
Two-stage precipitation process of iron and arsenic from acid leaching solutions
N. J. BOLIN, J. E. SUNDKVIST
Boliden Mineral AB, SE-936 81 Boliden, SWEDEN
Received 20 September 2008; accepted 5 November 2008
Abstract:
A leaching process for base metals recovery often generates considerable amounts of impurities such as iron and arsenic into the solution. It is a challenge to separate the non-valuable metals into manageable and stable waste products for final disposal, without loosing the valuable constituents. Boliden Mineral AB has patented a two-stage precipitation process that gives a very clean iron-arsenic precipitate by a minimum of coprecipitation of base metals. The obtained product shows to have good sedimentation and filtration properties, which makes it easy to recover the iron-arsenic depleted solution by filtration and washing of the precipitate. Continuos bench scale tests have been done, showing the excellent results achieved by the two-stage precipitation process.
Key words:
leaching; bioleaching; precipitation; iron removal;
1 Introduction
Boliden Mineral AB’s interest in bioleaching began in the late 1950s when a Swiss professor found arsenic resistant bacteria in the Boliden mine water.
Boliden Mineral AB has since then participated in different joint investigations on bioleaching and has also been a long-term supporter of basic research at the Universities of Lule? and Ume? in Sweden. Currently Boliden Mineral AB is a partner in the EC funded BioMine project.
Boliden started to study a selective precipitation processes for the recovery of base metals from bioleach solutions already in the beginning of 2000, as the existing iron-removal process was not acceptable, especially with respect to the zinc recovery and handling of waste products. However, it was found that a two-stage precipitation process could achieve the desired level of metal recovery and waste product quality. A patent[1] was granted to Boliden Mineral AB for its proposed flowsheet in 2002.
This paper gives a description of the precipitation method and presents some test data produced during the BioMinE project.
2 Two-stage precipitation method
The proposed two-stage iron removal process is based on precipitation using a controlled neutralization process employing limestone, lime or a similar alkaline material. A patent was granted on June 18, 2002 (US 6406676B1), which describes a method for precipitation of iron and arsenic from multi-element solutions in a selective way. It was found that at conditions giving an incomplete removal of iron from the solution, there was a minimum of co-precipitation of valuable metals such as zinc to the iron-gypsum precipitate. It was also found that the incomplete iron removal yields a very dense and more filterable precipitate compared to precipitates produced during complete iron removal. Based on this observation, the simplified flowsheet shown in Fig.1 was developed.
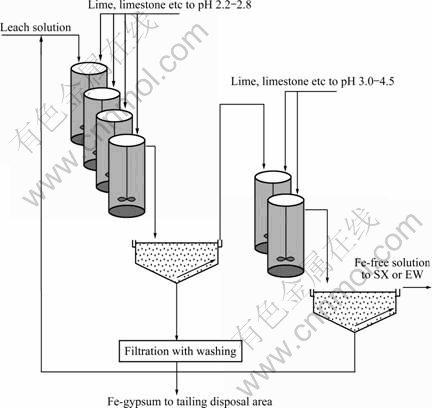
Fig.1 General flow sheet for precipitation of Fe from leach solution
The leach solution, which preferably contains iron in the ferric state, is fed into the first precipitation stage. Recycled pulp streams from downstream apparatus are also fed into the first reactor in stage 1. The first stage comprises of a number of reactors in series. The pH of solution is raised step by step by controlling the alkali addition. The partially neutralised solution from the last reactor in stage 1 discharges into the first thickener. At this point the solution will preferably contain a few hundreds milligrams of residual ferric per litre. The iron-gypsum precipitate is allowed to settle in the thickener without any flocculant addition. The net percentage of iron removal in the first stage will depend on flowrate from recycled streams and concentration of residual ferric concentration in the overflow from think-ener No.1. The target is to remove at least 90% of the iron in the first stage. The net production of precipitate is discharged from thickener No.1. The overflow from the first thickener is diverted to the first reactor of the secondary precipitation stage. The required number of reactors in the second stage is anticipated to be less than in the first stage, since the pH control is not so critical. However, the overall retention time might be more of a critical issue in obtaining a high quality overflow from the secondary thickener. In the second stage the pH is raised to a level where the ferric iron and arsenate are completely removed from the leach solution. The pulp from the last reactor in stage 2 discharges into thickener No.2, where the precipitate is allowed to settle. Flocculant is added to obtain a clear overflow, containing the valuable base metals for downstream processing. The relative high pH maintained in the second precipitation stage ensures that some of valuable elements might be co-precipitated. The precipitate will also contain un-reacted alkali. Therefore the second precipitate is returned to the first reactor in the first precipitation stage. The lower pH in this first step ensures that any alkali that has not reacted is reused and any co-precipitated elements are dissolved.
Other alkali additives can be used, if available, and if they have sufficiently high reactivity. Mesa calcium carbonate is such an alkali that can be used. Limestone seems to give better dewatering properties to the precipitate than slaked lime and is also a cheaper reagent.
The temperature in the precipitation should preferably be as high as possible for getting a good crystalline precipitate with good sedimentation and filtration properties. However, it has been shown that acceptable dewatering properties have been obtained at 35 ℃ with the proposed circuit.
3 Test results
The two-stage precipitation flowsheet has been studied in a mini-pilot circuit.
A view of the mini-pilot circuit is shown in Fig.2.
Fig.2 Mini-pilot circuit
The mini-circuit consists of 4 tanks in the primary stage and 2 tanks in the secondary stage. Each tank has a volume of approximately 1 L. The double-jacketed tanks were temperature controlled by circulating hot water. pH was automatically controlled by pulse additions of a limestone slurry in the second, third and fifth tank via a ring main. The pH was measured in the fourth and sixth tank. The net production of precipitate from the second thickener was circulated to the first tank. There was also a recycle stream of precipitate from the secondary thickener to the first tank in the secondary precipitation stage.
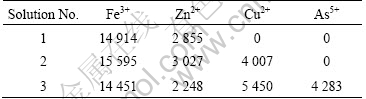
Synthetic solutions with 15 g/L ferric iron (Fe3+) and 3 g/L zinc (Zn) were used as basic feed. The content of copper and arsenic were varied in accordance to Table 1. The pH was adjusted with sulphuric acid to 1.5, and the temperature was controlled at 35 ℃. The solution was prepared from technical quality sulphate salts, except for solution No.3 where a cupric arsenate salt was used for introduction of copper and arsenate into the solution.
Table 1 Assay of synthetic feed solutions tested in two-stage precipitation mini-circuit (mg/L)
The aim of the study was to generate preliminary data for a preliminary evaluation of the process, together with design parameters for the equipment to be used in the larger scale testwork. To sustain the flow throughout the circuit, especially to maintain the thickener performance, a pretty high flow rate of recycled pulp was used. The feed rate to the continuous mini-circuit was maintained at only 10-11 mL/min. The influence of circulating load of precipitate was not investigated since pulp flows are difficult to regulate in such small test scale.
Each test was run during a period of about 4-5 days in order to stabilize the conditions. In Tables 2-4 the final conditions in the respective stages are summarized.
Table 2 Final conditions for test solution No.1
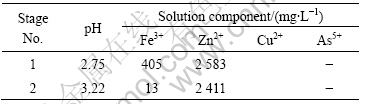
Table 3 Final conditions for test solution No.2
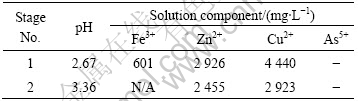
Table 4 Final conditions for test solution No.3
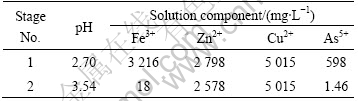
It is interesting to note that the presence of arsenic in the solution seems to increase the solubility of iron significantly at pH 2.7.
Filtration test was done on pulp from the thickener No.1 underflow, which contains the final iron-precipitate from circuit. The underflow was more diluted that can be expected from a commercial scale operation, since the mini-circuit had to be operated with a high circulating load to sustain the pulp flow. The collected pulp was therefore allowed to settle in a measuring cylinder over-night before it was subjected to filtration testwork. The filtration and washing testwork was carried out in accordance to standard procedure. 100 mL of wash water with a pH value of 3.5 (by sulphuric acid) was used in wash cycles 1-3. In the fourth wash 200 mL of wash water was used. For the ferric-arsenate precipitate, an additional pure water wash of 200 mL was used. 100 mL corresponds roughly to the volume of the filter cake.
The results from the filtration tests with wash water additions are given in Tables 5-7. The results are given as specific filtration rate with L/(m2?h) for the slurry feed, cake (dry solids) formation rate as kg/(m2?h) and filtration rate as kg/(m2?h). The pulp solid content in the feed to respective tests, as well as the obtained cake thicknesses, are also given in the tables.
Table 5 Results from filtration tests with several wash water cycles (Solution No.1: Fe-Zn)
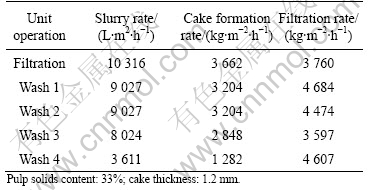
Table 6 Results from filtration tests with several wash water cycles (Solution No.2: Fe-Zn-Cu)
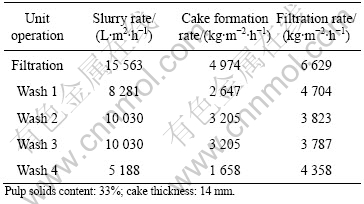
Table 7 Results from filtration tests with several wash water cycles (Solution No.3: Fe-Zn-Cu-As)
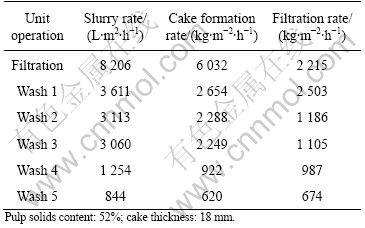
As can be seen from Tables 5-7, the filtration rate is generally high. The arsenic-containing precipitate is the
most compact precipitate and unlike the other precipitates, compacted successively during the various wash cycles.
The final moisture contents in the filter cakes are 45%, 50% and 27%, respectively. The rather low moisture contents make a final disposal easier to accomplish.
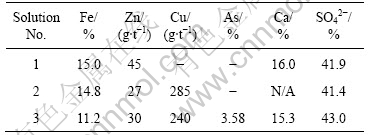
In Table 8 the assays of the washed precipitates are shown.
Table 8 Assays of final precipitates
An overall base metal balance was performed. The results are given in Tables 9-11 as metal recovery to the liquid from each iron removal test.
As can seen from Tables 9-11, the overall losses of zinc and copper to the precipitate are very small, already after four wash cycles.
Table 9 Metal recovery to liquid from each filtration and wash cycles (Fe-Zn solution)
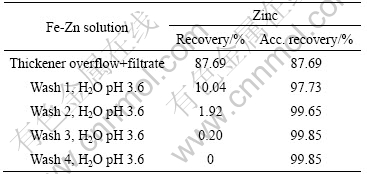
Table 10 Metal recovery to liquid from each filtration and wash cycles (Fe-Zn-Cu solution)
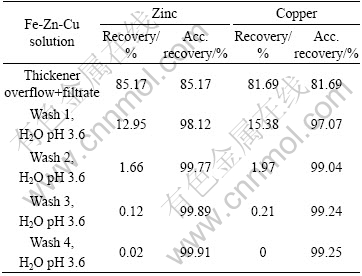
Table 11 Metal recovery to liquid from each filtration and wash cycles (Fe-Zn-Cu-As solution)
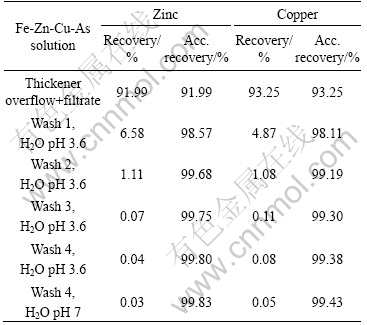
Fig.3 shows the calculated zinc losses vs m2 of filter area per tonne of solids to be filtered and washed per hour.
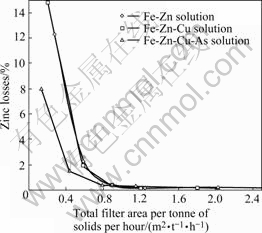
Fig.3 Zinc losses vs specific filter area
As one can see from Fig.3, about 1 m2 per tonne of dry solids to be handled per hour is required to obtain a high recovery of zinc for all types of precipitate. The drawback of the compact iron-arsenic precipitate seems to be fully compensated by the reduction in solution volume, which needs to be removed in the filtration and wash cycle.
4 Discussion
The presented method allows for selective disposal of iron and arsenic in a form that will easily settle and filter, with high recovery of the valuable metals to the final purified solution. The two-stage precipitation circuit gives a possibility to optimize the pH profile for different temperatures and metal concentrations in the feed in a flexible way. The proposed circuit can be integrated in any hydrometallurgical process for iron removal. One application of this process is when iron is removed from a bioleaching process for zinc rougher concentrates with high arsenic. Provided the filter cake is thoroughly washed the zinc losses to the iron-arsenic precipitate will be negligible. By optimization of the overall pH profile and the recycling system, it is believed that the performance of the circuit can be further improved. Besides raising the pH, addition of an oxidant such peroxide in the second stage will ensure that all iron and arsenic is removed from solution. The purified solution from the proposed iron removal process can go to a similar process for precipitation of zinc, in two-stages, to produce a zinc hydroxide precipitate that can be dissolved by spent electrolyte from a conventional electrowinning circuit. One option would be to recover the zinc using solvent extraction and electrowinning.
One example of reported problems can be found in Ref.[2] where iron was incomplete precipitated in order to avoid losses of cobalt in a bioleaching process in Uganda. Another example[3] is problems with co-precipitation of zinc with the iron precipitate that necessitated re-dissolving and re-filtration in order to ensure good process results.
5 Conclusions
The conclusions of the results are that the two-stage precipitation process will
1) minimize the losses of valuable metals;
2) give the precipitate of iron and arsenic a high settling rate;
3) give the precipitate of iron and arsenic a high filtration rate;
4) remove the iron and arsenic totally from the solution;
5) utilize the neutralization agents more and less totally.
References
[1] SUNDKVIST J E, Boliden Mineral AB. Method of purifying acid leaching solution by precipitation and oxidation. US Patent No 6406676B1 [P]. 2002-06-18.
[2] BRIGGS A P, MILLARD M. Int Biohydrometallurgy Symposium SBS97 [C]. Sydney, 1997.
[3] STEEMSON M L, WONG F S, GOEBEL B. Int Biohydrometallurgy Symposium SBS97 [C]. Sydney, 1997.
Corresponding author: N. J. BOLIN; Tel: +46-910-774251; Fax: +46-910-774296; E-mail: nils-johan.bolin@boliden.com
(Edited by YUAN Sai-qian)


