DOI:10.19476/j.ysxb.1004.0609.2019.01.12
三维多级结构V2O5锂离子阴极材料的研究进展
陈鹏宇,郑国涛,汤杰,李嵩,文钟晟,季世军,孙俊才
(大连海事大学 交通运输装备与海洋工程学院,大连 116026)
摘 要:
针对V2O5材料电导率低和锂离子扩散动力性能差等缺点,通过元素掺杂、表面包覆和微观形貌调控等方法可以有效进行改善。相比于一维和二维结构材料,三维多级结构材料既有高比表面积,又由于有良好的自支撑性,而不容易发生团聚。利用不同合成方法将V2O5材料微纳米化,制成具有不同形貌的三维多级结构,材料表现出优良的电化学性能。综述不同形貌三维多级结构V2O5材料的合成方法、形成机理及电化学性能的研究进展,并着重介绍制备工艺与V2O5材料微观形貌以及V2O5材料微观形貌和电化学性能之间的关系。
关键词:
文章编号:1004-0609(2019)-01-0100-15 中图分类号:TM912 文献标志码:A
锂离子电池具有环境友好、输出电压高、容量大、自放电小及循环寿命长和无记忆效应等优点[1-3],在智能手机、笔记本电脑、平板电脑等小型电器领域得到广泛应用[4]。然而,为满足电动汽车和大规模储能设备的应用要求,需要开发更高能量密度和功率密度的锂离子电池[5]。而锂离子电池所需的高容量和高能量密度在很大程度上取决于阴极材料的性能,因此,合理设计与开发高性能阴极材料有着重要的意义[6-7]。传统的阴极材料LiCoO2、LiMn2O4、LiFePO4等理论比容量不足200 mA·h/g[8-10],而V2O5作为阴极材料理论比容量明显高于上述材料的,最大可达440 mA·h/g(脱嵌3 mol Li+)[11-13],并且兼具资源丰富、成本较低以及安全性能良好等优点[14],被认为是具有光明前景的高性能锂离子电池阴极材料[15]。
V2O5虽然比容量高,但是其本身电导率低且有着较差的稳定性和电化学动力性能(锂离子扩散系数低)等缺点[16-17]制约了其发展和应用。近年来,大量研究表明,一方面,掺杂[18]和包覆[19]等方法可以有效改善上述问题;另一方面,将V2O5微纳米化也是一种有效的方法,成为近年来研究的热点。电极材料纳米化一方面可以缩短电子传输和Li+的扩散距离以及增大了电极与电解液之间的接触面积,另一方面,在锂离子嵌入/脱出引起的应变时,材料的纳米结构也能够起到良好的调节作用[20-21]。不同维度的V2O5纳米材料在电化学性能上有很大的差异,零维结构如纳米颗粒[17],一维结构如纳米棒[22]、纳米带[23]、纳米线[24]、纳米管[25]等。二维结构如纳米片[26-27]、纳米环[28]等。上述一维和二维结构的V2O5纳米材料虽然具有较大的比表面积和较高的比容量,但由于其具有高的比表面能,易于发生自团聚,从而降低了V2O5材料的有效活性面积,导致V2O5材料循环稳定性和倍率性能下 降[29]。而三维结构的微纳米V2O5材料是由不同维度的V2O5纳米材料组成的微纳米多级结构。因此,三维结构的微纳米V2O5材料不仅保持了V2O5纳米材料的高比表面积,而且通过自组装成为有序结构。一方面避免了V2O5纳米材料之间团聚,另一方面由于自身的自支撑性,可以使V2O5材料在反复脱嵌Li+过程中不容易发生形变和坍塌。大量研究结果表明,具有三维多级结构V2O5材料的微观形貌对其电化学影响极其重要,已成为当前研究的热点[30]。
本文作者课题组长期以来一直从事锂离子电池正负极材料的研究和开发[31-32],研究材料制备工艺对材料微观结构和形貌的影响,以及材料微观结构和形貌与电化学性能之间的关系。在此基础上结合国内外文献的相关报道,本文主要对不同形貌的三维多级结构V2O5材料的合成方法,形成机理以及电化学性能作出分析和总结。
1 V2O5的晶体结构与特性
V2O5晶体为正交晶系,具有二维的层状结构,有利于锂离子的嵌入和脱出。在充放电的过程中,锂离子嵌入会形成不同的相LixV2O5。当x<0.01时,生成α相的LixV2O5;当0.35<x<0.7,生成ε相的LixV2O5;当0.7<x≤1时,ε相转变为δ相;当x=1时,相完全转变为δ相[33]。当1<x<3时,δ相转变为γ相[34]。当x=3时,γ相转变为岩盐结构的ω相,使其失去了可逆储存锂的能力[35]。因此,在V2O5材料充放电过程中,电压的选择极其重要,当电压为1.5~4 V时,理论比容量为440 mA·h/g(脱嵌3 mol Li+);当电压为2~4 V时,理论比容量为294 mA·h/g(脱嵌2 mol Li+);当电压为2.4~4 V时,理论比容量为147 mA·h/g(脱嵌1 mol Li+)[11-13]。
2 三维结构V2O5作为锂离子电池阴极材料
众所周知,电极材料的电化学性能主要取决于电极材料元素组成和相结构。近年来,大量研究表明电极材料的微观结构与形貌也是影响材料性能的关键因素[36]。采用不同合成方法,通过控制工艺参数可以制备出不同形貌的三维多级结构V2O5材料,主要有花状多级结构、多孔结构和中空结构等。
2.1 V2O5花状多级结构
V2O5花状多级结构主要是指由纳米片或纳米带等组成的具有花状三维微纳米结构。近年来,常用溶剂热法和水浴方法等得到该结构。
PAN等[37]将V2O5与H2C2O4按摩尔比1:3水浴得到产物VOC2O4,采用VOC2O4作为反应物,溶剂热得到前驱体VO2,再经过煅烧得到微花状多级结构的V2O5。随着反应时间的增加,组成微花结构的纳米片逐渐长大变成纳米带,且厚度也随之增加;随着VOC2O4浓度的增加,组成微花结构的纳米片也逐渐变成纳米带 ,但厚度却逐渐减小,而且微花结构尺寸也逐渐减小。当VOC2O4浓度为0.33 mol/L时,反应2 h后,得到的微花状结构的V2O5的电化学性能最优,比表面积能达到33.64 m2/g。当电压为2~4 V,电流密度300 mA/g时,首次放电比容量达274 mA·h/g,50次循环后容量为219 mA·h/g,库伦效率接近100%。表明Li+脱嵌的过程中有良好可逆性。
除了用反应物溶液的浓度和反应时间控制纳米片/带的形成和厚度,通过加入添加剂和改变溶剂的方法也可以控制着纳米片/带的形成和厚度。LI等[38]使用乙酰丙酮氧钒为原料,溶剂热并且经过煅烧制备出花状V2O5多级结构如图1(a)所示。通过改变配体尿素浓度可以有效地调节V2O5的微观结构和形貌[39]。无尿素时,材料由纳米颗粒组成;随尿素用量增加,材料由纳米带向纳米片过渡,当尿素量继续增大时,材料变成由纳米片组成的团聚体。另一方面溶剂也是起到控制纳米片厚度的关键因素,当溶剂是乙醇/水时,纳米片厚度为100~200 nm;当溶剂是异丙醇/水时,纳米片厚度为50~100 nm;而当溶剂是乙二醇/水时,纳米片厚度小于10nm时,此时得到的V2O5比表面积为20.75 m2/g(溶剂是异丙醇/水和乙醇/水时比表面积分别是18.50 m2/g和4.35 m2/g),且性能最优。当电流密度是100 mA/g时,电压在2~4 V,首次放电比容量为248 mA·h/g,250次循环后,首次放电比容量仍为184 mA·h/g;电压为2~3V时,首次放电比容量为157 mA·h/g,500次循环后维持在102.6 mA·h/g。该结构形成机制可以解释为:乙酰丙酮氧钒和乙二醇通过各自羟基相互配位作用,迅速形成固体微粒。固体微粒通过奥斯瓦尔德熟化作用,彼此相互聚集形成聚集体颗粒,在此过程中尿素分解产生的CO32-和NH4+也进入到聚集体中,引起结构不稳定。为释放应力和降低体系能量,聚集体分裂成小的纳米片[40],在溶剂热环境中,通过范德华力、氢键以及溶剂疏水性的作用,纳米片/带自组装成花状多级结构。
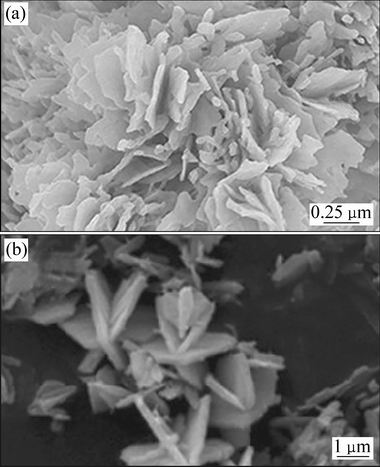
图1 花状V2O5样品[38]和海星状V2O5样品[41]的FESEM像
Fig. 1 FESEM images of flower-like[38] (a) and starfish-like[41] (b) V2O5 samples
LIANG等[41]以商业V2O5粉末为原料通过加热回流的方法制备出V2O5溶胶,并加入乙醇和水溶剂热并且经过煅烧得到由纳米片组成的多级海星状结构的V2O5如图1(b)所示。该结构形成机制如图2所示:V2O5溶胶在过量水中易于快速水解[42],形成氧化钒纳米颗粒。由于V—O键在a和b方向生长速度不同,通过羟连作用沿着H2O—V—OH方向生长速率更快,在b方向形成链结构;通过氧连作用沿着HO—V—OH生长速度较慢,在a方向形成多链结构。在小链的聚合过程中,氧连作用使得颗粒变大变厚;同时,羟连作用依然存在,细长的线状颗粒通过部分氧连作用聚集成簇。随着反应时间增加,当片状颗粒逐渐长大到一定尺寸时,该颗粒表面可作为形成新的片状颗粒生长点,新颗粒通过氧双键键合作用更趋向于沿着c方向的两个斜向生长,而氧连作用使片状颗粒变宽,最终形成多级海星状结构。而且多级海星结构的数量随着水热反应时间增加不断增多。当电压在1.5~4 V,电流密度为200 mA/g,首次放电比容量达到468 mA·h/g(商业V2O5粉末首次放电比容量仅为336 mA·h/g),超过了理论比容量440 mA·h/g,可能是由于存在氧空位和低价态钒离子V4+,其中氧空位可以提供锂离子的嵌入位点,而低价态钒可能在提高电极的锂离子嵌入能力中起重要作用[43]或在电极材料中作为锂储存的空位[44]。循环60次放电比容量保持在214 mA·h/g(商业V2O5粉末保持在200 mA·h/g),循环性能不理想是因为有不可逆相的生成[35]。当电流密度为1000 mA/g、首次放电比容量296 mA·h/g,50次循环容量保持在163 mA·h/g。同时,该海星状结构V2O5的电荷转移电阻为158 Ω,远小于商业V2O5粉末的电荷转移电阻258.5 Ω,且比表面积为33.9 m2/g远大于商业V2O5粉末的比表面积14.2 m2/g。
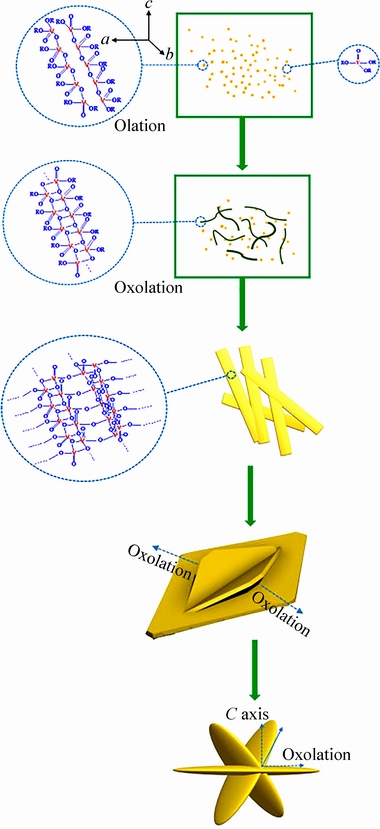
图2 多极海星状结构生长示意图[41]
Fig. 2 Schematic diagram of hierarchical starfish-like structure growth [41]
JIA等[45]通过将V2O5溶于草酸中,所得到的VOC2O4作为反应物,溶剂热并且经过煅烧后得到由纳米带组成的放射状结构的V2O5。研究表明,醇种类是影响着纳米带结构的关键因素[46]。当溶剂是乙二醇时,得到纳米带束组成的放射状结构的V2O5如图3(a)所示;当溶剂是乙醇时,得到纳米带组成的放射状结构的V2O5。研究认为,主要原因是一方面乙二醇有更大的极性,而且两个—OH可以同时发生反应,有利于沿着一侧形成纳米带,并组成束,形成径向结构。另一方面,尿素作为配体也是影响纳米带结构的重要因素,当尿素量增加1.5倍得到的是卷曲的小麦叶状纳米带组成的结构。通过用其他试剂(如表面活性剂P123)代替尿素,得到的是纳米粒子组成的氧化钒微球。研究分析认为,首先在溶剂热反应初期,钒氧离子将与乙二醇和尿素相互作用大量形核并快速生长成纳米带束前驱体。然后,由于尿素作为结构导向剂作用,由纳米带组成的束逐渐增长,新的束不断形成。为降低体系能量,已存在的束也可作为新束生长位点,最终完全形成结构一致的放射状多级结构。
当电压在2~4 V,以1/3C电流密度放电,首次放电容量285 mA·h/g,当10C放电时,容量为 157 mA·h/g;采用1C电流密度充放电100次循环后,容量为197 mA·h/g。当电压2.5~4 V时,以 1/3C放电,首次放电容量145 mA·h/g;以 40C放电,容量仍为105 mA·h/g,如图3(b)所示,采用1C电流充放电循环200次后,容量保持102 mA·h/g。PANG等[47]将(NH4)2V3O8溶解在水中,加入乙醇搅拌,随后加入氨水调节pH=10,40 ℃水浴制备出微米花状(NH4)2V3O8前驱体,煅烧后得到微米花状V2O5材料。前驱体的大小和厚度可以通过乙醇/水的比例以及水浴时间来调节。研究认为,花状结构的形成机制可能与(NH4)2V3O8在水和乙醇中的溶解度差异有关,这将促使其沿着(NH4)2V3O8晶粒和乙醇之间的界面方向再结晶。当电压2~4 V、电流密度为300 mA/g时,首次放电容量是289 mA·h/g,100次循环后,容量为200 mA·h/g。
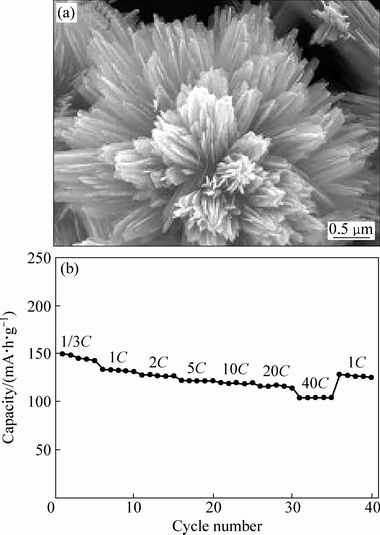
图3 纳米带束组装的放射状结构V2O5的SEM像及电化学性能[45]
Fig. 3 SEM images (a) and electrochemical properties (b) of V2O5 architecture assembled by nanoribbons bundle[45]
改变溶剂种类和控制反应时间可以调控多级结构中的纳米片/带的形成和厚度,而溶剂中醇种类影响着纳米片/带的结构。而反应物溶液的浓度除了可以控制合成纳米片/带的形成和厚度以外,还可以控制组成其多级结构的大小和尺寸。而配体尿素可能在某些体系下作为形貌控制剂和结构导向剂,能够促进纳米片/带的生成和改变纳米片/带的结构,从而更好地促进多级结构的形成。
花状多级结构材料具有良好的电化学性能归因于由纳米带/片等结构组成的花状多级结构具有高比表面积,使电极材料与电解液之间的接触面积增加,同时该结构在锂离子脱嵌过程中避免了纳米带/片的聚集和应力诱导致使结构崩溃,减少了充放电过程中体积的变化[48]。而组成多级结构的薄的纳米带/片可以有效地缩短Li+扩散距离和电子进入活性材料的传导路径,导致锂离子快速扩散和电子有效转移[49]。并且可以使电解液与电极界面的电荷转移电阻减小,导致电极反应动力学的增强[50]。此外,组成多级结构的纳米片和纳米带等二维结构存在着各向异性,二维结构的晶格在各个方向的Li+电导率有所差异,目前研究者对这方面没有深入研究。如果能利用好Li+电导率在晶格各个方向不同这一特点,控制晶面的优先生长,使材料具有更好的Li+传导和扩散能力,对于进一步提高V2O5电极材料电化学性能有着重要的意义。
2.2 V2O5多孔结构
为了提高电极材料的电化学性能,将其制成多孔结构是提高其电化学性能最有力手段之一[51]。多孔结构包括多种形貌的微纳米结构,有球形、八面体和立方体等。多孔结构是由纳米颗粒与孔隙空间结合得到的特殊结构[52]。近年来常用多孔结构的合成方法有 水/溶剂热法,静电喷雾沉积和喷雾热解法等等。
WANG等[53]以NH4VO3和草酸为原料通过静电喷雾沉积的方法,制备出三维多孔的V2O5薄膜正极材料如图4(a)所示。通过调节沉积时间和沉积时的温度(在260 ℃稳定多孔网状结构形成)可以控制材料的生长。溶剂对多孔膜的形成起到重要的作用。当溶剂是乙醇和1,2-丙二醇时,可以得到多层笼结构。如果用丙酮代替乙醇或者其他溶剂体系,导致膜表面粗糙且没有多孔结构的形成。假如溶剂中的含水量增加到 10%(质量分数)时,多孔膜也不能形成,研究者认为其原因主要为:首先在基板上形成2D网状层,随着沉积进行,厚度增加并堆叠在一起,最终形成三维多层-笼状微球。该材料在无粘结剂和导电剂条件下,在电流密度是0.5C,电压在2.5~4 V时,首次放电比容量为142 mA·h/g,100次循环后容量保持在130 mA·h/g,展现出良好的循环性能。当电压范围增加到2~4 V,在10C下,循环200次后,容量为110 mA·h/g,基本没有衰减,并且在循环一次后,电荷转移电阻稳定在小于50 Ω。此外,该材料有很好的倍率性能,当电流密度增加到56C时,还能提供86.7 mA·h/g的放电比容量如图4(b)所示。LONG等[54]将 NH4VO3和NH4NO3溶于水中通过喷雾热解方法制备出多孔球结构的V2O5。随着NH4NO3浓度增加,组成多孔结构纳米颗粒变小,形成介孔+大孔-V2O5,浓度继续增加,有利于大孔结构的形成。这种多孔结构形成可能由于NH4NO3的分解产生大量气体,促进纳米介孔+大孔- V2O5结构的产生。当NH4NO3浓度为0.272 mol/L时,材料的电化学性能最优,在电压为1.5~4 V时,当电流密度100 mA/g时,首次放电比容量为395 mA·h/g,循环50次后,放电比容量为218 mA·h/g,循环性能衰减过快同样是因为有不可逆相的生成[35]。当电流密度增大到1200 mA/g,放电比容量仍有180 mA·h/g。XIE等[55]用溶剂热方法得到巢状多孔V2O5球结构。将V2O5和草酸水浴得到VOC2O4溶液,通过乙二醇溶剂热得到的前驱体,煅烧后得到巢状多孔V2O5球。该结构材料在电压2~4 V,电流密度100 mA/g下,50次循环后容量保持240 mA·h/g。
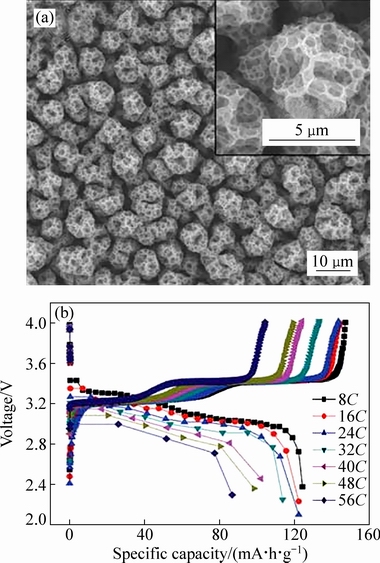
图4 三维多孔材料的SEM像及电化学性能[53]
Fig. 4 SEM image (a) and electrochemical properties (b) of 3D porous microspheres[53]
除了球形结构,研究者近年来制备了其他特殊形貌的多孔结构。AN等[56]用NH4VO3和1,2-丙二醇溶剂热得到钒酸铵前驱体,煅烧后得到多孔V2O5八面体。研究认为,尿素的添加会对某些晶面有选择性吸附作用,造成了特殊晶面的反应面积减少,使其生长缓慢,有利于获得的八面体形状[57]。而且尿素显示出一定的改善颗粒聚集的能力[58]。另一方面,在溶剂热反应过程中,由于尿素分解产生的氨,有效地抑制了偏钒酸铵的水解,最终形成钒酸铵八面体前驱体。在煅烧过程中,体积收缩和内部产生的氨的释放,钒酸铵转变成其相应的多孔结构的V2O5。相应的结构形成如图5所示。随着煅烧温度增加,孔径增加,但是比表面积和孔体积减小,表明了孔结构发生了变化。在350 ℃下得到的V2O5性能最优,并且比表面积最大为17.3 m2/g。在电流密度为100 mA/g,电压为2.4~4 V,首次放电比容量为135 mA·h/g时,循环60次后,容量保持141 mA·h/g;当电流密度增大到2 A/g,首次放电比容量96 mA·h/g,500次循环保持93 mA·h/g,容量基本不衰减。
ZHANG等[59]采用V2O5溶解在乙二醇中,加入PVP(聚乙烯吡络烷酮)溶剂热反应得到前驱体(PVP-VEG),煅烧后得到高度多孔V2O5立方体。研究认为材料形成机制如图6所示:溶剂乙二醇有利于前驱体的优先生长;PVP用作表面活性剂,高极性部分固定在金属或氧化物表面上,低极性的部分延伸到溶剂中,保持了立体结构的稳定性[60]。VEG是一种一维链状结构,氧钒基和配体之间发生低聚反应形成VEG纳米板[61]。PVP控制颗粒聚集的纵横比,随着反应进行,为降低其界面能,纳米板进行自组装和重新排列向更有利的立方体晶体生长,最后将前驱体煅烧,除去有机组分,形成高度多孔的立方体,比表面积为24.7 m2/g。在电压2.5~4 V、电流密度为100 mA/g时,首次放电比容量为143 mA·h/g,100次循环容量保持135 mA·h/g;当电流密度升到1.5 A/g,容量还能达到90 mA·h/g,1000次循环后,只有0.02%的衰减。并且与不加入PVP形成的V2O5相比,加入PVP后电荷转移电阻由259 Ω减小至144.9 Ω。研究认为,PVP-V2O5电极具有较好的电化学性能主要是由于如下原因造成的:由高孔隙介孔纳米板组装的立方体扩大了电极与电解液之间的接触面积,为电解液渗透提供了便利途径;连接的纳米板提高了活性材料的电子导电性,并且具有暴露的(110)面的单个纳米板的择优取向为Li+储存提供了优先的扩散通道;并且由1000次循环后只有0.02%的衰减可以看出多孔立方体具有耐应力,使其在脱嵌锂的过程中能稳定住整体结构,有利于循环稳定性的提高。
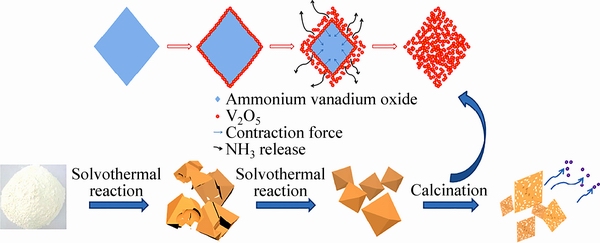
图5 钒酸铵八面体热分解形成三维多孔V2O5八面体的示意图[56]
Fig. 5 Schematic diagram of formation of 3D porous V2O5 octahedrons through thermal decomposition of ammonium vanadium oxide octahedrons[56]
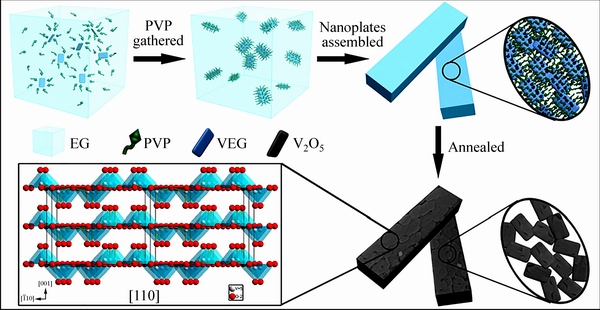
图6 高度多孔V2O5立方体形成示意图[59]
Fig. 6 Schematic diagram of highly porous V2O5 cuboids formation[59]
对于多孔材料来说,在合成过程中控制溶剂种类和比例有利于多孔结构的形成,同时反应时间和温度对于结构的生长也有着重要的影响。在有些体系下加入添加剂对于多孔结构的生长起到了重要的作用,例如加入NH4NO3有助于多孔结构的形成,其浓度来控制多孔材料的孔径大小;表面活性剂(如PVP)会限制纳米材料的形状和尺寸,并且强烈影响纳米颗粒的合成和组装[62];尿素作为配体,对于结构的形成也起到关键的作用。此外煅烧温度也可以控制多孔材料孔径的大小。
多孔结构材料良好的电化学性能归因于其独特的超高的颗粒堆积密度和高比表面积。并且组成多孔结构的纳米颗粒有着各向同性的特点,此外在多孔结构空间内有着高孔隙率,在脱嵌锂的过程中可以缓解内应力,保持了结构的稳定性,并且其内部的孔通道确保活性颗粒表面有效接触电解液,以及电解液有效渗透到颗粒内部,增加了电解液和电极之间的接触面积,缩短了Li+离子的扩散距离,从而改善电极内的扩散动力学,使Li+离子快速的扩散和加快了有效电荷的转移[63-65]。
2.3 V2O5中空结构
中空结构V2O5近年来一直被认为是一种有前景的阴极材料。目前,中空结构的V2O5主要有多层空心球阵列、中空单双壳结构、中空多壳结构、卵黄-壳结构、中空微线团结构等。其制备方法通常分为模板法和无模板法。
CHEN等[66]采用模板法制备出多层V2O5空心球阵列如图7(a)所示。通过垂直沉积工艺将聚苯乙烯球(PS)模板组装在石墨纸基底上,再用电沉积(ED)的方法将V2O5沉积到模板上,然后通过甲苯去除模板,经煅烧得到多层V2O5空心球阵列如图7(b)所示。当电压2~4 V,电流密度为0.5C时,首次比容量为293 mA·h/g,300次循环后,容量还能保持285 mA·h/g;即使当电流密度增大到20C,容量仍然有152 mA·h/g。
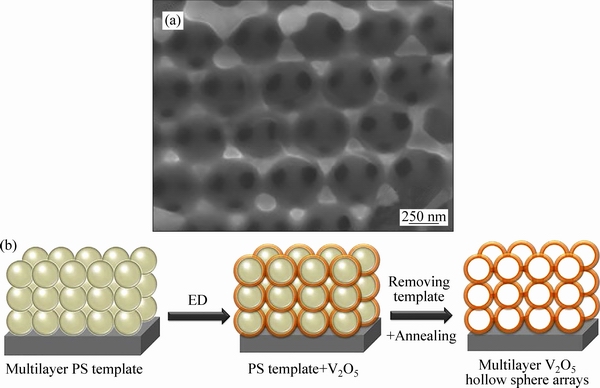
图7 多层V2O5空心球阵列的SEM像和制造工艺示意图[66]
Fig. 7 SEM images (a) and schematic diagram of fabrication process (b) of multilayer V2O5 hollow sphere arrays[66]
良好的电化学性能源于独特的中空结构连接的三维多孔内部空间有利于活性物质和电解液之间有效的接触,使Li+快速扩散,为电化学反应提供更多的活性位点,而且该结构可以缓解循环过程中体积膨胀引起的结构变化。此外,空心球阵列直接生长在集流体上确保了良好的机械粘附和活性物质与集流体的连接,由于不使用聚合物粘合剂和导电添加剂,因此可以避免死料(Dead mass)。此外,采用碳球、金属等作为模板[67-68]也可以得到中空结构的V2O5。
无模板法制备中空材料被认为是更加省时便利和价格低廉的方法。近年来,采用无模板法制备了各种中空材料,如中空单双壳结构、中空多壳结构、卵黄-壳结构、中空微线团结构等。PAN等[69]用V2O5和草酸微波辅助法得到的前驱体如图8(a)所示,煅烧后得到的由纳米棒组成的V2O5中空微球,空心球的直径随浓度升高而变大。研究认为,空心球形成过程中,结晶首先发生在固-液界面[70],从而在无定型固体表面上形成薄的结晶壳球体。内部的无定型核有很高的溶解度,一旦表面结晶壳与无定型核存在扩散路径,核将溶解在晶体壳附近,形成核-壳球体。随着晶体生长和内部物质的消耗,结晶壳的厚度不断增加直到无定型核全部耗尽。实际上,中空球的形成取决于非晶核的溶解速率和晶体壳的生长速率,如果非晶核的溶解速度太慢,则会形成固体结晶球,如果结晶壳的生长速度太慢,非晶核的溶解在晶体生长之前,产物的形态是不确定的。只有这两个速率是适当的,中空球才能形成[71]。当V2O5中空微球直径为440 nm时,电化学性能最优,并且比表面积高达54.996 m2/g。在电压2~4 V、电流50 mA/g时, 50次循环后容量为285 mA·h/g;当电流密度为2 A/g,容量达到133.9 mA·h/g。
MA等[72]将乙酰丙酮氧钒分散在异丙醇中,溶剂热得到卵黄-壳结构的VO2前驱体,经煅烧得到卵黄-壳结构的V2O5如图8(b)所示。在合成过程中,过氧化氢用量对材料的微观结构和形貌有着重要作用。当无过氧化氢时,材料为由纳米片组成的不规则颗粒,随着过氧化氢的增加,逐渐形成亚微球,当过氧化氢为2 mL时,形成卵黄-壳结构,而过氧化氢继续增加,材料逐渐变成由纳米板组成的不规则结构。这种卵黄- 壳结构的形成是由于乙酰丙酮氧钒的水解,首先在过饱和异丙醇溶液中形成具有光滑表面的固体亚微球。随着反应进行,球体受到由内向外奥斯瓦尔德熟化作用,颗粒的外部晶体壳继续长大,消耗内部核心以产生卵壳结构。在此基础上,随着不稳定的内部结构的彻底溶解,可以获得完全中空的亚微球体。由于氧化钒在溶剂中的溶解性差,以及大部分来自内部核心溶解的离子物质仍然保留在中空球内,随着连续溶解和重结晶的进行,当中空球内部的钒离子浓度达到VO2的成核密度,中空结构内部会发生再结晶,形成内部核心,再次形成卵黄-壳结构。最后,随着反应时间增加,外壳逐渐长大,中间芯变得更加明显,形成卵黄-壳结构。当电压在2~4 V、电流密度为300 mA/g时,第二次放电比容量是270.5 mA·h/g,80次循环后,容量保持166.8 mA·h/g;当电流密度增大12C,放电比容量仍然有87.3 mA·h/g。GUO等[73]将NH4VO3分散在甘油和异丙醇的混合溶液中,溶剂热得到氧钒甘油酯的前驱体,煅烧温度为350 ℃、450 ℃和550 ℃分别得到双壳中空球如图8(c)所示,单壳中空球和纳米颗粒。双壳中空结构形成机制被认为可能由于在煅烧的过程中,氧钒甘油酯的分解和V2O5的形成同时发生。氧钒甘油酯的氧化反应首先在颗粒-空气界面发生,形成核-壳结构,此时有两个相反的力,一个是有机组分分解引起的收缩力,另一个是生成的V2O5团聚过程中产生的粘附力阻止其向内收缩。这两个相反力相互作用,最终达到平衡形成空隙。随着加热时间的进行,内部的核心将发生二次演变得到双壳结构,表面粗糙和多孔可能由于CO2和水蒸汽的释放[74]。双壳中空结构,单壳中空球和纳米颗粒电荷转移电阻分别230、300和380 Ω,通过比较双壳中空结构电荷转移电阻最小且电化学性能最优。当电压在2~4 V、电流密度为500 mA/g时,首次放电比容量为256.7 mA·h/g;50次循环后,容量为197.6 mA·h/g;当电流密度升到5C,容量变为100 mA·h/g左右。良好的电化学性能由于纳米级结构组装的双壳中空结构可以提供更多的反应位点促进Li+的传导和电子的运输。
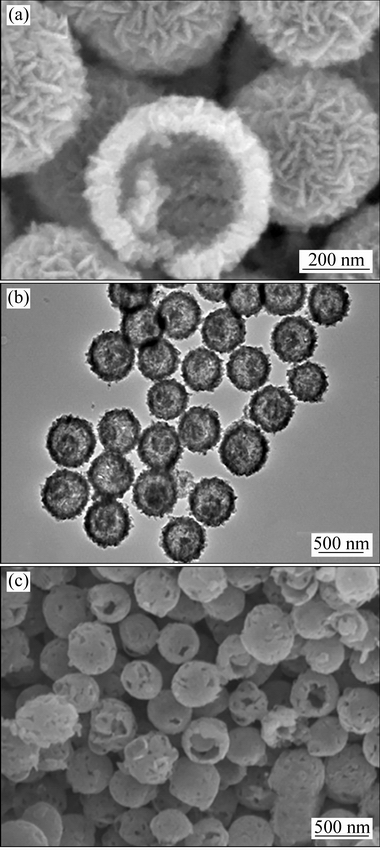
图8 中空结构[69]、卵黄-壳结构[72]和双壳结构[73]样品的图像
Fig. 8 FESEM images of hollow spheres[69] (a), TEM images of yolk-shell spheres[72] (b) and SEM images of double-shelled spheres[73] (c)
ZHANG等[75]将熔融V2O5直接水冷得到V2O5溶胶。加入异丙醇和三羟甲基氨基甲烷超声处理后,溶剂热并且经过煅烧后得到V2O5中空微线团结构(V2O5-HM),该结构形成机制如图9所示:V2O5溶胶电离得到[H2V10O28]4-, NH2C(CH2OH)3电离得到[NH2C(CH2OH)3]+,由于这两个物质之间的静电相互作用,导致了系统的凝结和纳米线的形成[76]。为了实现表面能最小化,纳米线经历卷曲和自滚过程,形成纳米线组成的具有中空腔的微线团结构[77]。随着纳米线之间越来越近,吸附在相邻纳米线上的[NH2C(CH2OH)3]+物质之间的排斥占主导地位,避免了纳米线进一步聚集[46]。在煅烧除去有机物质后,在纳米线之间形成孔或间隙,其中NH2C(CH2OH)3在反应中起到凝结作用。总的来说,首先超声处理后得到V2O5纳米线结构。溶剂热反应过程中,纳米线逐渐聚集并交织,形成中空微线团结构,最终煅烧得到V2O5中空微线团结构,比表面积为20.2 m2/g。当电压为2.4~4 V、电流密度为0.67C时,首次放电容量145.3 mA·h/g,50次循环后容量保持率为94.4%;当电流密度为13.3C,首次放电111.6 mA·h/g,100次循环容量基本无衰减;当电流密度为65C,容量还能达到94.8 mA·h/g,并且当电流密度回到1C,容量完全恢复,展现出极好的可逆性。而且与Li3VO4-CNT组成全电池,当电压为1~3.5 V、电流密度0.67C时,首次放电138.6 mA·h/g,200次循环后,容量为100 mA·h/g,当电流密度为20C,容量还能保持70 mA·h/g。良好的电化学性能由于纳米线之间的孔或间隙增加了电极和电解液之间的接触面积,并且有助于从各个方向插入Li+,缩短了Li+的扩散距离。确保了电极材料有效的利用。而且相互缠绕的纳米线构成的中空结构具有优异的抗粉碎和抗聚集能力,可以很好地维持循环过程中结构的完整性。
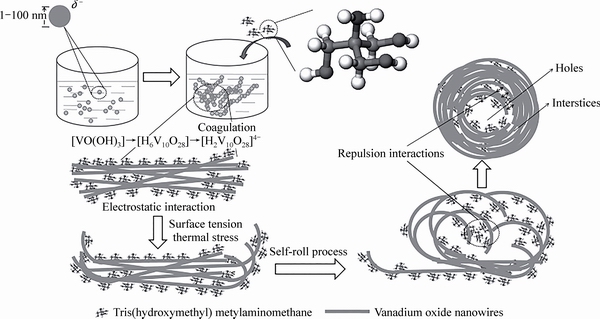
图9 V2O5-HM前驱体的形成机制[75]
Fig. 9 Proposed formation mechanism of V2O5-HM precursor[75]
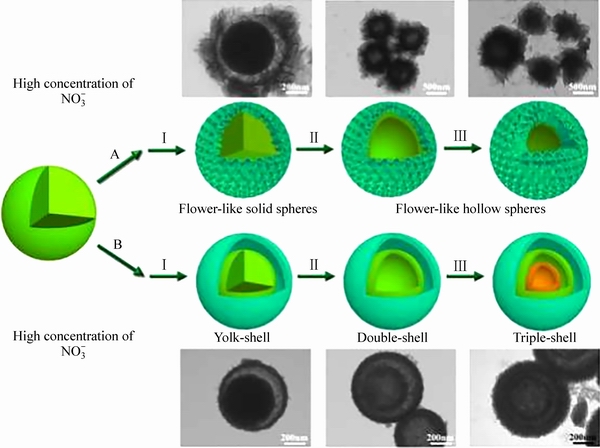
图10 花状空心结构(路线A)和多壳微球(路线B)的形成过程的示意图[78]
Fig. 10 Schematic illustration of formation process of flower-like hollow structure (Route A) and multi-shelled microspheres (Route B)[78]
REN等[78]通过将NH4VO3溶解到无水乙醇中,加入聚乙二醇-400(PEG-400)和浓硝酸,溶剂热并且经过煅烧后得到中空结构的V2O5。通过控制HNO3和PEG-400的使用量以及溶剂热反应时间获得如多壳微球和花状空心微球等中空结构。PEG-400有利于微球表面纳米片的形成,而HNO3对形成球状结构影响很大,在无HNO3时,材料由不规则形状形成[76]。该结构形成如图10所示:当HNO3浓度较低时,在初始反应阶段,固体微球首先形成。随着反应的进行,部分HNO3被消耗,纳米片在PEG-400的帮助下逐渐形成,PEG-400包裹固体微球来降低表面能(路线A,阶段Ⅰ)。奥斯特瓦尔德熟化作用使固体微球形成空心微球(路线A,阶段Ⅱ)。随着溶剂热反应时间的增加,外壳表面的纳米片充分生长(路线A,阶段Ⅲ)。当HNO3的量足够时,它将抑制纳米片的形成,奥斯瓦尔德熟化作用发生在预先生成的固体内部核心上,导致形成多层结构(路线B,阶段Ⅱ和Ⅲ)。因此,通过优化HNO3的浓度和反应时间,可以有效地控制中空材料的内部结构和微观形貌。最后发现花状空心微球电化学性能最优,当电压在2.5~4 V、电流密度为1C时,首次放电比容量为146.8 mA·h/g,350次循环容量为124 mA·h/g;甚至当电流密度增大到20C,容量为107.2 mA·h/g, 3000次循环后容量为73.5 mA·h/g。将钛酸锂(Li4Ti5O12)作为阳极进行全电池测试,电压在1~2.5 V,电流密度为147 mA/g,首次放电比容量133.5 mA·h/g, 100次循环后,容量保持105 mA·h/g,容量保持率为80%。显示出良好的可逆性和循环性能。
合成中空结构时,调节溶剂的浓度对中空球直径影响很大,前驱体浓度越高,直径越大。而控制反应时间,也可以有效地控制中空材料的内部结构和微观形貌。在某些体系下适当加入添加剂有利于材料的合成:比如过氧化氢用量对材料的微观形貌和结构也有显着影响,并且适当量的过氧化氢对于形成中空结构至关重要;聚乙二醇-400可以调节微球表面纳米片的大小;三羟甲基氨基甲烷有利于中空微线团的合成;浓HNO3影响着球状结构的合成。此外煅烧温度也可以影响中空材料的内部结构(单壳或双壳中空结构)。
中空结构材料良好的电化学性能归因于由纳米结构单元组成的中空结构具有高比表面积,增大了电极和电解液之间的接触面积。并且中空结构存在的孔隙保证了电解液的有效渗透,有利于电极材料与电解液接触,缩短Li+扩散距离。同时,内部中空结构对Li+脱嵌时发生的体积变化具有良好的应变力,有利于其循环稳定性[79]。
3 结论与展望
通过对三维不同形貌多级结构V2O5材料合成方法的总结发现,影响形貌的因素主要来源于:钒源和溶剂体系的选择和体系中不同溶剂的比例,反应物的浓度和反应时间,添加剂的种类及其分解的产物(如CO32-、NH4+和OH-等)之间的配位作用将控制着结构的生长,而且有些添加剂对于晶面有选择吸附作用,从而形成不同形貌的结构。前驱体转化为产物的煅烧温度。
不同形貌三维多级结构V2O5材料拥有高的比表面积,从而增加了电极和电解液之间的接触面积,缩短了Li+的扩散距离,并能在多次脱嵌锂过程中保持结构的稳定性,从而展现出优异的电化学性能。然而其在电压为1.5~4 V时嵌入3个锂形成不可逆的相使容量迅速衰减,并且V2O5电压平台过多也制约了其发展和实际应用,因此,今后可以加大其充放电机理的研究以及提出更有效的控制微观形貌的方法,并对其进行元素掺杂和表面包覆进一步的提高材料电化学性能,使其能进行大规模工业生产和商业化应用,进而成为新一代高能量密度锂离子电池的阴极材料。
REFERENCES
[1] GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries[J].Chemistry of Materials, 2010, 22(3): 587-603.
[2] 张 勃, 何 珺, 华正伸, 鲁豪祺, 王 新, 彭会芬. MoO42-取代对Nasicon型Li3Fe2(PO4)3正极材料电化学性能的影响[J]. 无机化学学报, 2016, 32(12): 2109-2116.
ZHANG Bo, HE Jun, HUA Zheng-shen, LU Hao-qi, WANG Xin, PENG Hui-fen. Effect of MoO42- substitution on electrochemical properties of Nasicon Li3Fe2(PO4)3 cathode[J]. Chinese Journal of Inorganic Chemistry, 2016, 32(12): 2109-2116.
[3] 杨绍斌, 董 伟, 沈 丁, 李思南, 王中将, 张佳民, 孙 闻, 张 琴. 钠离子电池负极材料的研究进展[J]. 中国有色金属学报, 2016, 26(5): 1054-1064.
YANG Shao-bin, DONG Wei, SHEN Ding, LI Si-nan, WANG Zhong-jiang, ZHANG Jia-min, SUN Wen, ZHANG Qin. Research progress of anode material for sodium-ion batteries[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(5): 1054-1064.
[4] 刘 黎, 焦丽芳, 袁华堂, 王先友. 锂离子电池电极材料 Li1+xV3O8研究进展[J]. 化工进展, 2011, 30(1): 189-195.
LIU Li, JIAO Li-fang, YUAN Hua-tang, WANG Xian-you. Research advances in Li1+xV3O8 for lithium ion batteries[J]. Chemical Industry and Engineering Progress, 2011, 30(1): 189-195.
[5] LI Zhe-fei, ZHANG Hang-yu, LIU Qi, LIU Ya-dong, STANCIU L, XIE Jian. Hierarchical nanocomposites of vanadium oxide thin film anchored on graphene as high-performance cathodes in li-ion batteries[J]. ACS Applied Materials & Interfaces, 2014, 6(21): 18894-18900.
[6] XU Kang. Electrolytes and interphases in Li-ion batteries and beyond[J]. Chemical Reviews, 2014, 114(23): 11503-11618.
[7] ELLIS B L, LEE K T, NAZAR L F. Positive electrode materials for Li-ion and Li-batteries[J]. Chemistry of Materials, 2010, 22(3): 691-714.
[8] YI Ting-feng, ZHU Yan-rong, ZHU Xiao-dong, SHU J, YUE Cai-bo, ZHOU An-na. A review of recent developments in the surface modification of LiMn2O4 as cathode material of power lithium-ion battery[J]. Ionics, 2009, 15(6): 779-784.
[9] LUO Shu, WANG Ke, WANG Jia-ping, JIANG Kai-li, LI Qun-qing, FAN Shou-shan. Binder-free LiCoO2/carbon nanotube cathodes for high-performance lithium ion batteries[J]. Advanced Materials, 2012, 24(17): 2294-2298.
[10] 郑贞苗, 唐新村, 汪 洋, 晋 媛, 孟 佳, 刘文明, 王 涛. 溶剂热法合成花状分级结构LiFePO4及其电化学性能研究[J]. 无机化学学报, 2015, 31(4): 731-738.
ZHENG Zhen-miao, TANG Xin-cun, WHANG Yang, JIN Yuan, MENG Jia, LIU Wen-ming, WANG Tao. Solvothermal synthesis and electrochemical performance of flowerlike LiFePO4 hierarchically microstructures[J]. Chinese Journal of Inorganic Chemistry, 2015, 31(4): 731-738.
[11] QU Qun-ting, ZHU Yu-song, GAO Xiang-wen, WU Yu-ping. Core-shell structure of polypyrrole grown on V2O5 nanoribbon as high performance anode material for supercapacitors[J]. Advanced Energy Materials, 2012, 2(8): 950-955.
[12] CHAO Dong-liang, XIA Xin-hui, LIU Ji-lei, FAN Zhan-xi, NG C F, LIN Jian-yi, ZHANG Hua, SHEN Ze-xiang, FAN Hong-jin. A V2O5/conductive-polymer core/shell nanobelt array on three-dimensional graphite foam: A high-rate, ultrastable, and freestanding cathode for lithium-ion batteries[J]. Advanced Materials, 2014, 26(33): 5794-5800.
[13] SONG Huan-qiao, LIU Chao-feng, ZHANG Chang-kun, CAO Guo-zhong. Self-doped V4+-V2O5 nanoflake for 2 Li-ion intercalation with enhanced rate and cycling performance[J]. Nano Energy, 2016, 22: 1-10.
[14] LI Zhuo-yu, ZHANG Chang-kun, LIU Chao-feng, FU Hao-yu, NAN Xi-hui, WANG Kan, LI Xin-yuan, MA Wen-da, LU Xian-mao, CAO Guo-zhong. Enhanced electrochemical properties of Sn-doped V2O5 as a cathode material for lithium ion batteries[J]. Electrochimica Acta, 2016, 222: 1831-1838.
[15] YU Rui-xiang, ZHANG Chao-feng, MENG Qing, CHEN Zhi-xin, LIU Hua-kun, GUO Zai-ping. Facile synthesis of hierarchical networks composed of highly interconnected V2O5 nanosheets assembled on carbon nanotubes and their superior lithium storage properties[J]. ACS Applied Materials & Interfaces, 2013, 5(23): 12394-12399.
[16] YU Dan-mei, CHEN Chang-guo, XIE Shu-hong, LIU Yan-yi, PARK K, ZHOU Xiao-yuan, ZHANG Qi-feng, LI Jiang-yu, CAO Guo-zhong. Mesoporous vanadium pentoxide nanofibers with significantly enhanced Li-ion storage properties by electrospinning[J]. Energy & Environmental Science, 2011, 4(3): 858-861.
[17] HU Yong-sheng, LIU Xi, MULLER J O, SCHLOGL R, MAIER J, SU Dang-sheng. Synthesis and electrode performance of nanostructured V2O5 by using a carbon tube-in-tube as a nanoreactor and an efficient mixed-conducting network[J]. Angewandte Chemie-International Edition, 2009, 48(1): 210-214.
[18] ZHENG Yan-zhen, DING Hai-yang, UCHAKER E, TAO Xia, CHEN Jian-feng, ZHANG Qi-feng, CAO Guo-zhong. Nickel-mediated polyol synthesis of hierarchical V2O5 hollow microspheres with enhanced lithium storage properties[J]. Journal of Materials Chemistry A, 2015, 3(5): 1979-1985.
[19] LIU Jin-yun, ZHENG Qi-ye, GOODMAN M D, ZHU Hao-yue, KIM J, KRUEGER N A, NING Hai-long, HUANG Xing-jiu, LIU Jin-huai, TERRONES M. Graphene sandwiched mesostructured Li-ion battery electrodes[J]. Advanced Materials, 2016, 28(35): 7696-7702.
[20] BRUCE P G, SCROSATI B, TARASCON J M. Nanomaterials for rechargeable lithium batteries[J]. Angewandte Chemie-International Edition, 2008, 47(16): 2930-2946.
[21] XIA Xin-hui, CHAO Dong-liang, NG C F, LIN Jian-yi, FAN Zhan-xi, ZHANG Hua, SHEN Ze-xiang, FAN Hong-jin. VO2 nanoflake arrays for supercapacitor and Li-ion battery electrodes: performance enhancement by hydrogen molybdenum bronze as an efficient shell material[J]. Materials Horizons, 2015, 2(2): 237-244.
[22] PAN Jing, LI Ming, LUO Yuan-yuan, WU Hao, ZHONG Li, WANG Qiang, LI Guang-hai. Microwave-assisted hydrothermal synthesis of V2O5 nanorods assemblies with an improved Li-ion batteries performance[J]. Materials Research Bulletin, 2016, 74: 90-95.
[23] RUI Xian-hong, TANG Yu-xin, MALYI O I, GUSAK A, ZHANG Y Y, NIU Z Q, TAN H T, PERSSON C, CHEN X D, CHEN Z. Ambient dissolution-recrystallization towards large-scale preparation of V2O5 nanobelts for high-energy battery applications[J]. Nano Energy, 2016, 22: 583-593.
[24] MAI Li-qiang, XU Lin, HAN Chun-hua, XU X, LUO Y Z, ZHAO S Y, ZHAO Y L. Electrospun ultralong hierarchical vanadium oxide nanowires with high performance for lithium ion batteries[J]. Nano Letters, 2010, 10(11): 4750-4755.
[25] ZHOU Xiao-wei, WU Guang-ming, GAO Guo-hua, WANG Ji-chao, YANG Hui-yu, WU Jian-dong, SHEN Jun, ZHOU Bin, ZHANG Zhi-hua. Electrochemical performance improvement of vanadium oxide nanotubes as cathode materials for lithium ion batteries through ferric ion exchange technique[J]. Journal of Physical Chemistry C, 2012, 116(41): 21685-21692.
[26] HUANG Jian-feng, QIAO Xiao-ning, XU Zhan-wei, CAO Li-yun, OUYANG Hai-bo, LI Jia-yin, WANG Rui-yi. V2O5 self-assembled nanosheets as high stable cathodes for Lithium-ion batteries[J]. Electrochimica Acta, 2016, 191: 158-164.
[27] 李延伟, 温胜山, 姚金环, 梁晓丽, 张灵志. 2D结构V2O5材料的制备与储锂性能[J]. 中国有色金属学报, 2015, 25(1): 158-164.
LI Yan wei, WEN Sheng shan, YAO Jin huan, LIANG Xiao li, ZHANG Ling zhi. Preparation and lithium storage performance of two dimensional V2O5 material[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(1): 158-164.
[28] NAGARAJU G, ASHOKA S, MANJUNATHA R, SRINIVASAN S, LIVAGE J, CHANDRAPPA G T. Vanadium oxide nanorings: Facile synthesis, formation mechanism and electrochemical properties[J]. Materials Research Bulletin, 2016, 83: 542-549.
[29] CHENG Jian-li, WANG Bin, XIN Huo-lin L, YANG Guang-cheng, CAI Hua-qiang, NIE F D, HUANG Hui. Self-assembled V2O5 nanosheets/reduced graphene oxide hierarchical nanocomposite as a high-performance cathode material for lithium ion batteries[J]. Journal of Materials Chemistry A, 2013, 1(36): 10814-10820.
[30] CHEN Liang, GU Xin, JIANG Xiao-lei, WANG Na-na, YUE Jie, XU Hua-yun, YANG Jian, QIAN Yi-tai. Hierarchical vanadium pentoxide microflowers with excellent long-term cyclability at high rates for lithium ion batteries[J]. Journal of Power Sources, 2014, 272: 991-996.
[31] CUI Jin-long, CUI Yong-fu, LI Shao-hui, SUN Hong-liang, WEN Zhong-sheng, SUN Jun-cai. Microsized porous SiOx@C composites synthesized through aluminothermic reduction from rice husks and used as anode for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(44): 30239-30247.
[32] CUI Jin-long, SUN Hong-Liang, LUO Ze-lin, SUN Jun-cai, WEN Zhong-sheng. Preparation of low surface area SiO2 microsphere from wheat husk ash with a facile precipitation process[J]. Materials Letters, 2015, 156: 42-45.
[33] YANG Yang, LI Lei, FEI Hui-long, PENG Zhi-wei, RUAN Ge-deng, TOUR J M. Graphene nanoribbon/V2O5 cathodes in lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2014, 6(12): 9590-9594.
[34] BADDOUR-HADJEAN R, MARZOUK A, PEREIRA-RAMOS J P. Structural modifications of LixV2O5 in a composite cathode (0≤x<2) investigated by Raman microspectrometry[J]. Journal of Raman Spectroscopy, 2012, 43(1): 153-160.
[35] LEGER C, BACH S, SOUDAN P, PEREIRA-RAMOS J P. Structural and electrochemical properties of ω-LixV2O5(0.4≤x≤3) as rechargeable cathodic material for lithium batteries[J]. Journal of the Electrochemical Society, 2005, 152(1): A236-A241.
[36] FERGUS J W. Recent developments in cathode materials for lithium ion batteries[J]. Journal of Power Sources, 2010, 195(4): 939-954.
[37] PAN An-qiang, WU Hao-bin, YU Le, ZHU Ting, LOU Xiong-wen. Synthesis of hierarchical three-dimensional vanadium oxide microstructures as high-capacity cathode materials for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2012, 4(8): 3874-3879.
[38] LI Gui-zhu, QIU Yong-cai, HOU Yuan, LI Hong-fei, ZHOU Li-sha, DENG Hong, ZHANG Yue-gang. Synthesis of V2O5 hierarchical structures for long cycle-life lithium-ion storage[J]. Journal of Materials Chemistry A, 2015, 3(3): 1103-1109.
[39] LI Yan-wei, YAO Jin-huan, UCHAKER E, YANG Jian-wen, HUANG Yun-xia, ZHANG Ming, CAO Guo-zhong. Leaf-like V2O5 nanosheets fabricated by a facile green approach as high energy cathode material for lithium-ion batteries[J]. Advanced Energy Materials, 2013, 3(9): 1171-1175.
[40] XIAO Ting, TANG Yi-wen, JIA Zhi-yong, LI Da-wei, HU Xiao-yan, LI Bi-hui, LUO Li-juan. Self-assembled 3D flower-like Ni2+-Fe3+ layered double hydroxides and their calcined products[J]. Nanotechnology, 2009, 20(47): 475603.
[41] LIANG Xing, GAO Guo-hua, WU Guang-ming, YANG Hui-yu. Synthesis and characterization of novel hierarchical starfish-like vanadium oxide and their electrochemical performance[J]. Electrochimica Acta, 2016, 188: 625-635.
[42] ZENG Yi, GAO Guo-hua, WU Guang-ming, YANG Hui-yu. Nanosheet-structured vanadium pentoxide thin film as a carbon- and binder-free cathode for lithium-ion battery applications[J]. Journal of Solid State Electrochemistry, 2015, 19(11): 3319-3328.
[43] LIU Da-wei, LIU Yan-yi, GARCIA B B, ZHANG Qi-feng, PAN An-qiang, JEONG Y H, CAO Guo-zhong. V2O5 xerogel electrodes with much enhanced lithium-ion intercalation properties with N2 annealing[J]. Journal of Materials Chemistry, 2009, 19(46): 8789-8795.
[44] ZHAO Kang-ning, LIU Feng-ning, NIU Chao-jiang, XU Wang-wang, DONG Yi-fan, ZHANG Lei, XIE Shao-mei, YAN Meng-yu, WEI Qiu-long, ZHAO Dong-yuan. Graphene oxide wrapped amorphous copper vanadium oxide with enhanced capacitive behavior for high-rate and long-life lithium-ion battery anodes[J]. Advanced Science, 2015, 2(12): 1500154.
[45] JIA Gui-qi, DENG Zong-nan, LIU Xue, JIANG Hao, LI Chun-zhong. Building radially oriented architecture by tailorable V2O5 nanoribbons toward enhanced lithium storage[J]. Chemical Engineering Journal, 2016, 304: 194-200.
[46] WEI Qiu-long, TAN Shuang-shuang, LIU Xiao-yi, YAN Meng-yu, WANG Feng-chao, LI Qi-dong, AN Qin-you, SUN Rui-min, ZHAO Kang-ning, WU Hen-gan. Novel polygonal vanadium oxide nanoscrolls as stable cathode for lithium storage[J]. Advanced Functional Materials, 2015, 25(12): 1773-1779.
[47] PANG Hong-chang, SONG Qiang, TIAN Peng, CHENG Jun, ZOU Nan, NING Gui-ling. Non-hydrothermal synthesis of (NH4)2V3O8 hierarchical flowers and their conversion into V2O5 for lithium ion battery[J]. Materials Letters, 2016, 171: 5-9.
[48] LIU Hai-mei, YANG Wen-sheng. Ultralong single crystalline V2O5 nanowire/graphene composite fabricated by a facile green approach and its lithium storage behavior[J]. Energy & Environmental Science, 2011, 4(10): 4000-4008.
[49] PALANISAMY K, UM J H, JEONG M, YOON W S. Porous V2O5/RGO/CNT hierarchical architecture as a cathode material: Emphasis on the contribution of surface lithium storage[J]. Scientific Reports, 2016, 6: 31275.
[50] LIU Yong-mei, ZHOU Xue-chou, GUO Yong-lang. Effects of fluorine doping on the electrochemical properties of LiV3O8 cathode material[J]. Electrochimica Acta, 2009, 54(11): 3184-3190.
[51] LI Yu, FU Zheng-yi, SU Bao-lian. Hierarchically structured porous materials for energy conversion and storage[J]. Advanced Functional Materials, 2012, 22(22): 4634-4667.
[52] 郑 卓, 吴振国, 向 伟, 杨秀山. 多孔微纳结构富锂正极材料0.6Li2MnO3·0.4LiNi0.5Mn0.5O2的制备及其电化学性能[J]. 无机化学学报, 2017, 33(3): 479-486.
ZHENG Zhuo, WU Zhen-guo, XIANG Wei, YANG Xiu-shan. Preparation and electrochemical performance of porous micro- nano structure layered Li-rich 0.6Li2MnO3· 0.4LiNi0.5Mn0.5O2 cathode for lithium-ion batteries[J]. Chinese Journal of Inorganic Chemistry, 2017, 33(3): 479-486.
[53] WANG Su-qing, LI Si-rong, SUN Yi, FENG Xu-yong, CHEN Chun-hua. Three-dimensional porous V2O5 cathode with ultra high rate capability[J]. Energy & Environmental Science, 2011, 4(8): 2854-2857.
[54] LONG Kong, TANIGUCHI I. Correlation between porous structure and electrochemical properties of porous nanostructured vanadium pentoxide synthesized by novel spray pyrolysis[J]. Journal of Power Sources, 2016, 312: 36-44.
[55] XIE Chong-yao, CHENG Chuan-jun, YANG Jin-gang, HOU Jun-ke, LIU Ding-yu, LIU San-gui, HAN Jin, ZHANG Yan, XU Mao-wen. A nest-like hierarchical porous V2O5 as a high-performance cathode material for Li-ion batteries[J]. Ceramics International, 2016, 42(15): 16956-16960.
[56] AN Qin-you, ZHANG Peng-fei, XIONG Fang-yu, WEI Qiu-long, SHENG Jin-zhi, WANG Qin-qin, MAI Li-qiang. Three-dimensional porous V2O5 hierarchical octahedrons with adjustable pore architectures for long-life lithium batteries[J]. Nano Research, 2015, 8(2): 481-490.
[57] KANG Long-tian, FU Hong-bing, CAO Xin-qiang, SHI Qiang, YAO Jian-nian. Controlled morphogenesis of organic polyhedral nanocrystals from cubes, cubooctahedrons, to octahedrons by manipulating the growth kinetics[J]. Journal of the American Chemical Society, 2011, 133(6): 1895-1901.
[58] ZHANG Xiao-fei, WANG Kai-xue, WEI Xiao, CHEN Jie-sheng. Carbon-coated V2O5 nanocrystals as high performance cathode material for lithium ion batteries[J]. Chemistry of Materials, 2011, 23(24): 5290-5292.
[59] ZHANG Cheng, FANG Guo-zhao, LIANG Cai-wu, ZHOU Jiang, TAN Xiao-ping, PAN An-qiang, LIANG Shu-quan. Template-free synthesis of highly porous V2O5 cuboids with enhanced performance for lithium ion batteries[J]. Nanotechnology, 2016, 27(30): 305404.
[60] XIA You-nan, XIONG Yu-jie, LIM B, SKRABALAK S E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics?[J]. Angewandte Chemie-International Edition, 2009, 48(1): 60-103.
[61] WEEKS C, SONG Y N, SUZUKI M, CHERNOVA N A, ZAVALIJ P Y, WHITTINGHAM M S. The one dimensional chain structures of vanadyl glycolate and vanadyl acetate[J]. Journal of Materials Chemistry, 2003, 13(6): 1420-1423.
[62] RABKIN A, SAMUHA S, ABUTBUL R E, EZERSKY V, MESHI L, GOLAN Y. New nanocrystalline materials: A previously unknown simple cubic phase in the SnS binary system[J]. Nano Letters, 2015, 15(3): 2174-2179.
[63] MAHMOOD N, ZHANG Chen-zhen, HOU Yang-long. Nickel sulfide/nitrogen-doped graphene composites: Phase-controlled synthesis and high performance anode materials for lithium ion batteries[J]. Small, 2013, 9(8): 1321-1328.
[64] CHOI B G, CHANG Sung-jin, LEE Y B, BAE J S, KIM H J, HUH Y S. 3D heterostructured architectures of Co3O4 nanoparticles deposited on porous graphene surfaces for high performance of lithium ion batteries[J]. Nanoscale, 2012, 4(19): 5924-5930.
[65] MAHMOOD N, ZHANG Chen-zhen, LIU Fei, ZHU Jing-han, HOU Yang-long. Hybrid of Co3Sn2@Co nanoparticles and nitrogen-doped graphene as a lithium ion battery anode[J]. ACS Nano, 2013, 7(11): 10307-10318.
[66] CHEN Ming-hua, XIA Xin-hui, YUAN Jie-fu, YIN Jing-hua, CHEN Qing-guo. Free-standing three-dimensional continuous multilayer V2O5 hollow sphere arrays as high-performance cathode for lithium batteries[J]. Journal of Power Sources, 2015, 288: 145-149.
[67] HU Jing, CHEN Min, FANG Xiao-sheng, WU Li-min. Fabrication and application of inorganic hollow spheres[J]. Chemical Society Reviews, 2011, 40(11): 5472-5491.
[68] WU Hao-bin, PAN An-qiang, HNG H H, LOU Xiong-wen. Template-assisted formation of rattle-type V2O5 hollow microspheres with enhanced lithium storage properties[J]. Advanced Functional Materials, 2013, 23(45): 5669-5674.
[69] PAN Jing, LI Zhong, LI Ming, LUO Yuan-yuan, LI Guang-hai. Microwave-assisted solvothermal synthesis of VO2 hollow spheres and their conversion into V2O5 hollow spheres with improved lithium storage capability[J]. Chemistry-A European Journal, 2016, 22(4): 1461-1466.
[70] PAN An-qiang, ZHU Ting, WU Hao-bin, LOU Xiong-wen. Template-free synthesis of hierarchical vanadium-glycolate hollow microspheres and their conversion to V2O5 with improved lithium storage capability[J]. Chemistry-A European Journal, 2013, 19(2): 493-499.
[71] YU Jia-guo, ZHANG Jun. A simple template-free approach to TiO2 hollow spheres with enhanced photocatalytic activity[J]. Dalton Transactions, 2010, 39(25): 5860-5867.
[72] MA Yi-ning, HUANG Ai-bin, ZHOU Huai-juan, JI Shi-dong, ZHANG Shu-ming, LI Rong, YAO He-liang, CAO Xun, JIN Ping. Template-free formation of various V2O5 hierarchical structures as cathode materials for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2017, 5(14): 6522-6531.
[73] GUO Fei-fan, FAN Mei-hong, JIN Pan-pan, CHEN Hui, WU Yuan-yuan, LI Guo-dong, ZOU Xiao-xin. Precursor-mediated synthesis of double-shelled V2O5 hollow nanospheres as cathode material for lithium-ion batteries[J]. CrystEngComm, 2016, 18(22): 4068-4073.
[74] SHEN Lai-fa, YU Le, WU Hao-bin, YU Xin-yao, ZHANG Xiao-gang, LOU Xiong-wen. Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties[J]. Nature Communications, 2015, 6: 6694.
[75] ZHANG Peng-fei, ZHAO Lu-zi, AN Qin-you, WEI Qiu-long, ZHOU Liang, WEI Xiu-juan, SHENG Jin-zhi, MAI Li-qiang. A high-rate V2O5 hollow microclew cathode for an all- vanadium-based lithium-ion full cell[J]. Small, 2016, 12(8): 1082-1090.
[76] AN Qin-you, WEI Qiu-long, ZHANG Peng-fei, SHENG Jin-zhi, HERCULE K M, LV F, WANG Qin-qin, WEI Xiu-juan, MAI Li-qiang. Three-dimensional interconnected vanadium pentoxide nanonetwork cathode for high-rate long-life lithium batteries[J]. Small, 2015, 11(22): 2654-2660.
[77] MCNULTY D, BUCKLEY D N, O’DWYER C. Synthesis and electrochemical properties of vanadium oxide materials and structures as Li-ion battery positive electrodes[J]. Journal of Power Sources, 2014, 267: 831-873.
[78] REN Xiao-chuan, ZHAI Yan-jun, ZHU Lin, HE Yan-yan, LI Ai-hua, GUO Chun-li, XU Li-qiang. Fabrication of various V2O5 hollow microspheres as excellent cathode for lithium storage and the application in full cells[J]. ACS Applied Materials & Interfaces, 2016, 8(27): 17205-17211.
[79] LAI Xiao-yong, HALPERT J E, WANG Dan. Recent advances in micro-/nano-structured hollow spheres for energy applications: From simple to complex systems[J]. Energy & Environmental Science, 2012, 5(2): 5604-5618.
Recent progress of three-dimensional hierarchical structure V2O5 Li ion cathode materials
CHEN Peng-yu, ZHENG Guo-tao, TANG Jie, LI Song, WEN Zhong-sheng, JI Shi-jun, SUN Jun-cai
(College of Transportation Equipment and Ocean Engineering, Dalian Maritime University, Dalian 116026, China)
Abstract: In view of the low electric conductivity and the poor lithium-ion diffusion kinetics, the element doping, surface coating and microstructure controlling were demonstrated as an effective strategy to improve these problems. Compared to the one-dimensional and two-dimensional structures materials, the three-dimensional hierarchical structure materials have both high specific surface area, and are not prone to agglomeration because of its good self-supporting properties. The cathode materials V2O5, which possess different morphology three-dimensional hierarchical structure made by different synthetic methods, show excellent electrochemical performances. In this review, the new progress of the synthesis methods, the formation mechanism and the electrochemical performance of different morphology three-dimensional hierarchical structure V2O5 materials were summarized. And the relationship between the preparation process and microscopic morphology as well as between microscopic morphology and electrochemical properties was introduced.
Key words: V2O5; three-dimensional hierarchical structure; morphology-controlling; formation mechanism; electrochemical performance
Foundation item: Project(21476035) supported by the National Natural Science Foundation of China; Project (3132016341) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2017-11-01; Accepted date: 2018-03-16
Corresponding author: LI Song; Tel: +86-411-84727971; E-mail: Lisong@dlmu.edu.cn
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(21476035);中央高校基本科研业务费(3132016341)
收稿日期:2017-11-01;修订日期:2018-03-16
通信作者:李 嵩,副教授,博士;电话:0411-84727971;E-mail:Lisong@dlmu.edu.cn
摘 要:针对V2O5材料电导率低和锂离子扩散动力性能差等缺点,通过元素掺杂、表面包覆和微观形貌调控等方法可以有效进行改善。相比于一维和二维结构材料,三维多级结构材料既有高比表面积,又由于有良好的自支撑性,而不容易发生团聚。利用不同合成方法将V2O5材料微纳米化,制成具有不同形貌的三维多级结构,材料表现出优良的电化学性能。综述不同形貌三维多级结构V2O5材料的合成方法、形成机理及电化学性能的研究进展,并着重介绍制备工艺与V2O5材料微观形貌以及V2O5材料微观形貌和电化学性能之间的关系。


