
Effect of degree of substitution of carboxymethyl starch on diaspore depression in reverse flotation
LI Hai-pu1, ZHANG Sha-sha1, JIANG Hao2, LI Bin1, LI Xing1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 16 December 2010; accepted 26 April 2011
Abstract: Carboxymethyl starchs (CMS) with low and high degrees of substitution (CMSL and CMSH in short, respectively) were employed as depressants of diaspore in cationic reverse flotation using dodecylamine (DDA) as collector. The effect of degree of substitution of CMS on its depression performance was examined and the interaction mode and behavior were investigated in a comparative manner. Micro-flotation test showed that CMSL exhibited better performance in depressing diaspore than CMSH in a broad pH range. The adsorption of CMS on diaspore was studied by adsorption test, zeta potential measurement, and atomic force microscopy. It was found that CMSH corresponds to lower adsorption amount, thinner adsorption layer, and more negative charge than CMSL, resulting from the more chelating sites brought by the high degree of substitution. The surface tension measurement and DDA adsorption test further revealed that CMSL/DDA system gives a better depressing performance benefiting from the trapping effect by enveloping some DDA molecules inside the loop chains, while CMSH/DDA system is likely considered a quasi-surfactant.
Key words: carboxymethyl starch; diaspore; reverse flotation; depressant
1 Introduction
The bauxite reserves in China are roughly 2 500 million tons, ranking the fifth in the world. However, these aluminum resources are overwhelmingly of diasporic ore with low aluminum/silicon ratio (A/S), which fails to meet the necessary requirement of direct Bayer process [1-2]. Reverse flotation was proposed to practically and efficiently utilize the low A/S diasporic bauxite by upgrading the mineral through floating and removing the gangue parts, such as illite, pyrophyllite, and kaolinite, while leaving the desirable component, diaspore, in the tailing [3-4]. In this process, both collector and depressant, which play different roles and facilitate different functionalities, are essential for the realization of selective separation. As for the cationic reverse flotation desilication treatment, a number of depressants, natural or synthesized, were employed to work with the cationic collector for the purpose of depressing and accumulating diaspore.
Starch is a low cost abundantly available biopolymer [5-7]. The high number of hydroxyl groups on starch backbone provides excellent convenience in modifying the crude polymer into characteristic derivatives for the use of this renewable recourse in the context of sustainable development [6, 8-9]. In the authors’ laboratory, some of them, including hydroxamic acid starch [10-11], multi-group starch [12], amphoteric starch, cationic starch, and as well as carboxymethyl starch [13], have been developed as effective depressants of diaspore in reverse flotation. Therein, carboxymethyl starch (CMS) is of special interest because of its chemisorbed character of —CH2COO- group on the surface aluminum atoms of minerals by forming a chelate complex. It is well known that one of the most important goals of carboxymethylation of starch is to obtain water-soluble derivatives for the smooth operation of froth flotation in aqueous media. The total degree of substitution (DS) as a carboxymethylation ratio, which represents the average number of functional groups introduced in the polymer, mainly determines the properties of modified product, i.e. hydrophilicity, solubility, affinity to diaspore, and interaction with cationic collector [9, 14]. However, the related studies focusing on DS factor of starch in the field of reverse flotation still remain vague.
2 Experimental
The mineral samples were obtained from Xiaoyi, Shanxi Province of China. The mineral lumps were crushed, selected by hand and then ground to a diameter smaller than 76 ?m. Based on chemical analysis, the sample purity was calculated to be more than 98%. Two CMS samples with low DS (0.4) and high DS (0.8) as CMSL and CMSH in short, respectively, were purchased and used as received (Haixiang Biological Engineering Co. Ltd., Shandong Province, China). The starch solutions were freshly prepared by dispersing starch particles into a small amount of absolute ethanol, and then they were dissolved in hot distilled water. Dodecylamine (DDA) of analytical grade was used as the collector. The pH values of the respective suspension and solution were adjusted using 1 mol/L NaOH or 1 mol/L HCl solutions. The ionic strength of solutions was maintained at unity by suitable additions of a stock KCl solution.
0.4 g mineral sample was transferred to a 100 mL Erlenmeyer flask, and suspended in a known concentration of starch solution. The flask was then placed in a mechanical shaker at 25 °C for 12 h which is long enough for a complete adsorption. At last, the mineral suspension was centrifuged at 5 000 r/min for 10 min and the supernatant was piped out for the determination of starch concentration by spectro- photometric method [15]. The amount of starch absorbed on diaspore was calculated from the initial and residual concentrations of starch.
The amount of DDA adsorption was determined by the procedures described in Ref. [16] using bromophenol blue spectrophotometric method [17] in a visible spectrophotometer (S22, Shanghai Lengguang Technology Co., Ltd., China) at 410 nm. It was assumed that the amount of starch depleted from solution had adsorbed onto the mineral phase, and likewise for DDA (6.47×10-4 mol/L).
All the adsorption tests were carried out in KCl background electrolyte solution, and the ionic condition was kept at 1×10-3 mol/L.
The micro-flotation tests were carried out on an XFD-type laboratory flotation machine. The procedure was the same as that used in our earlier studies [11-13].
The diaspore with a diameter smaller than 5 ?m was dispersed in a desired amount of 1×10-3 mol/L KCl solution and stirred for 2 min. The pH value was adjusted and measured. The starch stock solution was added into this mixture, followed by stirring for 3 min. Then, the zeta potential was determined by a Delsa 440SX zeta potential analyzer (Beckman-Coulter).
A multimode V scanning probe microscope (SPM) with the TappingMode AFM (Veeco Instruments Inc., USA) was used for the images of the adsorbed CMS on mica substrates. The AFM image was shown in the height mode after flattening.
The surface tension of starch aqueous solution was measured by Du Nouy ring tensiometer (DT-102, Zibo Huakun Electrical Equipment Co., Ltd, China). The procedure was similar to that reported in Ref. [18].
3 Results and discussion
The recoveries of diaspore by the collector DDA (6.47×10-4 mol/L) in the absence and presence of CMS depressants (100 mg/L) are depicted as a function of pH value in Fig. 1. It is shown that diaspore is largely recovered (>90%) by DDA in the pH range of 6-10 without the addition of any depressant.
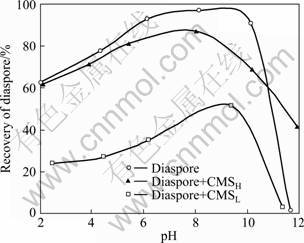
Fig. 1 DS effect of CMS (100 mg/L) on flotation of diaspore as function of pH
The presence of CMSH makes the recovery of diaspore only slightly reduced at pH<10, and it even somewhat activates the flotation of diaspore at pH>11. In contrast, diaspore is noticeably depressed by CMSL in the whole pH range investigated. Typically, at pH=9, the diaspore recovery is greatly decreased from the original 97% to as low as 51%. The above observation suggests that CMS with higher DS may bring up a large obstacle for the improvement of depression on diaspore under current conditions, despite its enhanced solubility. It has been already validated that the depressant action is mainly driven by the adsorption of depressant on the targeted mineral; therefore, the adsorption tests were carried out for a detailed investigation of DS effect on the interaction mode between CMS and diaspore.
Figure 2 shows the adsorption capacity of CMS with low and high DS on diaspore as a function of the initial starch concentration at pH=4.5, 6.5 and 10.5, respectively. It is evidently observed in Fig. 2 that CMSL corresponds to higher adsorption capacity on diaspore than CMSH under the same conditions, which could strongly account for the better performance of CMSL in depressing diaspore in micro-flotation test. On the other hand, the adsorption amount of CMSL on diaspore is found to increase steadily with the increase of the initial starch concentration at all three pH values, while the adsorption of CMSH seems to be approaching an equilibrium after the starch concentration reaches 120 mg/L. What’s more, the basic media results in lower adsorption than the acidic media in both the cases of CMSL and CMSH. A zeta potential measurement was carried out then for a further investigation.
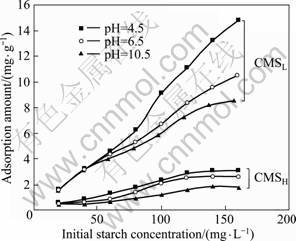
Fig. 2 Adsorption of CMS on diaspore as function of starch concentration
The DS effect of CMS on the zeta potential of diaspore is shown in Fig. 3. The isoelectric point (IEP) of diaspore is located around pH 7.8 in the absence of additives [19]. It means that diaspore particles exhibit a net positive zeta potential at pH lower than 7.8 due to the protonation of the surface Al—OH groups to form Al—OH2+ groups, while carry negative charges at pH higher than IEP because of the formation of Al—O- species by deprotonating the surface Al—OH groups. As shown in Fig. 3, the addition of CMS with either low or high DS decreases the zeta potential of diaspore to the negative domain in the whole pH range investigated and leads to a shift of IEP toward the acidic region. This observation robustly indicates that CMS is negatively charged and the concomitant electrostatic repulsion should occur between CMS and diaspore in pH region higher than 7.8, which is responsible for the lower adsorption of CMS on diaspore in basic media than that in acidic media. Meanwhile, the chemical interaction or hydrogen bonding could contribute largely to the adsorption of CMS on diaspore in basic pH to overcome the electrostatic repulsion [14].
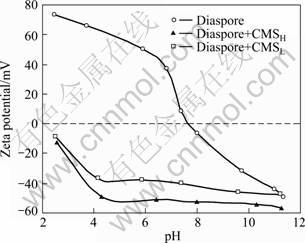
Fig. 3 Zeta potential of diaspore as function of pH in the presence and absence of CMS
In addition, it is noticeable from Fig. 3 that CMSH drags the zeta potential of diaspore more negatively than CMSL, which represents more negative charges residing on CMSH and then coincides with the higher degree of substitution of carboxymethyl groups. As a result, CMSH could provide more chelating sites for a stronger chemical interaction with surface aluminum atoms of diaspore. But why doesn’t it lead to higher adsorption on diaspore?
Atomic force microscopy is an informative tool for the observation of the adsorption state of polymers on mineral surface. The visualization of CMS adsorption onto mica substrate by AFM is shown in Fig. 4.
As seen in Fig. 4(a), CMSL adsorbs in the form of large islands with higher thickness. In comparison, the surface of mica substrate is covered by a much thinner and smoother layer of CMSH (Fig. 4(b)). The AFM measurement demonstrates that the change of DS of CMS would influence its adsorption state on mineral substrate, that is, a higher DS may correspond to a less adsorption.
It can be reasonably inferred that CMS with a higher DS, which contributes to more interaction sites, has better chances to drive the starch chains extended on mineral substrate and is preferably adsorbed in a flat manner, resulting in a thinner adsorption layer and a less adsorption amount. On the contrary, a less DS may lead CMS chains to be attached to the mineral surface through less chelating sites in so called loop fashion, which gives rise to a relatively thicker adsorption layer and higher adsorption amount.
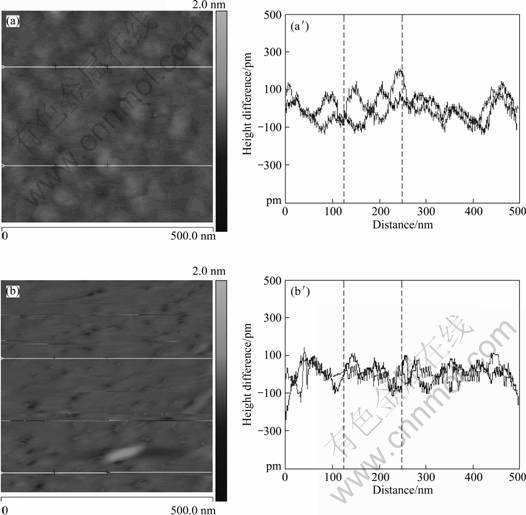
Fig. 4 AFM images of adsorption of CMSL (a) and CMSH (b) onto mica substrate (scan size 500 nm×500 nm, height difference 2.0 nm) and corresponding longitudinal section along solid line in respective images ((a′) CMSL; (b′) CMSH)
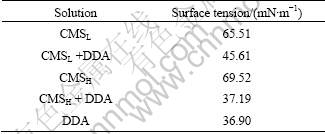
As mentioned above, the interaction between collector and depressant also plays an important role in determining the depression performance. The surface tension of CMS aqueous solution with and without the addition of collector DDA was examined accordingly, and the measured results are reported in Table 1.
It has been previously confirmed that the higher DS of CMS corresponds to the higher hydrophilicity and solubility in water [20]. Under such a condition, the surface tension of CMS solution with high DS would be larger than that with low DS, exactly as measured in this study for CMSH (69.52 mN/m) and CMSL (65.51 mN/m). When DDA was added into CMS solution, the surface tension of CMSH solution sharply decreased to as low as 37.19 mN/m, which is much close to the value for DDA solution (36.90 mN/m), while the surface tension of CMSL solution decreased moderately to 45.61 mN/m. The surface tension measurement indicated that DDA tended to bind with CMSH more than with CMSL, and this collector nearly modified CMSH into a quasi-surfactant in terms of the surface tension value (37.19 vs 36.90).
Table 1 Effect of DDA on surface tension of CMS solution
The adsorption of DDA on the treated diaspore in the presence of CMS was also examined to help further understand the DS effect of CMS on diaspore depression. As seen from Fig. 5, the amounts of adsorbed DDA on diaspore at pH=9 in the presence of CMSL and CMSH, respectively, increase with the increase of starch dosage and reach their maximum almost in parallel when the initial starch concentration is close to 62 mg/L. And, in both cases, CMS essentially enhanced the DDA adsorption by electrostatic interactions, in spite of a progressive drop occurring afterwards. It is impressively observed that CMSH rendered a much higher adsorption amount of DDA on diaspore than CMSL at intermediate starch concentration, while gave nearly identical amounts of DDA adsorption at low or high starch concentration compared with its low DS counterpart.
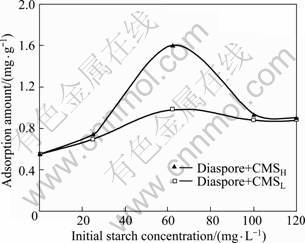
Fig. 5 Adsorption of DDA with fixed initial concentration (6.47×10-4 mol/L) on diaspore at pH=9 in the presence of CMS
The association between oppositely charged polymers and surfactants is complicated and it is influenced by many factors [21-22]. Nevertheless, the electrostatic interaction plays a major driving force therein, which could fundamentally explain the non-monotonic phase behavior. At low starch concentration, the number of charges of DDA exceeds that of CMS. The counterions of CMS either on diaspore surface or in bulk solution are fully exchanged by the DDA molecules due to the energetical favor, which leads to the formation of CMS/DDA complexes on diaspore surface and increases the adsorption of DDA with the increase of starch concentration in the initial stage. After achieving the peak value, DDA adsorption begins to decrease with further increase of starch concentration or, in other words, anionic polymer chains concentration. This could be attributed to the heavily accumulated surplus of wandering starch chains, which exhibit more flexible conformation in solution and are favored by DDA molecules than those pre-adsorbed on diaspore. Due to the fixed initial DDA concentration, CMSL and CMSH are consubstantial in terms of the opposite charge race at very low/high starch concentration, so as to lead to highly comparable results. However, the situation changes at moderate concentration, especially at the peak position where the opposite charges are supposed to maintain a balance. It means that almost every charge of the anionic polymer chains is neutralized by a cationic DDA molecule. Thus one would expect that the inter-molecular electrostatic repulsion among these uncharged CMS/DDA complexes would decrease and most likely result in macroscopic phase separation. This is particularly acute for CMSH system which has higher charge density and more pronounced hydrophobicity. The concomitant precipitate is eventually attached to the mineral particles and partly contributes to the adsorption of DDA.
Although CMSH and CMSL share nearly equal amounts of DDA adsorption at 100 mg/L starch concentration at pH=9 (Fig. 5), the latter indeed performs a much better depressant in micro-flotation (Fig. 1). One reason for this is that, as already addressed before, CMSL allows for far more adsorption on diaspore than CMSH under the same conditions (Fig. 2); another important reason is that the loop chains of CMSL pre-adsorbed on diaspore could trap some DDA molecules inside so as to partially exclude the enhancement of hydrophobicity of diaspore surface [7], which is consistent with the findings in surface tension measurement (Table 1). As a result, CMSL shows better performance in depressing diaspore than CMSH when working with DDA collector.
4 Conclusions
1) CMS with low DS exhibits better performance in depressing diaspore than CMS with high DS when working with cationic collector DDA.
2) CMS with high DS bears more chelating sites and tends to be absorbed on diaspore surface in flat manner, resulting in thinner adsorption layer and less adsorption amount. In contrast, the adsorption of CMS with low DS on diaspore surface is in loop fashion with thicker layer and higher amount.
3) CMS with low DS pre-adsorbed on diaspore can trap some collector molecules in its loop chains to alleviate the hydrophobicity of the mineral surface while CMS with high DS doesn’t.
References
[1] LIU W, YANG J, XIAO B. Review on treatment and utilization of bauxite residues in China [J]. International Journal of Mineral Processing, 2009, 93(3-4): 220-231.
[2] XU Z H, PLITT V, LIU Q. Recent advances in reverse flotation of diasporic ores—A Chinese experience [J]. Minerals Engineering, 2004, 17(9-10): 1007-1015.
[3] HU Yue-hua, WANG Yu-hua. Flotation chemistry of aluminum and silicate and desilication of bauxite [M]. Beijing: Science Press, 2004: 9. (in Chinese).
[4] WANG Y, HU Y, HE P, GU G. Reverse flotation for removal of silicates from diasporic-bauxite [J]. Minerals Engineering, 2004, 17(1): 63-68.
[5] RATH R K, SUBRAMANIAN S, PRADEEP T. Surface chemical studies on pyrite in the presence of polysaccharide-based flotation depressants [J]. Journal of Colloid and Interface Science, 2000, 229(1): 82-91.
[6] SHOGREN R L. Flocculation of kaolin by waxy maize starch phosphates [J]. Carbohydrate Polymers, 2009, 76(4): 639-644.
[7] L?PEZ VALDIVIESO A, CELED N, CERVANTES T, SONG S, ROBLEDO CABRERA A, LASKOWSKI J S. Dextrin as a non-toxic depressant for pyrite in flotation with xanthates as collector [J]. Minerals Engineering, 2004, 17(9-10): 1001-1006.
[8] PAVLOVIC S, BRANDAO P R G. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz [J]. Minerals Engineering, 2003, 16(11): 1117-1122.
[9] HEINZE T, KOSCHELLA A. Carboxymethyl ethers of cellulose and starch—A review [J]. Macromolecular Symposia, 2005, 223: 13-39.
[10] HU Yue-hua, LI Hai-pu, JIANG Yu-ren. Effect of hydroxamic acid starch on reverse flotation desilicate from diasporic bauxite [J]. Transactions of Nonferrous Metals Society of China, 2002, 12(5): 974-978.
[11] LI Hai-pu, HU Yue-hua, WANG Dian-zuo, XU Jing. Effect of hydroxamic acid polymers on reversed flotation of bauxite [J]. Journal of Central South University of Technology, 2004, 11(3): 291-294.
[12] LI Hai-pu, JIANG Yu-ren, CAO Xue-feng, HU Yue-hua, WANG Dian-zuo. Synthesis of modified starch and its performance [J]. Mining and Metallurgical Engineering, 2001, 21(4): 29-32 (in Chinese).
[13] LI Hai-pu, ZHANG Sha-sha, JIANG Hao, LI Bin. Effect of modified starches on depression of diaspore [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8): 1494-1499.
[14] CHEN Y X, LIU S Y, WANG G Y. Kinetics and adsorption behavior of carboxymethyl starch on alpha-alumina in aqueous medium [J]. Journal of Colloid and Interface Science, 2006, 303(2): 380-387.
[15] DUBIOS M, GILLES K A, HAMILTON J K, REBERS P A, SMITH F. Colorimetric method for determination of sugars and related substances [J]. Analytical Chemistry, 1956, 28(3): 350-356.
[16] LI H P, ZHANG S S, JIANG H, HU Y H, WANG D Z. Selective depression of diaspore with waxy maize starch [J]. Minerals Engineering, 2010, 23: 1281-1286.
[17] MUKERJEE P. Use of ionic dyes in analysis of ionic surfactants and other ionic organic compounds [J]. Analytical Chemistry, 1956, 28(5): 870-873.
[18] HUANG X F, GUAN W, LIU J, LU L J, XU J C, ZHOU Q. Characterization and phylogenetic analysis of biodemulsifier- producing bacteria [J]. Bioresource Technology, 2010, 101(1): 317-323.
[19] LIU X, HU Y, XU Z. Effect of chemical composition on electrokinetics of diaspore [J]. Journal of Colloid and Interface Science, 2003, 267(1): 211-216.
[20] ZHONG Zhen-sheng, SUN Ang. Performance of carboxymethyl starch paste and factors affecting paste transparency [J]. Journal of South China University of Technology, 2010, 38(2): 32-38 (in Chinese).
[21] BAIN C D, CLAESSON P M, LANGEVIN D, MESZAROS R, NYLANDER T, STUBENRAUCH C, TITMUSS S, von KLITZING R. Complexes of surfactants with oppositely charged polymers at surfaces and in bulk [J]. Advances in Colloid and Interface Science, 2010, 155(1-2): 32-49.
[22] CLAESSON P M, MAKUSKA R, VARGA I, MESZAROS R, TITMUSS S, LINSE P, PEDERSEN J S, STUBENRAUCH C. Bottle-brush polymers: Adsorption at surfaces and interactions with surfactants [J]. Advances in Colloid and Interface Science, 2010, 155(1-2): 50-57.
羧甲基淀粉的取代度对其在
反浮选中抑制一水硬铝石的影响
李海普1,张莎莎1,蒋 昊2,李 彬1,李 星1
1. 中南大学 化学化工学院,长沙 410083;
2. 中南大学 资源加工与生物工程学院,长沙 410083
摘 要:
研究取代度不同的两种羧甲基淀粉(CMSL和CMSH分别表示取代度低和取代度高的羧甲基淀粉)在以十二胺(DDA)为捕收剂的阳离子反浮选中对一水硬铝石的抑制性能。考察了CMS的取代度对其抑制性能及作用方式的影响。单矿物浮选实验表明,在广泛pH值范围内,CMSL对一水硬铝石的抑制能力要好于CMSH。借助吸附量测试、动电位测量和原子力显微镜对CMS在一水硬铝石表面的吸附进行研究。结果表明:相对于CMSL,CMSH分子中具有更多的吸附点,因而在一水硬铝石表面具有较小的吸附量和较薄的吸附层厚度,并使一水硬铝石表面具有较强的电负性。溶液表面张力的测定和捕收剂DDA的吸附实验进一步揭示,CMSL分子的环式吸附构象可以罩盖更多的DDA从而显示优良的抑制性能,而CMSH/DDA体系则表现得更像一种表面活性剂。
关键词:
(Edited by YUAN Sai-qian)
Foundation item: Projects (50804055, 50974134) supported by the National Natural Science Foundation of China; Project (09JJ3100) supported by the Natural Science Foundation of Hunan Province, China
Corresponding author: LI Hai-pu; Tel: +86-731-88830603; E-mail: lihaipu@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(11)60943-6


