yu. Precipitation selectivity of perovskite phase from Ti-bearing blast furnace slag under the dynamic oxidation condition [J]. Journal of Non-Crystal Solids, 2007, 353: 2214-2290.
[8] ZHANG Li, WANG Ming-yu, SUI Zhi-tong. Recovery of titanium compounds from Ti-bearing blast furnace slag under the dynamic oxidation condition [J]. Minerals Engineering, 2007, 20: 684-693.
[9] FU Nian-xin. The selective precipitation of Ti component in Ti-bearing blast furnace slag [D]. Shenyang: Northeastern University, 1997: 2. (in Chinese)
[10] LOU Tai-ping, SUI Zhi-tong. The isothermal growth of perovskite blast furnace slag bearing titania [J]. Acta Metallurgical Sinica, 1999, 35(8): 834-838. (in Chinese)
[11] LOU Tai-ping, LI Yu-hai, SUI Zhi-tong. Study of precipitation of perovskite phase from the oxide slag [J]. Acta Metallurgica Sinica, 2000, 36(2): 140-144. (in Chinese)
[12] XIA Yu-hu, SUI Zhi-tong. Computer simulation of phase separation in CaO-MgO-Fe2O3-Al2O3-SiO2 glass [J]. Journal of Northeastern University: Natural Science, 1999, 5: 511-514. (in Chinese)
[13] LI Yu-hai, LOU Tai-ping, SUI Zhi-tong. Kinetics of non-isothermal precipitate process of perovskite phase in CaO-TiO2-SiO2- Al2O3-MgO system [J]. Journal of Materials Science, 2000, 35: 5635-5637.
[14] SUI Zhi-tong, LOU Tai-ping, ZHANG Pei-xin. Precipitating selectivity of boron and titanium components from the slags [C]//Proceedings of 121th Japan Institute of Metals. Sendai, 1997: 471.
[15] SUI Zhi-tong. Precipitation selectivity of boron components from slags [J]. Acta Mater, 1999, 47(4): 1337-1344.
[16] WANG Ming-yu, ZHANG Li. Selective enrichment of TiO2 and precipitation behavior of perovskite phase [J]. Transactions of Nonferrous Metals Society of China, 2006: 16(2): 421-425.
低品位高钛渣中钛组分制备金红石
张 力1, 李光强2, 张 武1
1. 东北大学 材料与冶金学院,沈阳 110004;
2. 武汉科技大学 材料与冶金学院,武汉 430081
摘 要:介绍了以70%~75% TiO2的低品位高钛渣为原料制备人造金红石的分离工艺。低品位高钛渣中MgO, FeO, CaO, Al2O3 和SiO2进入杂质相,钛组分进入金红石相,金红石相中TiO2品位达到90%~95%,可满足流态化氯化对杂质的要求。1 050 °C的低温预氧化与1 510 °C的高温热处理促使渣中分散于各矿物相的钛组分选择性转移并富集于金红石相,金红石相析出与长大,用稀硫酸和稀盐酸实现金红石相的分离。 实验结果表明,金红石矿物相平均晶粒度可以达到25 μm,通过稀酸选择性浸出改性渣,可以获得95%TiO2品位的人造金红石。
关键词: 高钛渣;氧化;浸出;金红石
(Edited by YANG Hua)
Foundation item: Project (FMRU2007K10) supported by the Open Research Fund of Key Laboratory for Ferrous Metallurgy and Resources Utilization of Ministry of Education, China
Corresponding author: ZHANG Li; Tel: +86-24-83670456; E-mail: zhangl@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)61014-5


