Article ID: 1003-6326(2005)05-1194-05
Comparison of photocatalytic activity of
TiO2 film doped nonuniformly by Mn and Zn
XU Wei(徐 伟)1, 2, LI Xin-jun(李新军)1, ZHENG Shao-jian(郑少健)1,
WANG Jun-gang(王俊刚)1, 2, XU Zhong-kui(徐忠奎)3
(1. Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, China;
2. Graduate School of Chinese Academy of Sciences, Beijing 100039, China;
3. Xin Tianlong Alcohol Co Ltd, Jilin 132000, China)
Abstract:
The thin films of TiO2, doped by Mn or Zn with nonuniform distribution, were prepared by sol-gel method under process control. The actinic absorption of the catalyst thin films was evaluated by UV-vis spectrophotometry. And the activity of the photocatalyst was evaluated by photocatalytic degradation kinetics of aqueous methyl orange under UV radiation. The results show that the photocatalytic activity of the TiO2 thin film can be evidently enhanced by Mn non-uniformly doping in the bottom layer and can be decreased by Mn doping in the surface layer. The activity of TiO2 thin film can be evidently enhanced by Zn non-uniform doping in either the bottom or the surface layer. But the activity of TiO2 is less affected by uniformly Zn doping. The different mechanisms for enhanced photocatalytic activity of Mn or Zn non-uniformly doped titanium dioxide film were discussed in terms of the separation of photon-generated carrier in the TiO2 films.
Key words:
TiO2; Mn; Zn; non-uniform doping; photocatalysis CLC number: O643;
Document code: A
1 INTRODUCTION
Heterogeneous photocatalysis attracts much attention as a friendly environment technique valuable for water and air purification. However, there are some problems in application, and one of the key problems is the low photon-quantum efficiency. From 1990s, the modification of TiO2 by metal ions has become a hot topic, and the effect of metal (such as Cu, Fe, Ag, Au, Pt, W, V, Pb, Cr, Rh, Co and Ni) ions doping on photocatalytic activity of TiO2 has been studied widely[1-4]. However, there were some contradictory reports on the activity of TiO2 modified by metal ions. For example, Chot et al[4] considered that the activity of TiO2 could be improved by Fe3+ doping, but Brezova et al[5] and Navio et al[6] revealed that Fe3+ was harmful to the activity of TiO2. The photophysical mechanism of doped semiconductors is not always understood. According to the preparation process, the metal ion doping of TiO2 should be defined as uniform doping, in other words, the distribution of metal ions in TiO2 is uniform.
In the end of 2001, in order to improve the photocatalytic activity of the TiO2 films, the authors proposed a new idea on TiO2 modification, metal ions non-uniformly doping. It could not only promote the generation of current carriers, but also improve their separation. In this paper, the thin films of TiO2, doped nonuniformly by Mn or Zn, were prepared by sol-gel method under process control. The different mechanisms for enhanced photocatalytic activity of the titanium dioxide film by non-uniformly Mn or Zn doping were discussed in terms of the separation of photon-generated carrier in the TiO2 films.
2 EXPERIMENTAL
2.1 Preparation of Mn-doped, Zn-doped and pure TiO2 films
The TiO2-sol was prepared by the following method[7]: 68mL tetra-butyl-ortho-titanate and 16.5mL diethanolamine were dissolved in 210mL absolute ethanol, and then stirred vigorously for 1h (solution A). Under stirring, the mixture of 3.6mL water and 100mL absolute ethanol (solution B) was dropwise added into the solution A. The resultant alkoxide solution was left for 24h in the dark, then the TiO2-sol was formed.
The preparation of Mn/TiO2-sol was similar to that of the TiO2-sol. The difference was that some Mn(NO3)2 (AR) was added into 3.6mL H2O and different concentration sols were formed when solution B was made. The Mn/TiO2 sol contents were 0.23%, 0.57%, 0.80%, 1.15% and 1.72% (mass fraction), respectively. The Zn/TiO2-sol was prepared by the same method with Zn(CH3COO)2 (AR), and the contents of the Zn/TiO2 sol were 0.14%, 0.41% and 0.96%, respectively.
The doped or pure TiO2 films were coated on the soda lime glass substrates, which was pre-coated with two SiO2 layers, from the TiO2 sol or Mn/TiO2 sol by the following steps: 1) dipping-withdrawing at a speed of 2mm/s; 2) drying at 100℃ for 10min; 3) heating to 500℃ at a rate of 2℃/s; 4) keeping at 500℃ for 1h, then cooling. The films of TiO2 in different doping modes described in Table 1 were prepared by repeating the above steps.
Table 1 Doping modes of TiO2
The crystal form of the film was anatase, obtained by X-ray diffraction (XRD with D/MAX-ⅢA).
2.2 Photocatalytic activity test
The reactor was a glass cylinder (d=70mm, H=240mm), in which five pieces of glass with photocatalysis film were settled tightly near the inside container wall. Then, 400mL aqueous methyl-orange (prepared by reverse osmosis treatment water, pH=5.9, 10mg/L) was added into the cylinder, and the solution was aerated for 30min. A high-pressure mercury lamp (125W, λp=365nm) was used as a light-house and preheated for 30min. And then the high-pressure mercury lamp was put into the reactor center. The reactor was immersed in a thermostatic bath in order to obtain a constant temperature. The solution was sampled every 20min. The concentration of aqueous methyl orange is determined through measuring the absorptive characteristic at 464nm using a UV-visible spectrophotometer (UV-3010) within the scanning scope from 200nm to 600nm.
The photocatalytic decolorization of methyl orange is a pseudo-first order reaction and its kinetics can be expressed as follows[8]:
ln(c0/c)=K·tillum
where K is the apparent reaction rate constant, tillum is the reaction time, and c0 and c are the initial concentration and the reacting concentration of methyl orange, respectively.
3 RESULTS
3.1 Effect of doping mode
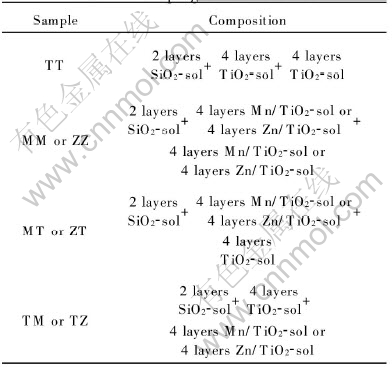
Fig.1 shows the effect of Mn doping mode on the photocatalytic activity of TiO2 films at the doping content of 0.80%. The sequence of activity of the TiO2 films is MT>TT>TM>MM. The photocatalytic activity of MT films is better than that of TT, but those of MM and TM are evidently worse than that of TT. This phenomenon is similar to that of the TiO2 doped by Mo[9].

Fig.1 Effect of Mn doping mode on photocatalytic activity of TiO2 films
Fig.2 shows the effect of Zn doping mode on the photocatalytic activity of TiO2 films at the doping content of 0.41%. The sequence of activity of TiO2 films is ZT>TZ>ZZ>TT. The photo-catalytic activity of ZT films is better than that of TT; and that of Zn doping in the surface layer is better. The activity of the films is little affected by Zn uniformly doping (ZZ).
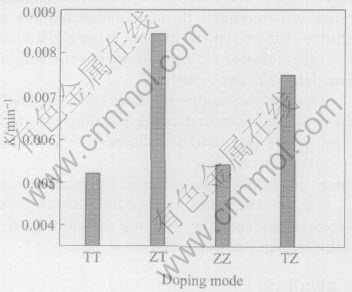
Fig.2 Effect of Zn doping mode on photocatalytic activity of TiO2 films
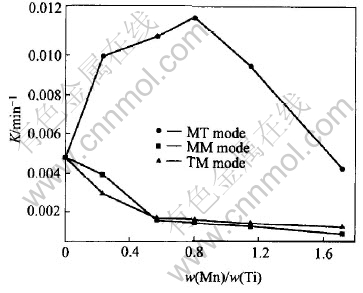
Fig.3 Photocatalytic activities of TiO2 films varied with Mn doping content
3.2 Effect of doping content
Fig.3 presents the apparent kinetics parameter (K) of methyl orange degradation as a function of the Mn-doped content under different doping modes. For the MT mode, the activity increases with the Mn content to the maximum at 0.80%, and then decreases with the more Mn addition. For the MM mode and TM mode, the activity is worse than that of pure TiO2 films and decreases with the Mn addition all the while.
Fig.4 presents the apparent kinetics parameter (K) of methyl orange degradation as a function of the Zn doped content under different doping modes. For the ZT mode and TZ mode, the activity increases with the Zn content to the maximum at 0.41%, and then decreases with the more Zn addition. For the ZZ mode, the activity is little affected by the doping content.
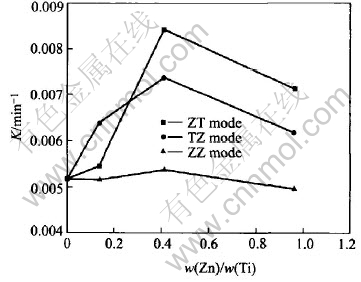
Fig.4 Photocatalytic activities of TiO2 films varied with Zn doping content
4 DISCUSSION
Heterogeneously photocatalytic oxidation is a surface reaction, and its efficiency depends on the separation of the photon-generated carrier and the quantity of the hole on the film surface to react with the organic substance or H2O absorbed on the surface[10]. Based on the above principle, the mechanism of the activity of the TiO2 films affected by Mn or Zn doping is discussed as follows.
4.1 Doping mode analysis
For Mn-doped films, because the radius of Mn is close to that of Ti, Mn ion can be easily embedded into the crystal lattice, and replace the Ti ion in the lattice point. Mn ion exists as Mn4+ after Mn-doped TiO2 is treated at 500℃[11]. The configuration of extra-nuclear electron of Mn4+ is 3s23p63d3, which is prone to return to that of 3s23p63d5 (Mn2+) (a stably-associated one with the half-filled subshells (3d5)).
For the MT mode, when the films are heat-treated, there will be a content gradient from the bottom layer to surface layer in the film, due to the diffusion and migration of Mn ions. With UV radiation, there are photon-generated carriers within the semiconductor. The Mn4+ in the bottom layer becomes the electron acceptor, and the photon-generated electrons transfer from the surface layer to the bottom layer in the films. Thus the N-type field of PN junction is formed in the bottom layer of the doped TiO2 film, and P-type field is formed in the surface layer (Fig.5). The whole systematic Fermi level is the same, but band near interface forms current carrier potential barrier[12, 13]. The potential barrier suppresses recombination of the electron-hole pairs. In the photocatalytic oxidation reaction, when the holes on the surface are consumed, the holes in the film will migrate to the surface layer due to the effect of the electric field of potential barrier, so the concentration of holes in the surface layer remains affluent and photocatalytic oxidation activity is enhanced. For the TM mode, it will form the reverse electric field in the interface. So the holes on the surface are fewer than those in TT mode. Because the holes are major factor in the photo-catalytic oxidation reaction, the photocatalytic oxidation activity is lower than that of pure TiO2. For the MM mode, the sites of dopant ions may become electron-hole recombination centers, which greatly decreases the lifetime of charge carriers. Furthermore it can not form potential barrier, accordingly the photocatalytic oxidation activity decreases.
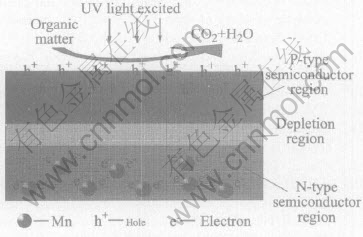
Fig.5 Schematic diagram of photocatalytic process in MT mode
For Zn-doped films, the radius of Zn ion is bigger than that of Ti, and it is difficult for the Zn ion to be embedded into crystal lattice. So the cluster of ZnO is formed in the TiO2 films. And ZnO has the characteristic of better electron-withdrawing[14].
For the ZT mode, there is also a Zn concentration gradient from the bottom layer to the surface layer in the film. With UV radiation, the electrons in the surface layer accumulate into the bottom layer of the films and leave behind the holes in the surface layer. In the same way, the concentration of holes in surface layer remains affluent, so the photocatalytic oxidation activity is enhanced.
For the TZ mode, the electrons on the surface increase, but the cluster of ZnO on the surface maybe form the effect of “Pt Island” analogously[15], which makes the electrons and holes separate effectively on the surface(Fig. 6), so the oxidation activity of the films is also enhanced. For the ZZ mode, it can not form electric potential in the film, then the oxidizing and reducing potential of TiO2 and ZnO have a little difference, so the photocatalytic oxidation activity of the films in the ZZ mode has been less affected.

Fig.6 Schematic diagram of photocatalytic process in TZ mode
4.2 Doping content analysis
For the MT and ZT modes, when Mn or Zn doping content in the bottom layer is low, the depletion layer can not form. So the photo-generated carrier can not be separated effectively in the titanium dioxide films[16]. Because more dopants in the bottom layer could decrease concentration gradient of dopant due to the driving force from field-aided diffusion in the process of heat treatment, there would be a maximum width of space-charge region along with an optimal dopant concentration. So there exists an optimal doping content in MT or ZT film on the photocatalytic activity. For the TM mode and MM mode, the electron-hole recombination centers increase with the doping content, which greatly decreases the lifetime of charge carriers. So the quantity of the effective carriers migrating to the surface of the films decreases, and the photocatalytic activity decreases evidently.
For TZ mode, in the similar manner, there exists an optimal charge separation on the surface, so there also exists an optimal content on the photocatalytic oxidation activity.
5 CONCLUSIONS
1) For Mn-doped TiO2 films, the MT mode can enhance the photocatalytic activity evidently. However, the activity of the films decreases in the TM mode or MM mode.
2) For Zn-doped TiO2 films, the photocatalytic activity of the films is enhanced evidently by the ZT mode or TZ mode. But the photocatalytic oxidation activity of the films in the ZZ mode has been less affected.
3) The optimal content of TiO2 films doped non-uniformly by Mn in MT mode is about 0.80%, and that by Zn in ZT and TZ mode is about 0.41% (mass fraction).
REFERENCES
[1]
[2]Di Paola A. García-López E. Ikeda S, et al. Photocatalytic degradation of organic compounds in aqueous systems by transition metal doped polycrystalline TiO2 [J]. Catalysis Today, 2002, 75: 87-93.
[3]Pea D A, Uphade B S, Smirniotis P G. TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3(Ⅰ)—Evaluation and characterization of first row transition metals [J]. Journal of Catalysis, 2004, 221: 421-431.
[4]Choi W, Termin A, Hoffmann M R. The role of metal ion dopants in quantum-sized TiO2: correlation between photoreactivity and charge carrier recombination dynamics [J]. J Phys Chem, 1994, 98: 13669-13679.
[5]Brezova V, Blazkova A, Karpinsky L, et al. Phenol decomposition using Mn+/TiO2 photocatalysts supported by the sol-gel technique on glass fibers[J]. J Photoch Photobio A—Chemistry, 1997, 109(2): 177-183.
[6]Navio J A, Colon G, Litter M I, et al. Heterogeneous photocatalytic reactions of nitrite oxidation and Cr(VI) reduction on iron-doped titania prepared by the wet impregnation method[J]. Appl Catal B, 1998, 16(2): 187-196.
[7]LI Xin-Jun, LI Fang-Bai, GU Guo-Bang. Preparation of nanometer-sized magnetic photocatalysts and their photocatalytic activity and properties [J].The Chinese Journal of Nonferrous Metals, 2001, 11(6): 971-976.(in Chinese)
[8]XU Yi-Ming, ZhU Zhi-Jie, CHEN Wen-Xing, et al. Photocatalytic degradation of phenol and chlorophenols on Pt/TiO2-coated photoreactor [J]. Chinese Journal of Applied Chemistry, 1991, 8(6): 28-32.
[9]YANG Ying, LI Xin-jun, CHEN Jun-tao, et al. Effects of doping modes on photocatalytic activities of Mo/TiO2 films [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(3): 509-514.(in Chinese)
[10]Assabane A, Yahia A I, Tahiri H. Photocatalytic degradation of polycarboxylic benzoic acids in UV-irradiated aqueous suspensions of titania: identification of intermediates and reaction pathway of the photomineralization of trimellitic acid (1,2,4-benzene tricarboxylic acid)[J]. Appl Catal B: Environ, 2000, 24(2): 71-87.
[11]DING Shi-Wen, WANG Li-Yong, ZHANG Shao-Ji, et al. Hydrothermal synthesis, structure and photocatalytic property of nano-TiO2-MnO2 [J]. Science in China(Series B), 2003, 46(6): 542-548.
[12]HUANG Kun, XIE Xi-de. Semiconductor Physics [M]. Beijing: Science Press, 1958. 311.
[13]Fendler J H. Nanoparticles and Nanostructured Films [M]. New York: John Wiley & Sons Inc, 1998.
[14]Hoyer P, Weller H. Potential-dependent electron injection in nanoporous colloidal ZnO films [J]. J Phys Chem, 1995, 99: 14096-14100.
[15]LIU Hong, WU Ming, WU He-Jin, et al. Photocatalytic activity and electrochemical impedance spectroscopy of TiO2 thermally treated by hydrogen [J]. Acta Phys Chim Sin, 2001, 17(3): 286-288.(in Chinese)
[16]Grove A S. Physics and Technology of Semiconductor Devices [M]. New York: John Wiley & Sons Inc, 1976. 158-188.
Foundation item: Project(32708) supported by the Natural Science Foundation of Guangdong Province, China; Project(2002C31621) supported by the Bureau of Science and Technology of Guangdong Province, China
Received date: 2004-12-29; Accepted date: 2005-07-12
Correspondence: LI Xin-jun, Professor, PhD; Tel: +86-20-87057615; E-mail: lixj@ms.giec.ac.cn


