蒙脱石对乙基钾黄药-铅复合污染体系的吸附平衡与动力学
郝艳,吴小莲,莫测辉,李彦文,黄献培,屈相龙,向垒,梁嘉华
(1. 暨南大学 环境工程系,广东省高校水土环境毒害性污染物防治与生物修复重点实验室,
广东 广州,510632)
摘 要:
金属复合污染体系的环境行为,以蒙脱石为吸附剂进行静态吸附试验,探讨乙基钾黄药-铅复合污染体系的吸附平衡与动力学特征。研究结果表明:乙基钾黄药在蒙脱石上的吸附作用很小,它通过与Pb2+反应形成悬浮态物质而严重影响Pb2+在蒙脱石上的吸附作用,使Pb2+的吸附速率常数由10.40 g/(mg·min)提高到54.76 g/(mg·min),而平衡吸附量(qe)则由9.80 mg/g降低到4.27 mg/g;影响程度随着乙基钾黄药浓度的升高而增大,随着Pb2+浓度、pH和蒙脱石投加量的升高而减弱。研究结果可为黄药-重金属复合污染环境的治理提供参考。
关键词:
中图分类号:X53 文献标志码:A 文章编号:1672-7207(2011)10-3213-07
Adsorption equilibrium and kinetics of potassium ethyl xanthate-lead combined pollutants on montmorillonite
HAO Yan, WU Xiao-lian, MO Ce-hui, LI Yan-wen, HUANG Xian-pei,
QU Xiang-long, XIANG Lei, LIANG Jia-hua
(1. Key Laboratory of Water/Soil Toxic Pollutants Control and Bioremediation, Department of Education
of Guangdong Province, Department of Environment Engineering, Jinan University, Guangzhou 510632, China)
Abstract: Adsorption equilibrium and kinetics of potassium ethyl xanthate-lead combined pollutants to montmorillonite by static adsorption experiments were investigated to reveal environmental behavior of xanthate and heavy metals in a combined pollution system. The results show that adsorption of potassium ethyl xanthate on montmorillonite is slight. Adsorption of Pb2+ to montmorillonite is seriously affected in the presence of potassium ethyl xanthate owing to a suspended product of the reaction between Pb2+ and potassium ethyl xanthate, adsorption rate constant increases from 10.40 g/(mg·min) to 54.76 g/(mg·min), while equilibrium adsorption capacity (qe) decreases from 9.80 mg/g to 4.27 mg/g. The impact degree improves with the increase of potassium ethyl xanthate concentration, but reduces with the increase of Pb2+ concentration, pH, and montmorillonite dosage. The findings may serve as an important reference for the control of xanthate and heavy metals combined pollution.
Key words: montmorillonite; potassium ethyl xanthate; lead; pollution control; adsorption isotherms; kinetics
黄药学名为黄原酸盐,又称为烃基二硫代碳酸盐,化学通式为ROCSSMe,其中Me为Na+或者K+。黄药被大量用于金属矿山选矿中作为浮选捕收剂[1-2],最终在尾矿库中形成高浓度残留,可通过地下渗漏、地表溢流等途径,进入矿区周边或流域的环境中,造成水体、土壤和农作物污染[3],对生态环境和人体健康构成严重威胁[4-7]。环境中黄药属于中-高毒污染物[8],对生物的神经系统、造血系统和肝脏等器官均有损害,甚至在较低质量浓度(5 mg/L)下即可造成大部分鱼类死亡。而且黄药能够与矿山环境中重金属发生相互作用而对其环境产生扩大危害[9]。目前国内外对于金属矿山环境问题的研究主要针对重金属[10-15],而对于黄药的环境行为及其与重金属相互作用的研究很 少 [16-20],对于黄药-重金属复合污染体系吸附行为的研究尚未见报道。为此,本文作者以广泛使用的蒙脱石为吸附剂进行静态吸附试验,探讨乙基钾黄药-铅复合污染体系的吸附平衡与动力学特征,以期为黄药-重金属复合污染环境的治理提供科学依据。
1 材料与方法
1.1 实验材料与仪器
实验材料为:吸附剂蒙脱石由华南理工大学提供,其粒径<2 μm,比表面积为700 m2/g,阳离子交换量为86 mmol/(100 g);乙基钾黄药(乙基黄原酸钾)购自上海润捷化学试剂有限公司,纯度≥95.0%;Pb标准品购自钢铁研究总院国家钢铁材料测试中心,介质为10%硝酸;实验用水为高纯水。
仪器为:AA-7000原子吸收分光光度计(岛津);雷磁PHS-3C精密pH计;TGL-16G型高速台式离心机;SHZ-82恒温震荡器;TU-1810 PC型紫外可见分光光度计。实验所用器皿主要为聚丙烯塑料管;玻璃仪器均以硝酸(1+5)浸泡过夜,用水反复冲洗,最后用高纯水冲洗干净后烘干备用。
1.2 实验方法
1.2.1 反应时间对吸附效果的影响
吸附实验参照 OECD guideline 106 批平衡方法进行[21]。称取蒙脱石0.250 0 g置于50 mL 聚丙烯塑料管中,分别加入25 mL的黄药溶液(处理1)、Pb2+溶液(处理2)、Pb2++黄药溶液(处理3)中,同时设置不含蒙脱石作为对照处理。黄药和Pb2+的质量浓度分别为20 mg/L和100 mg/L。在25 ℃下恒温振荡(250 r/min),分别在10,20,30,40和60 min时采集样品,离心10 min(转速为6 000 r/min)。取处理3及其对照处理的上清液1 mL于试管中稀释,作为黄药备测液。将剩余上层溶液移至容量瓶中,加入浓硝酸进行溶解,用高纯水定容后过孔径为0.45 μm的滤膜备测。处理1和处理2及其对照处理直接取上清液备测。Pb含量用原子吸收分光光度法测定,黄药含量用紫外可见分光光度计测定(波长为301 nm)[22]。每次实验均重复做3次(下同)。
蒙脱石对黄药或Pb2+的吸附量由以下公式进行计算:
![]()
其中:qe为吸附量,mg/kg;ρo为初始质量浓度,mg/L;ρe为平衡质量浓度,mg/L;V为溶液体积,L;M为吸附剂质量,g。
1.2.2 初始质量浓度对吸附效果的影响
设置黄药质量浓度为20,40,60,80和100 mg/L,Pb2+初始质量浓度分别为0和100 mg/L,振荡60 min,参照上述方法进行实验和分析;设置Pb2+质量浓度为50,100,200,400,600和800 mg/L,黄药质量浓度分别为0和20 mg/L,振荡60 min,参照上述方法进行实验和分析。
1.2.3 pH对吸附效果的影响
设置Pb2+质量浓度为100 mg/L,黄药质量浓度分别为0和20 mg/L,以3%盐酸溶液或0.5 mol/L氢氧化钠溶液调节pH分别为3,5,7,9和11,参照上述方法进行实验和分析,并测定平衡溶液pH。
1.2.4 蒙脱石用量对吸附效果的影响
设置蒙脱石用量为0,0.01,0.05,0.10,0.20和0.50 g,Pb2+质量浓度为100 mg/L,黄药质量浓度分别为0和20 mg/L,参照上述方法进行实验和分析。
1.3 分析方法
原子吸收分光光度法操作条件如下:波长为283.3 nm;狭缝宽为0.7 mm;点灯方式为BGC-D2;灯电流为10 mA;火焰类型为Air-C2H2;燃气流量为2.0 L/min;助燃气流量为15.0 L/min。
测定质量浓度为1,2,5,10和20 mg/L的黄药标准溶液及0.01,0.05,0.10,0.50,1.00,5.00和10.00 mg/L的Pb2+标准溶液并进行线性回归分析,相关系数均大于0.999 5。
2 结果与讨论
2.1 蒙脱石对黄药的吸附作用
2.1.1 反应时间和初始质量浓度对吸附作用的影响
黄药在水溶液中可因水解、光解等而被降解[16]。因此,为了考察水溶液中黄药的自然降解情况,设置了不投加蒙脱石作为对照处理。反应时间和黄药初始质量浓度对黄药平衡质量浓度的影响分别见图1和图2。结果表明:随着振荡时间的延长,不投加蒙脱石对照处理和投加蒙脱石处理溶液中黄药的质量浓度均呈逐渐降低趋势,前者在10 min和120 min时降解率分别为6.1%和11.2%,后者分别为3.9%和8.0%(图1)。在本实验过程中黄药的降解程度较低,在60 min内降解率均低于10%。
投加蒙脱石处理溶液中黄药的质量浓度高于不投加蒙脱石对照处理的质量浓度,但相差很小(<4%)(见图1和图2)。这说明投加蒙脱石形成的悬浊液降低了黄药的光解,同时也说明蒙脱石对黄药的吸附作用很微弱。这是由于乙基钾黄药在水溶液中本身带负电荷 (ROCS2-)[23],而蒙脱石本身也带负电荷[24]。但活性炭或改性黏土矿物对黄药具有较强的吸附能力[17-19]。
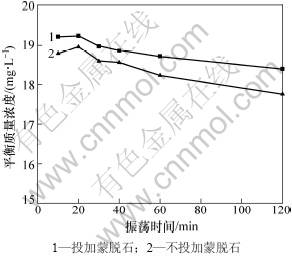
图1 反应时间对黄药平衡质量浓度的影响
Fig.1 Effect of time on xanthate equilibrium concentration
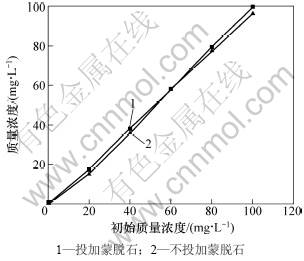
图2 黄药初始质量浓度对平衡质量浓度的影响
Fig.2 Effect of initial concentration on equilibrium concentration
2.1.2 pH和蒙脱石投加量对吸附作用的影响
pH和蒙脱石投加量对吸附作用的影响分别见图3和图4。从图3 和图4可见:pH对溶液中黄药的降解有明显影响。pH越小越有利于黄药的降解[16]。对于不投加蒙脱石对照处理,当pH由11降低到5时,黄药的降解率由5%上升为20%;而当pH下降到3时,黄药的降解率达到100%。投加蒙脱石处理中黄药的质量浓度、变化趋势与去除率均与不投加蒙脱石对照处理的相近。可见:不同pH和不同蒙脱石投加量条件下对黄药的吸附作用均很小。
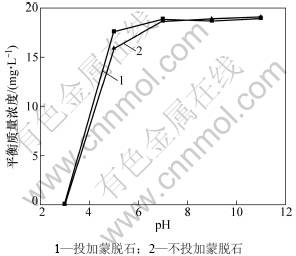
图3 pH对蒙脱石吸附黄药的影响
Fig.3 Effect of pH on xanthate adsorption
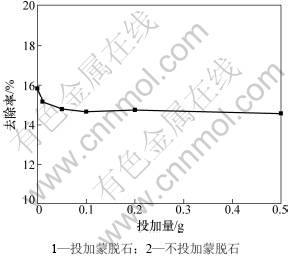
图4 投加量对蒙脱石吸附黄药的影响
Fig.4 Effect of montmorillonite dose on xanthate removal
2.2 黄药对蒙脱石吸附Pb2+的影响
2.2.1 黄药对Pb2+吸附动力学的影响
人们对黏土矿物对重金属的吸附研究较多[25-27]。实验表明:黏土矿物对Pb2+的吸附可以迅速达到平 衡[28-29]。反应时间对蒙脱石吸附Pb2+的影响见图5。从图5可见:无论溶液中是否存在黄药,蒙脱石对Pb2+均在10 min内达到吸附平衡。但当溶液中存在黄药时会严重影响蒙脱石对Pb2+的吸附,吸附量明显降低(降低57%)。主要是因为黄药与Pb2+之间发生化学反应,生成悬浮态的黄原酸铅,从而降低了蒙脱石对Pb2+的吸附量。
对吸附过程进行二级反应动力学拟合:
![]()
其中:k2为平衡速率常数,g/(mg·min);qe为平衡时吸附量,mg/g;qt为t时的吸附量。黄药对Pb2+吸附动力学特征的影响见图6。从图6可见:无论溶液中是否存在黄药,蒙脱石对Pb2+的吸附过程均符合二级动力学方程,具有很好的线性关系(R2>0.999)。吸附速率常数分别为10.404和54.756 g/(mg·min),计算得到2种情况下平衡时吸附量(qe)分别为9.804和4.273 mg/g,分别与实验测定值(9.720和4.205 mg/g)基本一致。
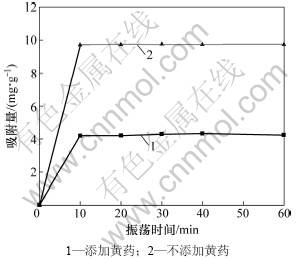
图5 反应时间对蒙脱石吸附Pb2+的影响
Fig.5 Effect of time on Pb2+ adsorption
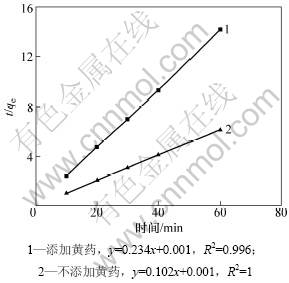
图6 蒙脱石对Pb2+的吸附动力学特征
Fig.6 Adsorption kinetics of Pb2+ to montmorillonite
2.2.2 黄药对Pb2+吸附等温线的影响
蒙脱石对Pb2+的吸附等温线依据Freundlich方程进行拟合:![]() ,两边取对数后为:
,两边取对数后为:
![]()
其中:qe为单位质量蒙脱石对Pb2+的吸附量,mg/g;ρe为平衡溶液中Pb2+质量浓度,mg/L;Kf和n为特征常数。lgKf越大表明吸附能力越强,当0.1<1/n<0.5时,表明吸附过程较易进行[30]。以Freundlich方程拟合所得到的Pb2+吸附等温线见图7。可见:Pb2+吸附等温线相关性较好。计算结果表明:不添加黄药和添加黄药时,lg Kf分别为0.843和0.044,1/n分别0.392和0.682,说明前者的吸附能力较强,也更容易发生吸附作用。黄药对蒙脱吸附Pb2+的影响见图8。可见:当黄药质量浓度一定时(20 mg/L),随着Pb2+质量浓度的提高,黄药对Pb2+在蒙脱石上吸附作用的影响也逐渐减弱,如Pb2+质量浓度为100 mg/L和800 mg/L,与不添加黄药对照相比,添加黄药对Pb2+在蒙脱石上吸附量的抑制率分别为72%和16%。当Pb2+质量浓度一定时(100 mg/L),黄药质量浓度越高,对Pb2+在蒙脱石上吸附作用的影响也越大。当黄药质量浓度为20 mg/L和100 mg/L,对Pb2+在蒙脱石上吸附量的抑制率分别为4%和14%。
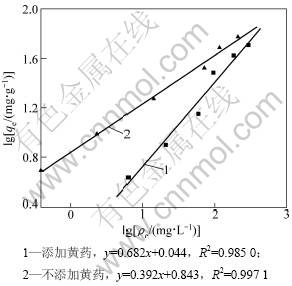
图7 Pb2+的Freundlich吸附等温线
Fig.7 Freundlich adsorption isotherms of Pb2+
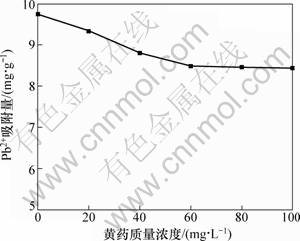
图8 黄药对蒙脱石吸附Pb2+的影响
Fig.8 Effect of xanthate on Pb2+ adsorption
2.2.3 不同pH下黄药对Pb2+吸附作用的影响
pH对Pb2+在蒙脱石上的吸附作用有较大影响[31],当黄药存在时影响尤其显著,见图9。从图9可见:pH越高,Pb2+的吸附量越大;当pH由3上升到11时,不添加黄药时Pb2+的吸附量提高了8.7%,而添加黄药时Pb2+的吸附量提高了75.6%。但pH越大,黄药对Pb2+在蒙脱石上吸附作用的影响越小。与不添加黄药对照相比,当pH为3时黄药对Pb2+吸附量的抑制率达74%,而当pH为11时抑制率仅为2%。不添加黄药时,在低pH条件下H+与Pb2+形成了竞争吸附,降低了Pb2+的吸附作用[32];随着pH升高,Pb2+与OH-生成Pb(OH)2,与蒙脱石发生共沉淀吸附作用[33],使Pb2+吸附量上升。添加黄药时,在低pH条件下,除了H+与Pb2+的竞争吸附以外,黄药水解产生的黄药离子(ROCS2-)[16]与Pb2+生成悬浮态黄原酸铅,导致蒙脱石对Pb2+的吸附量显著降低。随着pH升高,Pb2+的吸附量迅速提高。原因主要有3方面:一是黄药水解产生的黄药离子(ROCS2-)[16]及其与Pb2+生成的悬浮态黄原酸铅含量迅速减少;二是Pb2+与OH-生成Pb(OH)2,与蒙脱石发生共沉淀吸附作用[33];三是黄药在碱性条件下解离产生S-[34],与Pb2+反应生成PbS沉淀,与蒙脱石发生共沉淀吸附作用。
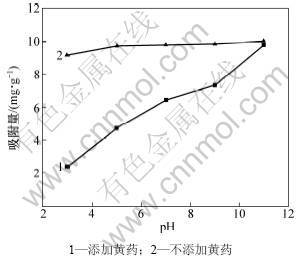
图9 pH对蒙脱石吸附Pb2+的影响
Fig.9 Effect of pH on Pb2+ adsorption
2.2.4 蒙脱石不同用量下黄药对Pb2+吸附作用的影响
蒙脱石不同用量下黄药对Pb2+在蒙脱石上的吸附作用见图10。可见:投加量对蒙脱石Pb2+的吸附作用有较大影响(图10)。对于不添加黄药对照处理,蒙脱石投加量为0.01 g时水溶液中Pb2+的去除率高达93%,投加量为0.05 g时Pb2+的去除率缓慢提高到97%。对于添加黄药处理,蒙脱石投加量为0.01 g时水溶液中Pb2+的去除率为32%,投加量为0.05 g时Pb2+的去除率迅速提高到75%,当投加量为0.5 g时Pb2+的去除率提高到96%。因此,当含Pb2+废水中存在黄药时,蒙脱石的投加量需加大,以达到Pb2+更高的去除率和净化废水的目的。
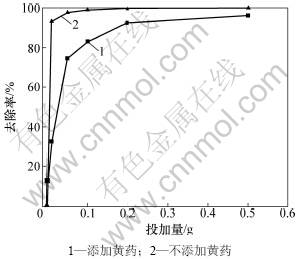
图10 投加量对蒙脱石吸附Pb2+的影响
Fig.10 Effect of montmorillonite dose on Pb2+ removal
3 结论
(1) 乙基钾黄药在蒙脱石上的吸附作用很小,它通过与Pb2+反应形成悬浮态物质而严重影响Pb2+在蒙脱石上的吸附作用,使Pb2+的吸附速率常数提高,而平衡吸附量下降,其影响程度随着乙基钾黄药质量浓度的增大而增大,随着Pb2+质量浓度、pH和蒙脱石投加量的增大而减弱。
(2) 研究结果可为矿山废水中黄药-重金属复合污染的治理提供科学依据和实用技术。
参考文献:
[1] Lotter N O, Bradshaw D J. The formulation and use of mixed collectors in sulphide flotation[J]. Minerals Engineering, 2010, 23: 945-951.
[2] Bulatovic S. Evaluation of alternative reagent schemes for the flotation of platinum group minerals from various ores[J]. Minerals Engineering, 2003, 16(10): 931-939.
[3] 史蓉蓉, 魏洽, 史少欣. 德兴铜矿大坞河至乐安江段水体浮选剂污染的调查[J]. 南昌航空工业学院学报, 1996, 2: 28-32.
SHI Rong-rong, WEI Qia, SHI Shao-xin. Investigation of the pollution of Dawu river-Lean River by floatation agent in Dexing copper mine[J]. Journal of Nanchang Institute of Aeronautical Technology, 1996, 2: 28-32.
[4] Brasel J M. Effects of environmentally relevant doses of cyanide on flight times in pigeons, Columba livia.Bull[J]. Environ Contam Toxicol, 2006, 76: 202-209.
[5] Donato D B. A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife[J]. Environment International, 2007, 33: 974-984.
[6] Griffiths S R, Smith G B, Donato D B, et al. Factors influencing the risk of wild life cyanide poisoning on a tailings storage facility in the Eastern Goldfields of Western Australia[J]. Ecotoxicology and Environmental Safety, 2009, 72: 1579-1586.
[7] Obiri S, Dodoo D K, Okai-Sam F, et al. Non-cancer health risk assessment from exposure to cyanide by resident adults from the mining operations of bogoso gold limited in Ghana[J]. Environmental Monitoring and Assessment, 2006, 118: 51-63.
[8] 赵玉娥. 黄药、黑药、二号油在水体中的降解试验研究[J]. 黄金, 1995, 16(7): 47-51.
ZHAO Yu’e. Experimental study on degradation of xanthate、aerofloat and turpentine in water body[J]. Gold, 1995, 16(7): 47-51.
[9] 栾和林, 陈彩霞, 田野, 等. 复合污染与尾矿区重金属释放和迁移[J]. 有色金属, 2006, 58(4): 124-127.
LUAN He-lin, CHEN Cai-xia, TIAN Ye, et al. Relationship of complex pollution to heavy metals release and migration from floatation tailings dam[J]. Nonferrous Metals, 2006, 58(4): 124-127.
[10] Lim H S, Lee J S, Chon H T. Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au-Ag mine in Korea[J]. Journal of Geochemical Exploration, 2008, 96: 223-230.
[11] Feng X B, Li P, Qiu G, et al. Human exposure to methylmercury through rice intake in mercury mining areas, guizhou province, China[J]. Environ Sci Technol, 2008, 42: 326-332.
[12] Schaider L A, Senn D B, Brabander D J, et al. Characterization of zinc, lead, and cadmium in mine waste: Implications for transport, exposure and bioavailability[J]. Environ Sci Technol, 2007, 41: 4164-4171.
[13] Sidenko N V, Khozhina E I, Sherriff B L, et al. The cycling of Ni, Zn, Cu in the system mine“tailings-ground water-plants”: A case study[J]. Applied Geochemistry, 2007, 22: 30-52.
[14] Conesa H M, Faz A, Arnaldos R, et al. Initial studies for the phytostabilization of a mine tailing from the Cartagena-La Union Mining District(SE Spain)[J].Chemosphere, 2007, 66: 38-44.
[15] Dermont G, Bergeron M, Richer M, et al. Remediation of metal-contaminated urban soil using ?oatation technique[J]. Science of the Total Environment, 2010, 408: 1199-1211.
[16] 赵永红, 成先雄, 谢明辉, 等. 选矿废水中黄药自然降解特性的研究[J]. 矿业安全与环保, 2006, 33(6): 33-35.
ZHAO Yong-hong, CHEN Xian-xiong, XIE Ming-hui, et al. Study on natural degradation of xanthate in mineral processing wastewater[J]. Mining Safety and Environmental Protection, 2006, 33(6): 33-35.
[17] 余江, 原小涛, 刘会洲, 等. 柱撑蒙脱石吸附与催化降解黄药的特性研究[J]. 环境化学, 2005, 24(4): 394-396.
YU Jiang, YUAN Xiao-tao, LIU Hui-zhou, et al. Studies on adsorption and photocatalytic degradation of xanthate by pillared montmorillonite[J]. Environmental Chemistry, 2005, 24(4): 394-396.
[18] 张建乐, 陈万雄, 林祥辉. 改性膨润土对黄药吸附性能的研究[J]. 矿产保护与利用, 1995, 5: 27-29.
ZHANG Jian-le, CHEN Wan-xiong, LIN Xiang-hui. A study on modified bentonite to xanthate adsorption property[J]. Conservation and Utilization of Mineral Resources, 1995, 5: 27-29.
[19] 程伟, 张覃, 马文强. 活性炭对浮选废水中黄药的吸附特性研究[J]. 矿物学报, 2010, 30(2): 262-267.
CHENG Wei, ZHANG Tan, MA Wen-qiang. A research on removal of xanthate from floation wastewater with activated carbon[J]. Acta Mineralogica Sinica, 2010, 30(2): 262-267.
[20] 栾和林, 喻晗, 邹畅, 等. 复合污染状态下尾矿区有害化学品的迁移研究[J]. 环境化学, 2006, 25(2): 207-210.
LUAN He-lin, YU Han, Zou Chang, et al. Study on the migration rule of compound mineral processing reagents in tailling area of compound contamination[J]. Environmental Chemistry, 2006, 25(2): 207-210.
[21] Organization for Economic Coorperation and Development. OECD guidelines for testing of chemicals, test guideline 106:adsorption/desorption using a batch equilibrium method[M]. Paris: Organization for Economic Coorperation and Development, 2000: 1-45.
[22] 贺心然, 曹雷, 展卫红, 等. 紫外分光光度法测定水中丁基黄原酸[J]. 环境污染与防治, 2007, 29(7): 552-554, 557.
HE Xin-ran, CAO Lei, ZHAN Wei-hong, et al. UV spectrophotometer for measurement of butyl xanthate in water [J]. Pollution and Prevention of Environment, 2007, 29(7): 552-554, 557.
[23] O’Dea A R, Prince K R, st Smart R C, et al. Secondary ion mass spectrometry investigation of the interaction of xanthate with galena[J]. Int J Miner Process, 2001, 61: 121-143.
[24] 吴平宵, 廖宗文. 蒙脱石层间域的性质及其环境意义[J]. 地球科学进展, 2000, 15(2): 184-189.
WU Ping-xiao, LIAO Zong-wen. Character of montmorillonite interlayer and its environmental significance[J]. Advance in Earth Sciences, 2000, 15(2): 184-189.
[25] 刘云, 吴平宵, 唐剑文, 等. 聚羟基铝柱撑蒙脱石吸附重金属离子实验研究[J]. 矿物岩石, 2005, 25(3): 122-126.
LIU Yun, WU Ping-xiao, TANG Jian-wen, et al. Study of heavy metal adsorption on hudroxyl-al pillared montmorillonite[J]. Mineral Petrol, 2005, 25(3): 122-126.
[26] 何宏平, 郭九皋, 朱剑喜, 等. 蒙脱石、高岭土、伊利石对重金属离子吸附容量的实验研究[J]. 岩石矿物学杂志, 2001, 20(4): 573-578.
HE Hong-ping, GUO Jiu-gao, ZHU Jian-xi, et al. An experimental study of adsorption capacity of montmorillonite, kaolinite and illite for heavy metals[J]. Acta Petrologica et Mineralogica, 2001, 20(4): 573-578.
[27] 何宏平, 郭九皋, 谢先德. 可膨胀性层状粘土矿物对铜离子吸附机理的模拟研究[J]. 环境科学, 2000, 21(4): 48-51.
HE Hong-ping, GUO Jiu-gao, XIE Xian-de. Adsorption mechanism of expansible layered clay minerals to copper ion[J]. Environmental Chemistry, 2000, 21(4): 48-51.
[28] 王宜鑫, 赵斌, 汤炎, 等. 不同粘土矿物材料对Pb2+的吸附特征[J]. 工业安全与环保, 2007, 33(2): 1-3.
WANG Yi-xin, ZHAO Bin, TANG Yan. The absorption characteristics of Pb2+ with different clay minerals[J]. Industrial Safety Environmental Protection, 2007, 33(2): 1-3.
[29] 谭光群, 李晖, 彭同江. 蛭石对重金属离子吸附作用的研究[J]. 四川大学学报: 工程科学版, 2001, 33(3): 59-61.
TAN Guang-qun, LI Hui, PENG Tong-jiang. Study on the absorption of heavy metals ion onto vermiculite[J]. Journal of Sichuan University: Engineering Science Edition, 2001, 33(3): 59-61.
[30] 唐受印, 戴有之, 汪大翚, 等. 废水处理工程[M]. 2版. 北京:化学工业出版社, 2004: 125.
TANG Shou-yin, DAI You-zhi, WANG Da-hui, et al. Wastewater treatment engineering[M]. 2nd ed. Beijing: Chemical Industry Press, 2004: 125.
[31] Altin O, Ozbelge O H, Dogu T.Effect of pH, flow rate and concentration on the sorption of Pb and Cd on montmorillonite I.Experimental[J]. Journal of Chemical Technology and Biotechnology, 1999, 74: 1131-1138.
[32] 李虎杰, 田煦, 易发成. 活化沸石对Pb2+的吸附性能研究[J].非金属矿, 2001, 24(2): 49-51.
LI Hu-jie, TIAN Xu, YI Fa-cheng. Study on the absorption capacity of Pb2+ with activated zeolite[J]. Non-Metallic Mines, 2001, 24(2): 49-51.
[33] 胡振琪, 杨秀红, 高爱林. 黏土矿物对重金属镉的吸附研究[J]. 金属矿山, 2004, 6: 53-55.
HU Zhen-qi, YANG Xiu-hong, GAO Ai-lin. Absorption of heavy metal cadmium with clay minerals[J]. Metal Mine, 2004, 6: 53-55.
[34] Duˇsica R, Vuˇcini?c, Predrag M, et al. Rosi?c.Ethyl xanthate adsorption and adsorption kinetics on lead-modified galena and sphalerite under flotation conditions[J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2006, 279: 96-104.
(编辑 陈灿华)
收稿日期:2010-10-15;修回日期:2010-12-27
基金项目:教育部博士点基金资助项目(200805590005);国家自然科学基金资助项目(41071211);中央高校基本科研业务费专项资金项目(21610410,21609709)
通信作者:莫测辉(1965-),男,广西柳州人,博士,教授,博士生导师,从事环境有机污染与控制研究;电话:020-85226615;E-mail:tchmo@jnu.edu.cn
摘要:为揭示黄药-重金属复合污染体系的环境行为,以蒙脱石为吸附剂进行静态吸附试验,探讨乙基钾黄药-铅复合污染体系的吸附平衡与动力学特征。研究结果表明:乙基钾黄药在蒙脱石上的吸附作用很小,它通过与Pb2+反应形成悬浮态物质而严重影响Pb2+在蒙脱石上的吸附作用,使Pb2+的吸附速率常数由10.40 g/(mg·min)提高到54.76 g/(mg·min),而平衡吸附量(qe)则由9.80 mg/g降低到4.27 mg/g;影响程度随着乙基钾黄药浓度的升高而增大,随着Pb2+浓度、pH和蒙脱石投加量的升高而减弱。研究结果可为黄药-重金属复合污染环境的治理提供参考。


