
Effect of photocatalyst on performance of LaNi5 hydrogen storage alloy electrodes
LI Min-shan(李民善), TANG You-gen(唐有根), WANG Jia-li(王佳力),
YANG Hai-hua(杨海华), WAN Wei-hua(万伟华)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Photocatalyst hydrogen storage alloys(PHSA) with different photocatalyst contents were synthesized by mechanical blend method. The effects of the photocatalyst on the electrochemical performance of the PHSA were studied. The results indicate that the PHSA electrodes show better activation performance when UV irradiation is present. The high rate discharge performance as well as cycle performance is improved compared with that of the AB5 alloys. The PHSA shows the best performance when the photocatalyst content is 20%.
Key words:
photocatalyst; hydrogen storage alloys; electrochemical performance;
1 Introduction
Under the pressure of resources and environment, many governments and organizations have attached great attention to the development and application of new power source. The solar energy and the hydrogen energy have attracted more and more attention in recent years[1-2]. Much work on solar energy photocatalytic hydrogen production has been carried out since 1972[3-6]. Photocatalysts for hydrogen production such as semiconductor oxide compounds, perovskite compounds and spinel compounds have been successfully developed.
For its high energy density, high rate charge-discharge capability, excellent hydrogen absorption-desorption ability and environmental acceptability, metal hydride has been widely applied in rechargeable alkaline batteries, hydrogen storage and transportation[7], hydrogen vehicles heat pumps, conversion between heat energy and mechanical energy, separation and purification of hydrogen[8] and catalyst, and so on. Preparing a kind of new electrode— photocatalytic hydrogen storage alloy(PHSA) electrode to make the hydrogen storage process of the hydrogen storage alloy run after the photo catalysis process of the photocatalyst immediately, realize the light charge of the hydrogen storage alloy, which presents a possibility of combining the energy band structure of photocatalyst and the property of hydrogen storage alloy together. This means another possible method of transforming the solar energy into the electrical energy. Researches in the field from BIGNOZZI et al[9], ZHOU et al[10], YANG et al[11], ZHANG et al[12] are original and effective. There is no report about the effect of photocatalyst on the charge-discharge performance of hydrogen storage alloy. The effect of spinel photocatalyst on the charge- discharge performance of low-Co-content LaNi5 hydrogen storage alloy is studied in this paper.
2 Experimental
2.1 Preparation of copper aluminate(CuAl2O4) photo- catalyst by citric acid complexation method
Copper aluminate was prepared by sol-gel method. Citric-acid(C6H8O7·H2O) as a complexing agent, Al(NO3)3·9H2O and Cu(NO3)2·3H2O as precursor materials for CuAl2O4. Stoichiometric amounts of Al(NO3)3·9H2O and Cu(NO3)2·3H2O (2?1, molar ratio) were weighed and placed in a beaker with proper amount deionized water, then the C6H8O7·H2O solution which dissolved in advance was added slowly and then a light blue mixed solution was obtained. The solution was heated to 40 ℃ and held for half an hour. Then ammonia was dropped slowly into the solution, adjusting the pH between 2-3, and the solution changed to a dark blue sol. Then the sol was heated to a constant temperature of 70 ℃ until dark blue gel appears[13], so the CuAl2O4 precursor was gained. The precursor was sintered at 750 ℃ for 6 h, cooled naturally. After ground carefully, a deep brown CuAl2O4 photocatalyst powder was obtained.
2.2 Morphology analysis and characterization of photocatalyst
The structure of CuAl2O4 catalyst dispersed in ethanol was examined with a KYKY2800-scanning electron microscope (Beijing) operating at 300 kV, and a X-ray diffractometer (Germany, Cu Kα radiation, scanning rate 4 (?)/ min) operating at 36 kV.
2.3 Preparation of hydrogen storage alloy electrode
Hydrogen storage alloy (MlNi4.1Co0.25Mn0.4Al0.25) powder and carbonyl nickel powder (conductive agent) (mass ratio 1?2) were mixed with proper 2% PTFE solution(adhesive). Repeating the above procedure for 2 times and three parallel samples were obtained. A certain amount of CuAl2O4 catalyst was added into the 2 samples (account for hydrogen storage alloy powder quality of 20%, 30%, recorded as 20%PHSA, 30%PHSA respectively as follows), then roasted in vacuum. Three 0.2 g samples picked out from the three mixtures were pressed to a slice round electrode (d 10 mm×1 mm) on the punch.
2.4 Electrochemistry impedance study
The electrochemistry impedance of electrodes was tested on the CHI660b electrochemistry station, by using hydrogen storage alloy electrode as an investigative electrode, big surface NiOOH piece as assistant electrode and Hg/HgO (6 mol/L KOH) as a reference electrode.
2.5 Electrochemical properties investigation
The electrochemical properties of electrodes were investigated in the ringent simulation cells. A large area of sintered nickel electrode as the cathode, 6 mol/L KOH solution as the electrolyte solution. A 300 W long-wave UV lamp was used in the illuminate experiments, and the distance between the lamp center and electrode surface was 20 cm.
3 Results and discussion3.1 Characterization of photocatalyst
Fig.1 presents the SEM image of the CuAl2O4 catalyst showing anomalistic particles with a uniform morphology. The material disperses well and shows a uniform size. The particle size is 10-20 nm, with an average particle size of 14 nm.
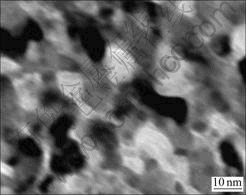
Fig.1 SEM image of CuAl2O4 catalyst
Fig.2 shows the XRD pattern of the catalyst sample. Compared with the standard spectrum, besides the characteristic peaks of CuAl2O4, there are a few of weaker impurity peaks in the sample spectrum. This indicates that the catalyst sample is CuAl2O4 spinel primarily, and a little CuO is detected.
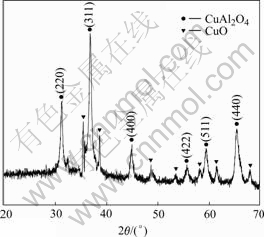
Fig.2 XRD pattern of CuAl2O4 catalyst
3.2 Electrochemical impedance
Fig.3 shows the electrochemical impedance pattern of the low-Co-content LaNi5 alloy electrode and PHSA electrodes with different CuAl2O4 contents in the UV light and without light. All the electrodes were charged with 12 mA current for 300 min before tests.
Fig.3 indicates that the addition of CuAl2O4 improves the kinetics properties of hydrogen storage alloy. The electrode electrochemical reaction resistance decreases when CuAl2O4 is added, but the interface resistance between the electrode, the membrane and the solution increases. It may be interpreted by the partial deoxidization of the CuAl2O4 to Cu and Al on the surface of the hydrogen storage alloy, and the Co could increase the hydrogen diffusion coefficient, reduce the electro- chemical reaction resistance[14-16]. The CuAl2O4 photocatalyst on the surface of the electrodes is excited by the ultraviolet radiation and facilitates the electron conduction, so the PHSA electrode charge transfer resistance decreases, which promotes the PHSA electrode electrochemical reaction. The R1 and Rr of the electrodes are listed in Table 1.
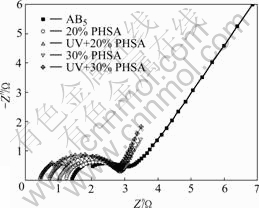
Fig.3 AC impedance plots of AB5 alloy and PHSA
Table 1 Impedance data of electrodes

3.3 Electrochemical property
3.3.1 Activation property
Fig.4 shows the activation property pattern of low-Co-content AB5 alloy electrode and PHSA electrode with different CuAl2O4 contents in the UV light irradiation or no irradiation. The test parameters are listed in Table 2.
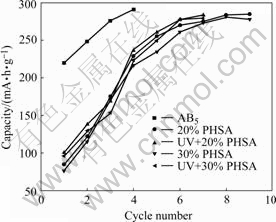
Fig.4 Comparison of activation property of electrodes
Table 2 Charge-discharge program in activation process of electrodes
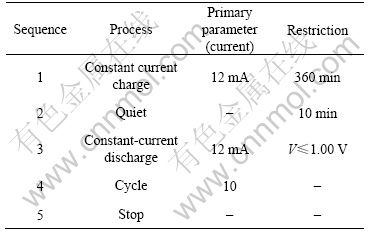
Fig.4 indicates that the electrochemical capacity of the low-Co-content AB5 alloy electrode reaches 220 mA?h/g after initial activation, and reaches the maximum discharge capacity of 291 mA·h/g when the 4th activation completes. Because of the bad conductivity of CuAl2O4, the initial activation capacities of the PHSA electrodes with CuAl2O4 are comparatively low: 85.126 mA·h/g for PHSA electrodes with 20%(mass fraction) CuAl2O4 and 76.524 mA·h/g for 30% CuAl2O4, and reach the maximum discharge capacity after no less than 9 and 8 activations. The UV irradiation improves the activation performance and the discharge capacity of PHSA electrodes, the initial activation capacity aggrandizes to 100.654 mA·h/g for PHSA electrodes with 20%(mass fraction) CuAl2O4 and 96.516 mA·h/g for 30%, and reaches the maximum discharge capacity after 7 and 6 activations.
3.3.2 Comparison of cycle performance of PHSA electrode and AB5 electrode
Fig.5 shows the cycle pattern of AB5 alloy electrode and PHSA electrode with different CuAl2O4 contents. The test parameters are listed in Table 3.
The discharge capacity of low-Co-content AB5 alloy electrode decays fast to 86.3% of the maximum capacity after 50 cycles. Due to the partial deoxidization of the CuAl2O4 to Cu and Al, the discharge capacity of PHSA electrodes with CuAl2O4 decays noticeably after 50 cycles[14-16]. The PHSA electrodes with 20% CuAl2O4 maintains 93.7% of the maximum capacity, and PHSA electrodes with 30% CuAl2O4 maintains 90.1%.
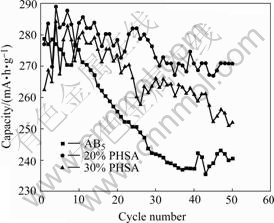
Fig.5 Comparison of cycle property of electrodes
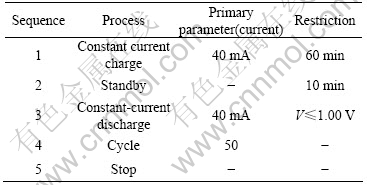
Table 3 Charge-discharge pattern in cycle process of electrodes
3.3.3 Comparison of high-rate discharge performance of PHSA electrode and AB5 electrode
Fig.6 shows the high-rate discharge performance pattern of AB5 alloy electrode and PHSA electrode with different CuAl2O4 contents.
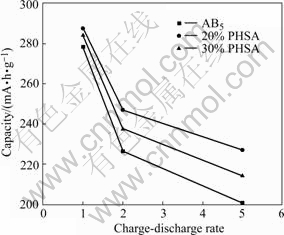
Fig.6 High-rate charge-discharge ability of electrodes
For the same reason[14-16], the CuAl2O4 improves the high-rate discharge performance of the low-Co-content AB5 alloy. Besides, the CuAl2O4 might enhance the hydrogen absorption-desorption speed of low-Co-content AB5 alloy, inhibiting the pulverization and embrittlement of the alloy[8]. But the addition of overload may lead to increased resistance of electrodes, which is partially offset by its low-cobalt alloy AB5 filling high-rate discharge performance improvement.
4 Conclusions1) AC impedance test results show that a proper amount of CuAl2O4 photocatalyst can reduce the electrochemical reaction resistance of low-Co-content AB5 alloy electrode, but also aggrandize the contact resistance between the electrode, membrane and the solution.
2) The CuAl2O4 photocatalyst reduces the activation performance of the low-Co-content AB5 alloy electrode. The PHSA electrodes need more than 8 charge-discharge activation cycles to reach the maximum discharge capacity. UV irradiation is helpful to improving the activation performance of the PHSA electrodes, making the activation cycle number decline remarkably.
3) The PHSA electrode shows better cycle performance and high-rate discharge performance compared with that of low-Co-content AB5 alloy electrode. The capacity retention keeps more than 90% with 20% catalyst additive after 50 cycles, while the low-Co-content AB5 alloy electrode keeps 86.3%. The PHSA electrode with 20% catalyst additive keeps 93.7% after 50 cycles, and shows better high rate discharge performance compared with the other two electrodes.
References
[1] WU Wen-jun, ZHAN Wen-hai, MENG Fan-shun, et al. Photoelectrochemical properties of three new carboxylated hemicyanine dyes on dye-sensitized solar cells [J]. Chemistry, 2007, 5: 356-360. (in Chinese)
[2] MU Jun-ying, XU Juan, et al. Low-temperature fabrication of nano-porous TiO2 films for flexible dye-sensitized solar cells [J]. New Chemical Materials, 2007, 35(1): 31-33. (in Chinese)
[3] FUJISHIMA A, HONDA K. Electrochemical proteolysis of water at a semiconductor electrode [J]. Nature, 1972, 5358(238): 37-38.
[4] NAKANISHI H, SANCHEZ C, HENDEWERK M, et al. Photochemical hydrogen production from a water-methanol mixture with small particles of iron oxide suspensions [J]. Materials Research Bulletin, 1986,21(2): 137-148.
[5] BESSEKHOUAD Y, TRARI M. Photocatalytic hydrogen production from suspension of spinel powders AMn2O4(A=Cu and Zn) [J]. International Journal of Hydrogen Energy, 2002, 27: 357-362.
[6] WANG De-fa, ZOU Zhi-gang, YE Jin-hua. A new spinel-type photocatalyst BaCr2O4 for H evolution under UV and visible light irradiation [J]. Chemical Physics Letters, 2003, 373: 191-196.
[7] HUANG Tai-zhong, WU Zhu, XU Nai-xin, et al. Structure and performances of TiCrMo ternary hydrogen storage alloys [J]. The Chinese Journal of Nonferrous Metals, 2007, 16(11): 1855-1860. (in Chinese)
[8] WAN Wei-hua, TANG You-gen, LU Zhou-guang, et al. Modifaction of LaNiAl hydrogen storage alloys [J]. J Cent South Univ (Science and Technology), 2007, 38(1): 107-111. (in Chinese)
[9] BIGNOZZI C A, ARGAZZI R, INDELLI M T, et al. Design of supramolecular systems for spectral sensitization of semiconductors [J]. Solar Energy Mater and Solar Cells, 1994, 32: 229-232.
[10] ZHOU Hua-jun, WANG Da-zhi, LIU Jin-hua, et al. The photo-absorbency effect of size and doping SnO2 in nano TiO2 [J]. Chin J Chem Phys, 2002, 15: 61-63. (in Chinese)
[11] YANG Guang, ZHAO Qian, ZHANG Liang, et al. Photogenerated current process of TiO2 nanostructured electrode sensitized by unsymmetrical cyanine dye [J]. Chin J Chem Phys, 2003, 16: 214-217. (in Chinese)
[12] ZHANG Wen-kui, GAN Yong-ping, HUANG Hui, et al. Photochargeable behaviors of hydrogen storage alloy’s electrode modified with nano-TiO2 photocatalyst [J]. Chin J Chem Phys, 2005, 18(5): 832-836. (in Chinese)
[13] JIANG Yan-yan, LI Jing-gang, NING Gui-ling, et al. Preparation and visible light photocatalytic property of spinel CuAl2O4 nanoparticles [J]. Journal of the Chinese Ceramic Society, 2006, 34(9): 1084-1087. (in Chinese)
[14] XIA Tong-chi, DONG Hui-chao, WANG Shu-xin, et al. Effects of micro-encapsulation on the performance and discharge mechanism of hydrogen storage alloy electrode [J]. Chinese Journal of Power Source, 2005, 29(10): 652-654. (in Chinese)
[15] ZHANG Sen, DENG Chao. A novel method of surface modification on AB5 hydrogen storage alloy and its mechanism analysis [J]. Acta Phys Chim Sin, 2005, 21(10): 1146-1150. (in Chinese)
[16] LI Qian, JIANG Li-jun, LIN Qin, et al. Influence of Ag and Al on hydrogen storage properties of Mg2Ni alloy [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(4): 864-870. (in Chinese)
(Edited by PENG Chao-qun)
Corresponding author: TANG You-gen; Tel: +86-731-8830886; E-mail: ygtang@l26.net


