DOI: 10.11817/j.issn.1672-7207.2015.06.006
溶胶-凝胶后硫化法制备铜锌锡硫薄膜太阳电池
韩自力1,苏正华1,孙凯文1,刘芳洋1, 2,赖延清1,李劼1,刘业翔1
(1. 中南大学 冶金与环境学院,湖南 长沙,410083;
2. 新南威尔士大学 光伏与可再生能源工程学院,悉尼,2052)
摘 要:
硫气氛中退火处理溶胶-凝胶法制备的薄膜前躯体,制备太阳电池光吸收层铜锌锡硫(CZTS)薄膜。采用X线能量色散谱、扫描电镜、X线衍射、拉曼光谱和紫外-可见-近红外分光光度计等对薄膜进行表征。研究结果表明:制备的CZTS薄膜为贫铜富锌成分,呈现锌黄锡矿结构;薄膜禁带宽度约为1.50 eV,在可见光区域内光吸收系数达到104 cm-1;制作的结构为Ag/ZnO:Al/i-ZnO/CdS/CZTS/Mo/SLG的薄膜太阳电池器件的电池开路电压、短路电流密度、填充因子和光电转换效率分别为658 mV,16.75 mA/cm2,0.47和5.18%,表明溶胶-凝胶法有望成为制备廉价高效的CZTS薄膜太阳电池的有效途径。
关键词:
中图分类号:TM615 文献标志码:A 文章编号:1672-7207(2015)06-2014-06
Cu2ZnSnS4 solar cells prepared with sulphurized sol-gel deposited precursors
HAN Zili1, SU Zhenghua1, SUN Kaiwen1, LIU Fangyang1, 2, LAI Yanqing1, LI Jie1, LIU Yexiang1
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. School of Photovoltaic and Renewable Energy Engineering, University of New South Wales, Sydney 2052, Australia)
Abstract: The solar cell light absorber Cu2ZnSnS4(CZTS) thin films were prepared by annealing precursor thin film in a sulfur atmosphere at 560 ℃ based on sol-gel method. The annealed films were characterized by energy dispersive X-ray spectroscopy, scanning electron microscopy, X-ray diffraction, Raman scattering and UV-vis spectroscopy. The results show that the prepared CZTS thin films have a pure kesterite structure with Cu-poor and Zn-rich. The band gap of CZTS films is about1.50 eV, and the optical absorption coefficient in visible light range is 104 cm-1.Thin film solar cells with a structure of Ag/ZnO:Al/i-ZnO/CdS/CZTS/Mo/SLG are tentatively fabricated. The solar cell shows an open-circuit voltage of 658 mV, a short-circuit current density of 16.75 mA/cm2, a fill factor of 0.47 and an efficiency of 5.18%,which demonstrates that the sol-gel technique is a successful method of preparing cheap thin film solar cells of CZTS.
Key words: CZTS; sol-gel; sulfurizing; thin film solar cell
化合物半导体薄膜太阳电池由于具有成本低且易于实现大面积生产的优点,因而应用前景广阔。其中CuIn1-xGaxSe2 (CIGS) 薄膜太阳电池具有高效率、性能稳定等优点,受到广泛关注,实验室最高光电转换效率达到21.7%[1]。但由于Ga和In为稀有元素,成本较高,CIGS商业化产量受到限制。锌黄锡矿结构的Cu2ZnSnS4(CZTS)晶体与黄铜矿结构的CIGS晶体结构相似,不同的是由地壳上蕴含量丰富的锌和锡元素代替了CIGS中的铟和镓元素,并且具有与太阳光谱非常匹配的禁带宽度(1.4~1.5 eV)和超过104 cm-1的光吸收系数[2-4],理论最高光电转化率可达32.2%,因而 CZTS被普遍认为是有望替代CIGS的最佳材料之一,已成为目前薄膜太阳电池领域的研究热点[5]。自Katagiri[6-9]研制了结构为Al/ZnO:Al/CdS/CZTS/Mo/ SLG的CZTS太阳电池以来,基于真空制备CZTS薄膜太阳电池的方法得到了较快发展[69]。采用溅射或蒸发沉积合金预制层,然后,在一定气氛下进行热处理制备的CZTS薄膜太阳电池最高光电转换效率分别达到9.3%[10]和8.4%[11]。然而,通过真空沉积法制备薄膜所需设备复杂并且昂贵,导致制造成本过高,所以,基于非真空液相法的薄膜沉积工艺成为低成本制备CZTS薄膜太阳电池的有效途径。目前,采用单相纳米颗粒或者多种纳米颗粒的混合物制成墨水涂覆的方法制备Cu2ZnSn(SxSe1-x)4(CZTSSe)薄膜太阳电池光电转换效率分别达到7.2%[12]和8.5%[13]。IBM公司将Cu,Zn和Sn的硫族化合物按照一定的化学计量比溶解分散在肼里,然后旋涂沉积在衬底上,并在540 ℃的陶瓷热板中退火,得到CZTSSe薄膜,制备的太阳电池的转化效率为12.7%[14],是迄今为止CZTSSe薄膜太阳电池的最高转换效率。然而,这些制备方法往往在制备的CZTS薄膜中采用有毒元素Se代替大部分S,或者在其制备过程中采用了高毒性的联氨溶液,对环境存在潜在的危害。Ahmed等[15]采用电沉积叠层金属预制层后硫化的方法制备了转换效率为7.2%的不含Se的CZTS太阳电池,对制备低成本、环境友好的CZTS太阳电池具有重要意义。溶胶-凝胶(sol-gel)法作为一种非真空溶液法具有设备简单、产品纯度高、化学均匀性好等特点,在制备薄膜方面得到广泛应用。Tanaka等[16-17]采用sol-gel法制备了接近于化学计量比为2:1:1:4的CZTS薄膜,并制备了完全非真空条件下制备的转换效率为2.23%的CZTS薄膜太阳电池,但其后续硫化热处理条件均是在高毒性的H2S气氛中进行。Ilari等[18]采用类似的方法,用毒性较温和的Se气氛代替H2S退火制备了Cu2ZnSnSe4太阳电池,获得的最高光电转化效率为2.76%。Ki等[19]采用sol-gel法先制备CZTS薄膜,然后在Se气氛下后处理制备了x(S)/(x(S)+x(Se))(其中,x为摩尔分数)CZTSSe太阳电池,最高转换效率为4.1%。Yeh等[20]在160~320 ℃的空气中采用退火处理sol-gel法制备薄膜前躯体,制备的CZTS薄膜的形貌以及结晶度均不理想。Jiang等[21]采用sol-gel法制备前躯体薄膜,热处理改为在550 ℃下氮气气氛中进行,得到的CZTS太阳电池转换效率仅为0.63%。本文作者采用sol-gel制备薄膜前躯体后硫化的方法制备纯CZTS薄膜,硫化过程在硫蒸气下进行,得到转换效率为5.18%的CZTS薄膜太阳电池,为制备高效率、廉价且环境友好的CZTS薄膜太阳电池提供了一条有效途径。
1 实验
CZTS薄膜的制备过程如图1所示。分别在镀鉬玻璃和钠钙玻璃上采用sol-gel法制备CZTS薄膜,在钠钙玻璃上制备CZTS薄膜以研究其光学性质。将一水醋酸铜Cu(CH3COO)2H2O (0.49 mol/L)、二水醋酸锌Zn(CH3COO)22H2O (0.32 mol/L)、二水合氯化亚锡SnCl22H2O (0.27 mol/L)和硫脲(2.16 mol/L )溶解在乙二醇甲醚中,并加入三乙醇胺作为稳定剂,于50 ℃水浴加热并搅拌1 h后得到均匀溶液。在清洁环境中采用旋涂(旋涂机KW-4A CHEMAT TECHNOLOGY)方法在基底上制备前躯体薄膜,旋涂速度为4 000~5 000 r/min,然后在空气中于250 ℃下预处理10 min。如此重复12次形成所需厚度的前躯体薄膜。前驱体薄膜在双温区管式炉中进行硫化退火。退火过程在过量硫蒸气气氛下进行,退火温度为560 ℃,退火时间为1 h。
实验中,采用X线能量色散谱仪(EDS,美国,型号为EDX-GENESIS 60S)检测薄膜的化学成分,采用扫描电镜(SEM,美国,型号为FEI Quanta-200)对退火前后薄膜的形貌进行分析,采用X线衍射仪(XRD,日本,型号为D/MAX-2000H X)和拉曼光谱仪(Raman,法国,型号为LabRAM HR800)分析薄膜的物相组成。采用紫外-可见-近红外分光光度计(HITACHI U-4100)测量薄膜的光透过率。采用NEWPORT太阳光模拟器测试太阳电池J-V特性曲线;采用美国KEITHLEY公司生产的2420数源表及相应的测试软件进行分析。
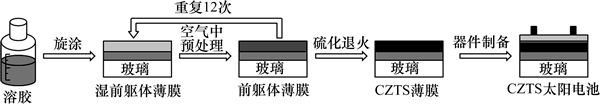
图1 溶胶-凝胶后硫化法制备CZTS太阳电池的示意图
Fig. 1 Schematic of formation mechanism of CZTS solar cell by sol-gel and sulfurizing methods
2 结果与讨论
2.1 薄膜成分分析
黄铜矿薄膜太阳电池吸收层中不同金属元素之间的比例对太阳电池的器件效率有很大影响。溶胶中金属元素的比例控制为x(Cu)/(x(Zn)+x(Sn))=0.84,x(Zn)/x(Sn)=1.2,用于形成贫铜富锌的薄膜,从而提高太阳电池转换效率。表1所示为溶胶中各金属元素的比例和退火后薄膜的成分组成。从表1可见:退火后薄膜中金属元素的比例相对于溶胶中没有太大变化,所以,sol-gel法为多元半导体化合物薄膜成分控制提供了一条简单易行的途径,很容易通过改变前躯体溶胶中各金属元素的比例来有效控制退火后薄膜金属元素的最终成分;比较退火前后Zn/Sn可知退火过程中Zn的损失略大于Sn的损失。Ilari等[18]在前躯体薄膜后Se化制备CZTSe的研究中也发现了类似现象。EDS结果表明:最终生成的CZTS薄膜成分为Cu1.74Zn1.13Sn1.02S4.11。金属元素比例Cu/(Zn+Sn)和Zn/Sn分别为0.81和1.10,表现为贫铜富锌成分,与文献[11,14]报道的高效率器件成分相同。电子结构计算结果表明[11, 14],在贫铜富锌材料中,施主缺陷能级VCu-和受主缺陷能级ZnCu+会相互结合,形成[VCu-+ZnCu+]缺陷对。这种缺陷对在样品中大量产生时,表现为CZTS的1个衍生相,它与纯的CZTS之间的能带排布呈II型,能带在两相的界面区弯曲,这有利于光生电子-空穴对分离,从而有利于太阳电池效率提高[22-23]。同时,由于退火过程在560 ℃和过量硫蒸气气氛下进行,退火后的薄膜略微富S,有利于P型半导体的形成以及提高太阳电池的效率[3]。
2.2 薄膜表面形貌
图2所示为退火前后薄膜表面形貌的SEM照片。与文献[20-21]报道的由sol-gel法制备的前躯体薄膜表面由于成分挥发导致表面常常会变得疏松且存在一定孔洞不同,本文制备的前躯体薄膜表面致密平整。薄膜主要由金属硫化物(CuxS, ZnxS和SnxS)的纳米微粒交联堆叠而成,无法分辨出明显的晶界。高温硫化退火促进薄膜结晶以及晶粒长大,退火后CZTS薄膜晶粒粒度为0.5~1.0 μm。
表1 CZTS薄膜EDS元素组分及比例
Table 1 Chemical composition of CZTS thin film

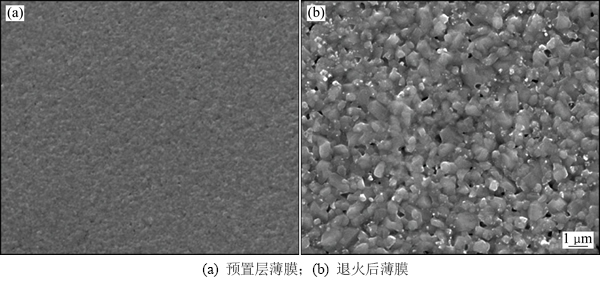
图2 退火前后薄膜表面SEM图
Fig. 2 SEM images of thin films before and after annealing treatment
2.3 物相结构分析
为了确定所制备的薄膜样品的物相组成,对在镀鉬玻璃上制备的CZTS薄膜进行XRD分析。图3所示为在560 ℃退火1 h所得产物的XRD图谱,基底Mo的衍射峰在图谱上已经标出。分析图3可知:所制备薄膜的所有衍射峰均与锌黄锡矿结构的CZTS标准卡片(PDF No.26-0575)相对应;2θ为28.5°,47.3°和56.1°的3个主要衍射峰分别对应其(112),(220)和(312)晶面,薄膜呈(112)面择优取向,与文献[24]报道的一致。
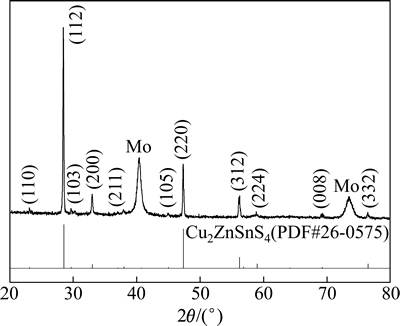
图3 薄膜结构为CZTS/Mo/SLG的XRD图谱
Fig. 3 XRD pattern of CZTS/Mo/SLG substrate structure
由于可能存在的二次相Cu2SnS3和ZnS与锌黄锡矿CZTS具有极其相似的XRD衍射图谱,为了表征制备的CZTS薄膜是否为纯相结构,对其进行拉曼检测。薄膜样品拉曼散射图谱如图4所示。从图4可见:退火后的CZTS薄膜在331 cm-1处有1个很强的峰,同时在286 cm-1处有1个较弱的特征峰,均与文献[25]报道的CZTS的特征峰相对应;未检测到二次相Cu2SnS3(337 cm-1和351 cm-1)和ZnS(356 cm-1)的特征峰[25-26];在406 cm-1处还出现了1个较弱的峰,对应为MoS2的特征峰[27],说明硫化退火过程中,在560 ℃和过量硫气氛下,一部分基底上的Mo已经被硫化,在CZTS与基底Mo接触处有MoS2生成。Fontane等[28]通过电沉积金属前躯体后硫化的方法制备CZTS薄膜,也通过拉曼检测出薄膜底层CZTS与基底Mo接触处有MoS2层。然而,在图3中的XRD检测结果中未发现MoS2特征衍射峰,这是由于MoS2的特征峰被锌黄锡矿CZTS的(220)面衍射峰和Mo的衍射峰所掩盖。Biccari等[29]采用蒸发法在Mo玻璃上制备了CZTS薄膜太阳电池,然后将CZTS薄膜机械剥离,通过比较剥离前后Mo玻璃上样品的XRD衍射图谱发现,剥离前只能检测到CdS,CZTS和Mo基底的衍射峰,剥离后除去CZTS薄膜,MoS2的特征峰才被检测出来。综合XRD和拉曼分析得知:所制得的薄膜样品为纯锌黄锡矿结构的CZTS,未检测到其他二元或三元化合物诸如ZnS和Cu2SnS3。
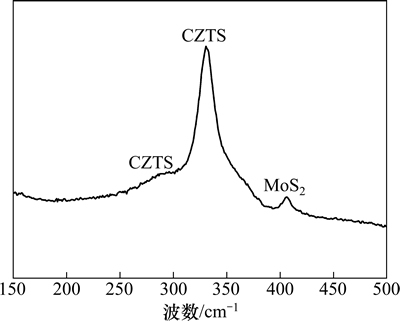
图4 薄膜结构为CZTS/Mo/SLG的拉曼图谱
Fig. 4 Raman spectrum of CZTS/Mo/SLG substrate structure
2.4 光学性能
为了研究薄膜的光学性能,在钠钙玻璃上制备了CZTS薄膜并测量了其在波长为500~2000 nm范围内的光吸收图谱。图5所示为制备的CZTS薄膜光吸收系数谱,其中α和hv分别为薄膜的光吸收系数与光子能量。从图5可见:在可见光范围内薄膜的光吸收系数达到104 cm-1,只需1~2 μm就可吸收90%以上的可见光,表明制备的CZTS是一种适用于薄膜太阳电池的材料。将(αhv)2-hv图谱线性部分延长线与横坐标轴的交点对应的数值即为薄膜的禁带宽度,如图5中插图所示。制备的CZTS薄膜禁带宽度为Eg=(1.50±0.01) eV,与单结太阳电池光吸收层材料的理论最佳值接近,体现出极大的光伏应用潜力。
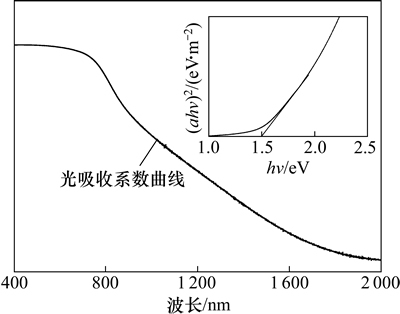
图5 CZTS薄膜的光吸收系数曲线
Fig. 5 Optical absorption coefficient of CZTS thin film
2.5 太阳电池
采用典型的Ag/ZnO:Al/i-ZnO/CdS/CZTS/Mo/SLG太阳电池结构制备了单个面积为0.25 cm2的CZTS薄膜太阳电池器件。采用化学水浴法(CBD)在CZTS薄膜上沉积一层CdS薄膜作为缓冲层,然后,采用磁控溅射沉积i-ZnO和ZnO:Al作为窗口层。图6所示为制备的CZTS薄膜太阳电池截面的SEM图片以及光照下的J-V特性曲线。吸收层CZTS薄膜的厚度约为1 μm,CZTS的晶粒粒度为0.5~1.0 μm,并且吸收层中还有少量微小孔隙存在;在基底Mo与吸收层之间存在一层明显的MoS2层,厚度为300 nm左右,与之前的拉曼检测结果相一致。Ahmed等[15]采用电沉积法制备了效率为7.2%的纯CZTS薄膜太阳电池器件,通过电池截面的TEM图片也观察到背接触处厚度为350 nm左右MoS2层。采用NEWPORT太阳光模拟器在光照AM1.5G和100 mW/cm2条件下测得太阳电池的J-V特征曲线如图6所示。从图6可见:太阳电池的转化效率为5.18%,为sol-gel法制备CZTS太阳电池的最高转换效率;电池的开路电压为658 mV,短路电流密度为16.75 mA/cm2,填充因子为0.47。Todorov等[14]报道的最高转换效率为11.1%的CZTS薄膜太阳电池,其开路电压为460.6 mV,本文制备的太阳电池开路电压要高很多。这是由于Se化后的CZTSSe吸收层禁带宽度一般为1.0~1.2 eV,而纯的CZTS薄膜禁带宽度一般在1.5 eV附近,导致纯CZTS太阳电池的开路电压往往较大,而短路电流较小,反之亦然[15]。因此,必须减少CZTS薄膜制备过程中微小孔隙的生成,因为这些孔隙会阻碍光生载流子的运输从而降低器件性能。从图6所示的电池截面SEM图可以看出:与高效率CZTS太阳电池的缓冲层(CdS厚度一般为50 nm左右)和窗口层(TCO厚度为500 nm左右)相比,本文所制备的太阳电池CdS和ZAO厚度都明显过大,这在一定程度上影响了太阳电池效率的提高。此外,填充因子仅为0.47,其原因主要是与电池背接触电学性质不理想导致串联电阻Rs过大有关。本文所制备的太阳电池并联电阻Rsh为412.82 Ω/cm2,而串联电阻Rs高达14.29 Ω/cm2。良好的背接触不仅使基底具有好的黏附性能,在电学方面形成良好的欧姆接触,而且作为扩散阻挡层抑制吸收层中不稳定相的生成。Wang等[8, 30]报道当前CZTS电池结构在背接触处往往会表现出Shottky barrier的性质,其原因很可能是在薄膜硫化退火过程中,吸收层与Mo基底之间会形成一层一定厚度的MoS2层。在图6中可以明显观察到一层厚度为300 nm左右的MoS2层,导致串联电阻较高。
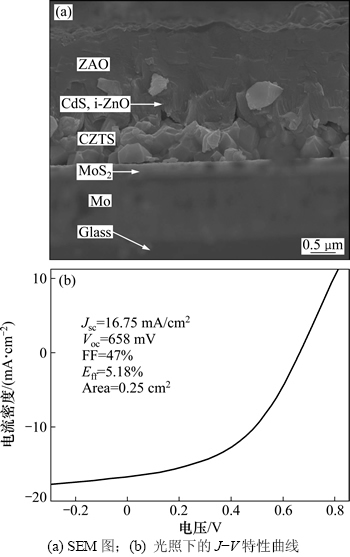
图6 CZTS太阳电池截面的SEM图以及光照下的J-V特性曲线
Fig. 6 SEM cross-section and J-V characteristics under light irradiation of CZTS solar cell
3 结论
1) 采用溶胶-凝胶法将包含Cu,Zn和Sn的金属盐和硫脲的溶胶旋涂在镀钼玻璃和钠钙玻璃上制备了前躯体薄膜,在560 ℃下硫化退火1 h得到CZTS薄膜。
2) 制备的CZTS薄膜检测为贫铜富锌成分,薄膜表面形貌致密平整,有贯穿整个薄膜截面的大晶粒生成。CZTS薄膜为纯的锌黄锡矿结构,无其他二次相生成。其在可见光区域内光吸收系数大于104 cm-1,禁带宽度为(1.50±0.01) eV,具有极大的应用潜力。
3) 所制作的结构为Ag/ZnO:Al/i-ZnO/CdS/ ZTS/Mo/SLG的薄膜太阳电池器件的开路电压、短路电流密度、填充因子和光电转换效率分别为658 mV,16.75 mA/cm2,0.47和5.18%。
参考文献:
[1] Jackson P, Hariskos D, Wuerz R, et al. Properties of Cu(In,Ga)Se2 solar cells with new record efficiencies up to 21.7%[J]. Physica Status Solidi (RRL)-Rapid Research Letters, 2014, 9(1): 28-31.
[2] Katagiri H. Cu2ZnSnS4 thin film solar cells[J]. Thin Solid Films, 2005, 480/481: 426-432.
[3] Jimbo K, Kimura R, Kamimura T, et al. Cu2ZnSnS4-type thin film solar cells using abundant materials[J]. Thin Solid Films, 2007, 515: 5997-5999.
[4] 李苗苗, 王天兴, 夏存军, 等. Cu2ZnSnS4/Cu2ZnSnSe4电子结构与光学特性的第一性原理计算[J]. 中国有色金属学报, 2012, 22(5): 1413-1420.
LI Miaomiao, WANG Tianxing, XIA Cunjun, et al. First-principles calculation of electronic structure and optical properties of Cu2ZnSnS4/Cu2ZnSnSe4[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(5): 1413-1420.
[5] Wadia C, Alivisatos A P, Kammen D M. Materials availability expands the opportunity for large-scale photovoltaics deployment[J]. Environmental Science & Technology, 2009, 43: 2072-2077.
[6] Katagiri H, Jimbo K, Yamada S, et al. Enhanced conversion efficiencies of Cu2ZnSnS4-based thin film solar cells by using preferential etching technique[J]. Applied Physics Express, 2008, 1: 041201.
[7] Katagiri H, Sasaguchi N, Hando S, et al. Preparation and evaluation of Cu2ZnSnS4 thin films by sulfurization of E-B evaporated precursors[J]. Solar Energy Materials and Solar Cells, 1997, 49: 407-414.
[8] Wang K, Gunawan O, Todorov T, et al. Thermally evaporated Cu2ZnSnS4 solar cells[J]. Applied Physics Letters, 2010, 97: 143508.
[9] 张坤, 刘芳洋, 赖延清, 等. 太阳电池用 Cu2ZnSnS4 薄膜的反应溅射原位生长及表征[J]. 物理学报, 2011, 60: 790-796.
ZHANG Kun, LIU Fangyang, LAI Yanqing, et al. In situ growth and characterization of Cu2ZnSnS4thin films by reactive magnetron co-sputtering for solar cells[J]. Acta Physica Sinica, 2011, 60: 790-796.
[10] Chawla V, Clemens B. Effect of composition on high efficiency CZTSSe devices fabricated using co-sputtering of compound targets[C]//Photovoltaic Specialists Conference (PVSC), 2012 38th IEEE Texas. USA, 2012: 2900-2902.
[11] Shin B, Gunawan O, Zhu Y, et al. Thin film solar cell with 8.4% power conversion efficiency using an earth-abundant Cu2ZnSnS4 absorber[J]. Progress in Photovoltaics: Research and Applications, 2013, 21: 72-76.
[12] Guo Q, Ford G M, Yang W C, et al. Fabrication of 7.2% efficient CZTSSe solar cells using CZTS nanocrystals[J]. J Am Chem Soc, 2010, 132: 17384-17386.
[13] Cao Y, Denny M S, Caspar J V, et al. High-efficiency solution-processed Cu2ZnSn(S,Se)4 thin-film solar cells prepared from binary and ternary nanoparticles[J]. Journal of the American Chemical Society, 2012, 134: 15644-15647.
[14] Kim J, Hiroi H, Todorov T K, et al. High efficiency Cu2ZnSn(S,Se)4 solar cells by applying a double In2S3/CdS emitter[J]. Advcanced Materials, 2014, 26(14): 7427-7431.
[15] Ahmed S, Reuter K B, Gunawan O, et al. A high efficiency electrodeposited Cu2ZnSnS4 solar cell[J]. Advanced Energy Materials, 2012, 2(2): 253-259.
[16] Tanaka K, Moritake N, Uchiki H. Preparation of Cu2ZnSnS4 thin films by sulfurizing sol-gel deposited precursors[J]. Solar Energy Materials and Solar Cells, 2007, 91: 1199-1201.
[17] Maeda K, Tanaka K, Fukui Y, et al. Influence of H2S concentration on the properties of Cu2ZnSnS4 thin films and solar cells prepared by sol-gel sulfurization[J]. Solar Energy Materials and Solar Cells, 2011, 95: 2855-2860.
[18] Ilari G M, Fella C M, Ziegler C, et al. Cu2ZnSnSe4 solar cell absorbers spin-coated from amine-containing ether solutions[J]. Solar Energy Materials and Solar Cells, 2012, 104: 125-130.
[19] Ki W, Hillhouse H W. Earth-abundant element photovoltaics directly from soluble precursors with high yield using a non-toxic solvent[J]. Advanced Energy Materials, 2011, 1(5): 732-735.
[20] Yeh M Y, Lee C C, Wuu D S. Influences of synthesizing temperatures on the properties of Cu2ZnSnS4 prepared by sol-gel spin-coated deposition[J]. Journal of Sol-Gel Science and Technology, 2009, 52: 65-68.
[21] Jiang M, Li Y, Dhakal R, et al. Cu2ZnSnS4 polycrystalline thin films with large densely packed grains prepared by sol-gel method[J]. Journal of Photonics for Energy, 2011, 1: 019501-019506.
[22] Chen S, Gong X, Walsh A, et al. Defect physics of the kesterite thin-film solar cell absorber Cu2ZnSnS4[J]. Applied Physics Letters, 2010, 96: 021902.
[23] Chen S, Yang J H, Gong X G, et al. Intrinsic point defects and complexes in the quaternary kesterite semiconductor Cu2ZnSnS4[J]. Physical Review B, 2010, 81: 245204.
[24] Ito K, Nakazawa T. Electrical and optical properties of stannite-type quaternary semiconductor thin films[J]. Japanese Journal of Applied Physics, 1988, 27: 2094-2097.
[25] Fernandes P A, Salomé P M P, Da Cunha A F. Growth and Raman scattering characterization of Cu2ZnSnS4 thin films[J]. Thin Solid Films, 2009, 517: 2519-2523.
[26] Fernandes P, Salomé P, Da Cunha A. A study of ternary Cu2SnS3 and Cu3SnS4 thin films prepared by sulfurizing stacked metal precursors[J]. Journal of Physics D:Applied Physics, 2010, 43: 215403.
[27] Windom B C, Sawyer W, Hahn D W. A Raman spectroscopic study of MoS2 and MoO3: Applications to tribological systems[J]. Tribology Letters, 2011, 42: 301-310.
[28] Fontane X, Calvo-Barrio L, Izquierdo-Roca V, et al. In-depth resolved Raman scattering analysis for the identification of secondary phases: Characterization of Cu2ZnSnS4 layers for solar cell applications[J]. Applied Physics Letters, 2011, 98: 181905.
[29] Biccari F, Chierchia R, Valentini M, et al. Fabrication of Cu2ZnSnS4 solar cells by sulfurization of evaporated precursors[J]. Energy Procedia, 2011, 10: 187-191.
[30] Gunawan O, Todorov T K, Mitzi D B. Loss mechanisms in hydrazine-processed Cu2ZnSnS4 solar cells[J]. Applied Physics Letters, 2010, 97: 233506.
(编辑 陈灿华)
收稿日期:2014-07-12;修回日期:2014-09-25
基金项目(Foundation item):国家自然科学基金资助项目(51204214);中国博士后科学基金资助项目(2012M511403)(Project (51204214) supported by the National Natural Science Foundation of China; Project (2012M511403) supported by the National Science Foundation for Post-doctoral Scientists of China)
通信作者:刘芳洋,博士,讲师,从事薄膜太阳电池研究;E-mail:liufangyang@csu.edu.cn;fangyang.liu@unsw.edu.au
摘要:在560 ℃的硫气氛中退火处理溶胶-凝胶法制备的薄膜前躯体,制备太阳电池光吸收层铜锌锡硫(CZTS)薄膜。采用X线能量色散谱、扫描电镜、X线衍射、拉曼光谱和紫外-可见-近红外分光光度计等对薄膜进行表征。研究结果表明:制备的CZTS薄膜为贫铜富锌成分,呈现锌黄锡矿结构;薄膜禁带宽度约为1.50 eV,在可见光区域内光吸收系数达到104 cm-1;制作的结构为Ag/ZnO:Al/i-ZnO/CdS/CZTS/Mo/SLG的薄膜太阳电池器件的电池开路电压、短路电流密度、填充因子和光电转换效率分别为658 mV,16.75 mA/cm2,0.47和5.18%,表明溶胶-凝胶法有望成为制备廉价高效的CZTS薄膜太阳电池的有效途径。


