DOI:10.19476/j.ysxb.1004.0609.2017.07.14
Ni掺杂CoFe2O4晶体电磁性质的第一性原理研究
侯世界1,侯育花1,黄有林1,欧阳义芳2
(1. 南昌航空大学 材料科学与工程学院,南昌 330063;
2. 广西大学 物理科学与工程技术学院,南宁 530004)
摘 要:
尖晶石型钴铁氧体因具有良好的电磁性质,广泛应用于电子装备、微波装备及高密度磁存储等领域。采用基于密度泛函理论(DFT)的第一性原理平面波赝势法,结合广义梯度近似(GGA+U),系统研究不同Ni掺杂量对CoFe2O4晶体结构、电子结构和磁性能的影响。结果表明:Ni离子倾向于占据八面体位置且随着Ni掺杂量的增加,晶格常数呈递减趋势。通过电子结构分析表明,带隙随着Ni掺杂量的变化而变化,大部分都呈绝缘体,且存在强烈的3d-2p轨道杂化。磁性能依赖于Ni掺杂量,随着Ni掺杂量的增加,晶格中的原子磁矩变化不明显,但总磁矩呈线性递减趋势,这主要是因为Ni离子最外层的3d未配对电子数与Co离子最外层3d未配对电子数不同,因而对磁性能的影响较大。
关键词:
文章编号:1004-0609(2017)-07-1426-07 中图分类号:TG142 文献标志码:A
尖晶石铁氧体是一类具有广泛用途的磁性功能材料,其中钴铁氧体(CoFe2O4)因其具有成本低、化学稳定性好、高电阻率、较高的磁晶各向异性和饱和磁化强度高等特点,广泛应用于微波装备、高密度磁存储和磁记录介质、生物医学和电子装备等领域[1-3]。随着其应用领域的不断拓展,高性能钴铁氧体的研究与开发成为研究热点。离子取代是一种调控钴铁氧体电磁性能的有效技术方法,如MAAZ等[4]通过共沉淀法制备了Co1-xNixFe2O4(0≤x≤1)尖晶石纳米晶铁氧体,结果表明:随着Ni掺杂量的增加,饱和磁化强度呈下降趋势。GUL等[5]通过共沉淀法制备了Co-Ni纳米晶铁氧体,结果表明,Ni离子倾向于取代八面体位置中的Co离子,以+2价存在于晶体中,且随着Ni2+掺杂量的增加,电阻率随Ni2+掺杂量的增加而增加。SINGHAL等[6]采用气溶胶法制备Co1-xNixFe2O4纳米晶铁氧体,研究表明:随着Ni2+掺杂量的增加,晶格常数呈下降趋势,且饱和磁化强度降低。理论方面,SZOTEK等[7]采用自相互作用修正的局域密度泛函(SIC-LSD)研究尖晶石铁氧体CoFe2O4和NiFe2O4的磁性能和电子结构,结果表明:从反尖晶石到正尖晶石结构,总磁矩呈增加趋势,所研究的结构都呈绝缘性,且反尖晶石结构最稳定。ANTONOV等[8]采用局域自旋密度近似(LSDA+U)方法研究Ni掺杂Fe3O4的电子结构,结果表明:反尖晶石结构的NiFe2O4最稳定,且结构呈现绝缘性质,带隙为0.99 eV。FRITSCH等[9]采用广义梯度近似GGA和GGA+U方法分别研究NiFe2O4的电子结构和磁性能,结果表明:采用GGA方法得到的带隙很小,仅为0.1 eV,计算的Ni2+离子的磁矩为1.36 μB,而采用GGA+U方法得到的带隙为0.97 eV,结构呈绝缘性质,计算的Ni2+离子磁矩为1.58 μB。尽管实验上和理论上对Ni掺杂CoFe2O4进行了相关研究,但是从理论上并没有详细和系统地研究不同Ni掺杂量对CoFe2O4磁性能和电子性能的影响。因此,本文作者从第一性原理计算出发,结合过渡族金属离子3d电子间的强相关作用系统研究不同Ni掺杂量对CoFe2O4晶体结构、电子结构和磁性能的影响并分析其影响机制。
1 计算模型和方法
尖晶石型铁氧体的单位晶胞中有8个AB2O4分子式,即尖晶石型铁氧体的晶胞中有8个四面体位置(A位)被占据,16个八面体位置(B位)被占据,32个氧离子,如图1所示。单位晶胞由氧离子面心立方密堆而成,具有立方对称性,空间群为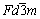 (No. 227)[10-11]。
(No. 227)[10-11]。
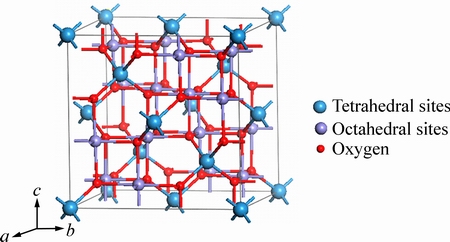
图1 尖晶石型铁氧体的晶胞图
Fig. 1 Crystal cell of spinel ferrite
尖晶石铁氧体一般有3种晶体结构,如正尖晶石型、反尖晶石型和混合尖晶石型结构,其表达式为(A2+1-xB3+x)[A2+xB3+2-x]O4,其中脚标x表示A2+离子占据八面体位置的百分比。当x=0时,表示所有的A2+离子都占据四面体位置,B3+离子全部占据八面体位置,称为理想正尖晶石结构;当x=1时,表示所有的A2+离子全部占据八面体位置,而B3+离子分别占据四面体位置和八面体位置,称为理想反尖晶石结构;当0<x<1时,表示在四面体位置和八面体位置同时分布两种不同价态的金属离子,称为混合型尖晶石铁氧体[10-11]。尖晶石结构铁氧体属于亚磁性材料,来源于被阴离子所隔开的磁性金属离子间的超交换作用,使处于不同晶格位置中的金属离子磁矩反向排列,即其晶体结构中四面体位置阳离子的自旋取向反平行八面体位置阳离子的自旋取向。
本研究采用基于密度泛函理论的第一性原理计算软件VASP(Vienna Ab initio Simulation Package)[12-14]软件包进行计算。其中波函数采用平面波基函数展开,交换关联能采用广义梯度近似(GGA)[15-16]下的PBE(Perdew-Burke-Ernzerhof)[17]方法,势函数采用PAW势[18],平面波截断能取500 eV,布里渊区积分采用5×5×5的Monkhorst-Pack型的K点网格进行优化。每一个结构都进行了全优化(即体积、原子位置和形状等)计算,直至原子间的力小于0.01 eV/ 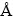 为止。由于传统的密度泛函理论计算(LSDA,GGA)并不能精确地用来描述包含3d电子的过渡族元素和4f电子的稀土族元素的氧化物。为了精确地描述原子间的强相关作用,本研究采用GGA+U [19]进行计算,通过多次测试以及前期的研究结果表明[20],尖晶石型CoFe2O4的反尖晶石结构最稳定,在此结构基础上,分别对Co离子,选用U=4.22 eV和J=0.80 eV,Fe离子,U=4.08 eV和J=0.79 eV,Ni离子U=4.22 eV和J=0.82 eV,所得到的结果与已有的实验值和理论值符合得较好。
为止。由于传统的密度泛函理论计算(LSDA,GGA)并不能精确地用来描述包含3d电子的过渡族元素和4f电子的稀土族元素的氧化物。为了精确地描述原子间的强相关作用,本研究采用GGA+U [19]进行计算,通过多次测试以及前期的研究结果表明[20],尖晶石型CoFe2O4的反尖晶石结构最稳定,在此结构基础上,分别对Co离子,选用U=4.22 eV和J=0.80 eV,Fe离子,U=4.08 eV和J=0.79 eV,Ni离子U=4.22 eV和J=0.82 eV,所得到的结果与已有的实验值和理论值符合得较好。
2 计算结果与讨论
2.1 结构稳定性
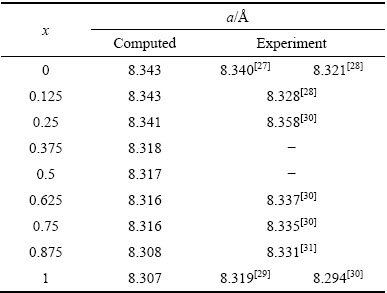
根据能量稳定性计算结果表明,当Ni离子取代Co离子占据八面体位置的能量比占据四面体位置的能量低1.8 eV, 即Ni离子占据八面体位置更稳定,说明Ni离子倾向于占据八面体位置,这与实验结果[21-22]一致。表1给出了不同成分NixCo1-xFe2O4稳定结构的晶格常数(x=0,0.125,0.25,0.375,0.5,0.625,0.75,0.875,1.0),为了比较,表中也给出了部分实验结果。从表1可以看出,本研究所计算的结果与实验值符合得较好,且随着Ni掺杂量的增加,晶格常数呈递减趋势,与实验[23-24]得到的变化趋势一致,这是由于过渡金属Ni2+(0.74 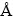 )[25]离子半径小于Co2+(0.78
)[25]离子半径小于Co2+(0.78 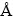 )[26]离子半径所引起的。
)[26]离子半径所引起的。
表1 NixCo1-xFe2O4的晶格常数
Table 1 Crystal constants of NixCo1-xFe2O4
2.2 磁性能
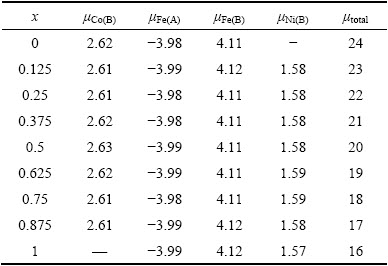
为了分析Ni掺杂对CoFe2O4磁性能的影响,表2给出了NixCo1-xFe2O4中的各原子磁矩和总磁矩。从表2可以看出,在所考虑的结构体系中,Co离子磁矩约为2.60 μB,Ni离子磁矩约为1.58 μB,八面体位置中的Fe离子磁矩约为4.11 μB,四面体位置中的Fe离子磁矩约为3.98 μB。随着Ni掺杂量的不同,各原子磁矩在不同掺杂体系中变化很小,说明在掺杂中金属离子价态未发生改变,即Ni2+取代了Co2+,这在已有实验中也得到了证实[33]。同时发现随着Ni掺杂量的增加,体系总磁矩呈减小趋势,这是因为在尖晶石铁氧体结构中,八面体位置中的离子自旋磁矩与四面体位置中的离子自旋磁矩反向平行,且Ni离子磁矩小于Co离子磁矩。
表2 NixCo1-xFe2O4的各原子磁矩和总磁矩
Table 2 Atomic and total magnetic moment of NixCo1-xFe2O4
2.3 电子结构
为了进一步分析Ni掺杂对CoFe2O4体系电子结构的影响,本研究给出了NixCo1-xFe2O4(x=0.125)的总态密度图(TDOS)及部分原子的局域态密度图(LDOS),如图2所示,其中虚线表示费米能级的位置。从图2分析得出,当Ni离子取代八面体位置中的Co离子时,晶体的电子结构仍呈绝缘性,带隙值为0.80 eV,比未掺杂前CoFe2O4的带隙(0.72 eV)宽。从原子的局域态密度可以看出,费米能级附近主要由Co和Ni的3d轨道及O的2p轨道构成,其中Co原子的3d轨道电子的贡献占主导作用,同时也说明存在3d电子和2p电子的杂化。从图中也可以看出,Ni原子态密度主要分布在价带中的-6.0~0 eV范围内,且自旋向上和自旋向下的态密度分布不对称,说明Ni离子具有未配对的3d轨道电子,即Ni离子对总磁矩的贡献较大,同时从Co和Fe的原子局域态密度图可以看出,其电子占据表现明显的局域性,且自旋向上和自旋向下占据态分布具有明显的不对称性,说明Co和Fe对总磁矩的贡献很大,这与表2给出的结果符合得较好。
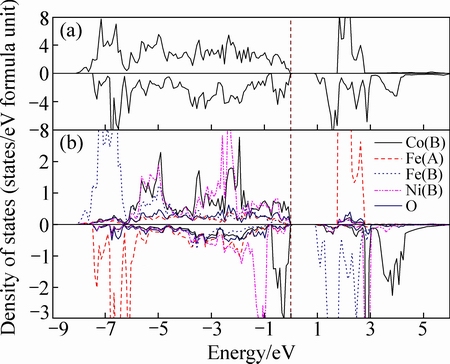
图2 Co1-xNixFe2O4(x=0.125)的总态密度图和原子态密度图
Fig. 2 TDOS (a) and LDOS (b) of Co1-xNixFe2O4 (x=0.125)
图3所示为NixCo1-xFe2O4(x=0.25)的总态密度图及部分原子的局域态密度图,其中虚线表示费米能级的位置。从图3分析得出,当Ni离子继续取代八面体中的Co离子时,费米能级向低能区移动,导致在费米能级处出现了少量的自旋向下的电子占据态,这主要由Co原子的3d轨道贡献,而费米能级处自旋向上态密度则没有电子占据,说明Ni0.25Co0.75Fe2O4具有半金属性。从原子局域态密度图可以看出Co、Ni和Fe原子的电子占据都表现明显的局域化,如Co 3d轨道电子主要分布在-6.0~0 eV;Ni 3d轨道电子主要分布在-5.5~-0.5 eV;Fe的3d轨道电子主要分布在价带处-8.0~-5.5 eV。同时O原子2p轨道电子主要分布在-7.5~0 eV, 说明存在强烈的3d-2p杂化。在金属原子局域态密度图中,其自旋向上和自旋向下占据态分布明显不对称,说明各金属原子对总磁矩的贡献很大,表2中给出的原子磁矩也得到了证实。
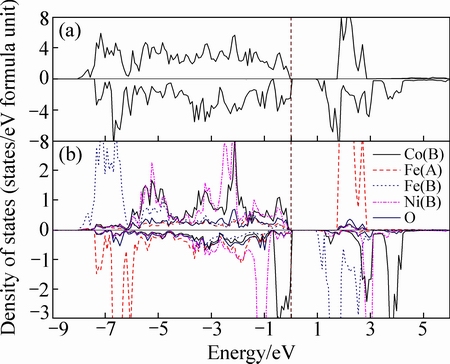
图3 Co1-xNixFe2O4(x=0.25)的总态密度图和原子态密度图
Fig. 3 TDOS (a) and LDOS (b) of Co1-xNixFe2O4 (x=0.25)
图4所示为NixCo1-xFe2O4(x=0.375~1.0)的总态密度图,其中虚线表示费米能级的位置。从图4分析可知,当Ni离子取代(x=0.375)八面体中的Co离子时总态密度图呈绝缘体性,带隙值为0.72 eV。当Ni离子取代(x=0.5)八面体中的Co离子时,在费米能级附近出现了一些不规则的小峰值,且带隙变窄,带隙值为0.31 eV。当Ni离子取代(x=0.625)八面体中的Co原子时,其态密度图分布与x=0.5比较相似,呈绝缘性且带隙值为0.30 eV。当x=0.75, 0.875, 1.0时,费米能级处自旋向上和自旋向下都没有电子占据,晶体的电子结构呈绝缘性,带隙分别为0.86、0.81和0.80 eV。不同成分Ni掺杂体系的原子局域态密度图与NixCo1-xFe2O4(x=0.125,0.25)相似,即Co、Ni和Fe原子的电子占据都表现明显的局域化,存在强烈的3d-2p杂化,且费米能级附近主要由Co原子的3d轨道的电子贡献,Co、Ni和Fe 具有3d 轨道未配对电子,对总磁矩的贡献较大。
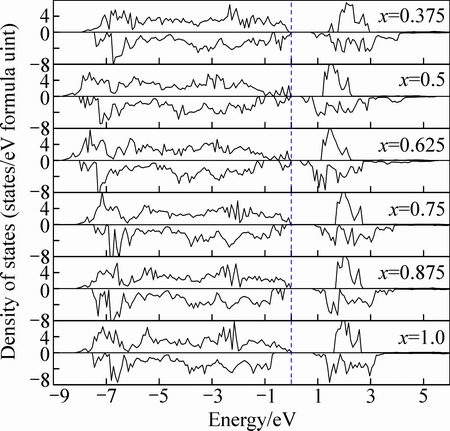
图4 Co1-xNixFe2O4(x=0.375,0.5,0.625,0.75,0.875,1.0)的总态密度
Fig. 4 TDOS of Co1-xNixFe2O4 (x=0.375, 0.5, 0.625, 0.75, 0.875, 1.0)
通过分析原子局域态密度图和原子磁矩结果可得出,在Ni掺杂CoFe2O4体系结构中,占据四面体位置和八面体位置的Ni 离子、Co离子和Fe离子具有高自旋态,而且Ni离子、Co离子和Fe离子的核外分别有2个、3个和5个未配对的3d轨道电子,因此,Ni 离子、Co离子和Fe离子对总磁矩的贡献较大,同时随着Ni掺杂量的增加,体系总磁矩呈递减趋势。其中八面体晶格中的Ni2+离子的t2g轨道被完全填充,而eg轨道由未配对的电子半填充,其d8电子分布为t2g3↑eg2↑t2g3↓,八面体晶格中的Co离子有一个未配对的电子占据在t2g轨道,同时有二个未配对的电子占据在eg轨道,其d7电子分布为t2g3↑eg2↑t2g2↓。在四面体晶格中的Fe3+离子的t2和e轨道及八面体晶格中的Fe离子的t2g和eg轨道都是由未配对的电子半填充,其d5电子结构排列为e2↑t23↑或t2g3↑eg2↑,如图5所示。
为了证实Ni、Co和Fe离子的高自旋态的电子分布,本研究给出了Co原子的3d轨道分波态密度图,如图6所示。从图6可以看出,Co离子3d轨道发生了劈裂,且5个3d电子自旋向上,2个3d电子自旋向下,且自旋向下电子占据dxy和dyz劈裂轨道。
根据图5可知,Ni离子、Co离子和Fe离子的理论磁矩应该分别是2 μB、3 μB和5 μB,但表2计算给出的原子磁矩比理论值小,这主要是因为过渡金属3d轨道发生了轨道劈裂(如图6所示),且与O 2p轨道之间发生了轨道杂化(如图2和3所示)导致原本没有磁性的O离子也携带了磁矩,造成晶体结构中阳离子的局域磁矩比理论分析值小。为了更清楚地阐明这个观点,图7给出了Co0.5Ni0.5Fe2O4铁氧体结构的空间自旋电荷密度分布图,同时也给出了该体系的原子占位结构图。从图7(a)可以看出,O原子中存在少量的自旋取向。
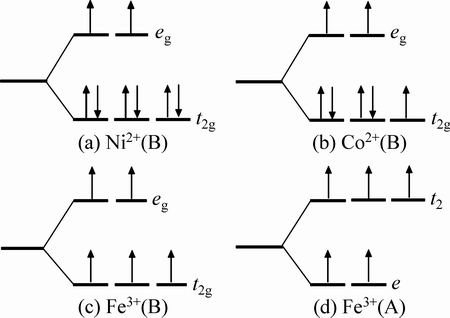
图5 Ni、Co和Fe离子分别在八面体和四面体晶体场中的电子分布
Fig. 5 Electron distribution of Ni, Co and Fe ions in octahedral and tetrahedral crystal field
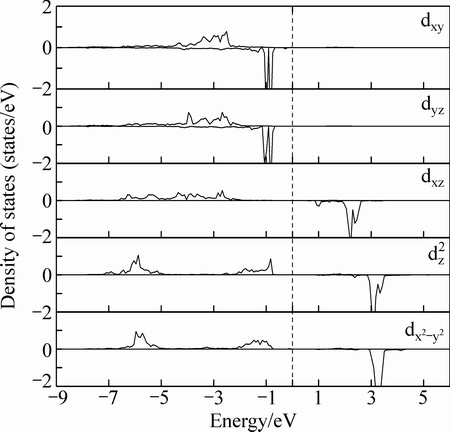
图6 Co原子的分波态密度图
Fig. 6 Partial density of state of Co atom
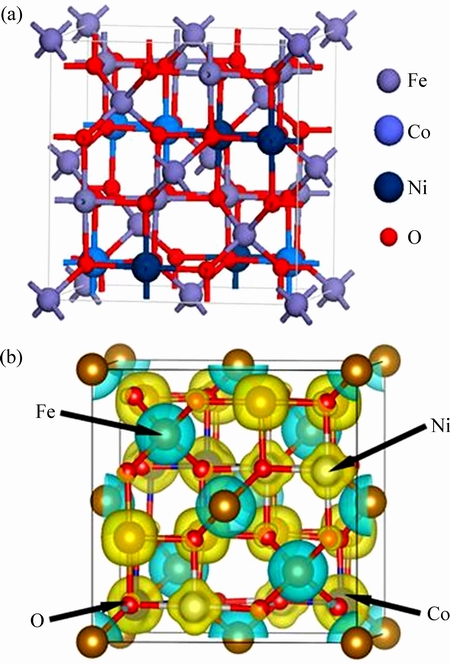
图7 Co0.5Ni0.5Fe2O4的晶体结构图和空间自旋电荷密度分布图
Fig. 7 Crystal structure (a) and space spin charge density distribution (b) of Co0.5Ni0.5Fe2O4
3 结论
1) Ni离子倾向于取代八面体位置中的Co2+离子,且随着掺杂量的增加,晶格常数呈递减趋势,这主要是因为Ni离子半径(0.74 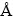 )小于Co离子半径(0.78
)小于Co离子半径(0.78 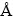 )。
)。
2) 由于Ni离子与Co离子价态一致,所以随着Ni掺杂量的变化,各原子磁矩几乎不变,但总磁矩呈线性降低趋势,这是由于Ni离子(d8)的自旋磁矩小于Co离子(d7)的自旋磁矩所导致的,所得结果与实验结果符合得较好。
3) 通过态密度图分析可知,带隙随着Ni掺杂量的变化而变化,总态密度图大部分都呈绝缘性,且存在强烈的3d-2p轨道杂化。
4) 通过各原子局域态密度图可知,阳离子(Ni、Co和Fe)的自旋向上与自旋向下占据态明显不对称,说明阳离子对磁矩的贡献较大,这从所给出的原子磁矩结果得到了证实。
REFERENCES
[1] 高海涛, 王建江, 赵志宁, 蔡旭东, 侯永伸. 铁氧体吸波材料吸波性能影响因素研究进展[J]. 磁性材料及器件, 2014, 45(1): 68-73.
GAO Hai-tao, WANG Jian-jiang, ZHAO Zhi-ning, CAI Xu-dong, HOU Yong-shen. Research progress of facters affacting absorbing property of ferrite materials[J]. Journal of Magnetic Materials and Devices, 2014, 45(1): 68-73.
[2] LUO G S, ZHOU W P, LI J D, JIANG G, TANG S L, DU Y W. Effect of Cu ion substitution on structural and dielectric properties of Ni-Zn ferrites[J]. Transactions of Nonferrous Metal Society of China, 2015, 25(11): 3678-3684.
[3] 刘 渊, 刘祥萱, 王煊军, 张泽洋, 李 茸, 郭 磊. 离子取代镍基铁氧体的性能及吸波涂层优化设计[J]. 中国有色金属学报, 2013, 23(1): 168-174.
LIU Yu-an, LIU Xiang-xuan, WANG Xuan-jun, ZHANG Ze-yang, LI Rong, GUO Lei. Performance of nickel ferrite replaced by different ions and optimization design of absorbing coating[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(1): 168-174.
[4] MAAZ K, KARIM S, MASHIATULLAH A, LIU J, HOU M D, SUN Y M, DUAN J L, YAO H J, MO D, CHEN Y F. Structural analysis of nickel doped cobalt ferrite nanoparticles prepared by coprecipitation route[J]. Physica B, 2009, 404: 3947-3951.
[5] GUL I H, AMIN F, ABBASI A Z, ANIS-UR-REHMAN M, MAQSOOD A. Physical and magnetic characterization of co-precipitated nanosize Co-Ni ferrites[J]. Scripta Materialia, 2007, 56: 497-500.
[6] SINGHAL S, SINGH J, BARTHWAL S K, CHANDRA K. Preparation and characterization of nanosize nickel-substituted cobalt ferrites (Co1-xNixFe2O4)[J]. Journal of Solid State Chemistry, 2005, 178: 3183-3189.
[7] SZOTEK Z, TEMMERMAN W M,  D, SVANE A, PETIT L, WINTER H. Electronic structures of normal and inverse spinel ferrites from first principles[J]. Physical Review B, 2006, 74: 174431(1-12).
D, SVANE A, PETIT L, WINTER H. Electronic structures of normal and inverse spinel ferrites from first principles[J]. Physical Review B, 2006, 74: 174431(1-12).
[8] ANTONOV V N, HARMON B N, YARESKO A N. Electronic structure and X-ray magnetic circular dichroism in Fe3O4 and Mn-, Co-, or Ni-substituted Fe3O4[J]. Physical Review B, 2003, 67: 024417(1-14).
[9] FRITSCH D, EDERER C. Epitaxial strain effects in the spinel ferrites CoFe2O4 and NiFe2O4 from first principles[J]. Physical Review B, 2010, 82: 104117(1-11).
[10] HANN N, QUY O K, THUY N P, TUNG L D, SPINU L. Synthesis of cobalt ferrite nanocrystallites by the forced hydrolysis method and investigation of their magnetic properties[J]. Physica B, 2003, 327: 382-384.
[11] SHI Y, DING J, YIN H. CoFe2O4 nanoparticles prepared by the mechanochemical method[J]. Journal of Alloys and Compounds, 2000, 308: 290-295.
[12] KOHN W, SHAM L J. Self-consistent equations including exchange and correlation effects[J]. Physical Review, 1965, 140: A1133-A1138.
[13] KRESSE G, HAFNER J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium[J]. Physical Review B, 1994, 49: 14251-14269.
[14] KRESSE G, FURTHMULLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Computational Materials Science, 1996, 6: 15-50.
[15] KRESSE G, FURTHMULLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Physical Review B, 1996, 54: 11169-11186.
[16] 李武会, 任凤章, 马战红, 贾淑果, 田保红, 郑茂盛. 电子理论在材料科学中的应用[J]. 中国有色金属学报, 2008, 18(3): 494-504.
LI Wu-hui, REN Feng-zhang, MA Zhan-hong, JIA Shu-guo, TIAN Bao-hong, ZHENG Mao-sheng. Application of electron theories in materials science[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(3): 494-504.
[17] HAMMER B, HANSEN L B,  J K. Improved adsorption energetics within density-founctional theory using revised Perdew-Burke-Ernzerhof functionals[J]. Physical Review B, 1999, 59: 7413-7421.
J K. Improved adsorption energetics within density-founctional theory using revised Perdew-Burke-Ernzerhof functionals[J]. Physical Review B, 1999, 59: 7413-7421.
[18] KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1998, 59: 1758-1775.
[19] DUDAREV S L, BOTTON G A, SAVRASOV S Y, HUMPHREYS C J, SUTTON A P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study[J]. Physical Review B, 1998, 57: 1505-1509.
[20] HOU Y H, ZHAO Y J, LIU Z W, YU H Y, ZHONG X C, QIU W Q, ZENG D C, WEN L S. Structural, electronic and magnetic properties of partially inverse spinel CoFe2O4: A first-principles study[J]. Journal of Physics D: Applied Physics, 2010, 43: 445003.
[21] 侯育花, 黄有林, 刘仲武, 曾德长. 稀土掺杂对钴铁氧体电子结构和磁性能影响的理论研究[J]. 物理学报, 2015, 64(3): 037501(1-7).
HOU Yu-hua, HUANG You-lin, LIU Zhong-wu, ZENG De-chang. Theoretical study on the influence of rare earth doping on the electronic structure and magnetic properties of cobalt ferrite[J]. Acta Physica Sinica, 2015, 64(3): 037501(1-7).
[22] UDAY BHASKER S, VIJAYAKUMAR Y, VENKATA R R M. Structural, electrical and magnetic characteristics of nickel substituted cobalt ferrite nano particles, synthesized by self combustion method[J]. Journal of Magnetism and Magnetic Materials, 2015, 374: 376-380.
[23] IVANOV V G, ABRASHEV M V, ILIEV M N, GOSPODINOV M M, MEEN J, AROYO M I. Short-range B-site ordering in inverse spinel ferrite NiFe2O4[J]. Physical Review B, 2010, 82: 3054-3056.
[24] YADAV S P, SHINDE S S, KADAM A A, RAJPURE K Y. Structural, morphological, dielectrical, magnetic and impedance properties of Co1-xMnxFe2O4[J]. Journal of Alloys and Compounds, 2013, 555: 330-334.
[25] HANKARE P P, SANADI K R, GARADKAR K M, PATIL D R, MULLA I S. Synthesis and characterization of nickel substituted cobalt ferrite nanoparticles by sol-gel auto-combustion method[J]. Journal of Alloys Compounds, 2013, 553: 383-388.
[26] SHAIKH P A, KAMBALE R C, RAO A V, KOLEKAR Y D. Effect of Ni doping on structural and magnetic properties of Co1-xNixFe1.9Mn0.1O4[J]. Journal of Magnetism and Magnetic Materials, 2010, 322: 718-726.
[27] HASHIM M, ALIMUDDIN, KUMAR S, SAGAR E S, KOTNALA R K, SHAH J, KUMAR R. Synthesis and characterizations of Ni2+ substituted cobalt ferrite nanoparticles[J]. Materials Chemistry and Physics, 2013, 139: 364-374.
[28] RANA K, THAKUR P, SHARMA P, TOMAR M, GUPTA V, THAKUR A. Improved structural and magnetic properties of cobalt nanoferrites: Influence of sintering temperature[J]. Ceramics International, 2015, 41: 4492-4497.
[29] VERMA K C, SINGH V P, RAM M, SHAH J, KOTNALA R K. Structural, microstructural and magnetic properties of NiFe2O4, CoFe2O4 and MnFe2O4 nanoferrite thin films[J]. Journal of Magnetism and Magnetic Materials, 2011, 323: 3271-3275.
[30] SUTKA A, PARNA R, MEZINSKIS G, KISAND V. Effects of Co ion addition and annealing conditions on nickel ferrite gas response[J]. Sensors and Actuators B:Chemical, 2014, 192: 173-180.
[31] ATI A A, OTHAMAN Z, SAMAVATI A. Influence of cobalt on structural and magnetic properties of nickel ferrite nanoparticles[J]. Journal of Molecular Structure, 2013, 1052: 177-182.
[32] KUMAR A, YADAV N, RANA D S, KUMAR P, ARORA M, PANT R P. Structural and magnetic studies of the nickel doped CoFe2O4 ferrite nanoparticles synthesized by the chemical co-precipitation method[J]. Journal of Magnetism and Magnetic Materials, 2015, 394: 379-384.
[33] RAO P, GODBOLE R V, BHAGWAT S. Copper doped nickel ferrite nano-crystalline thin films: A potential gas sensor towards reducing gases[J]. Materials Chemistry and Physics, 2016, 171: 260-266.
[34] TUDORACHE F, POPA P D, DOBROMIR M, IACOMI F. Studies on the structure and gas sensing properties of nickel-cobalt ferrite thin films prepared by spin coating[J]. Materials Science and Engineering B, 2013, 178: 1334-1338.
First-principle study of electronic and magnetic properties of Ni doped CoFe2O4
HOU Shi-jie1, HOU Yu-hua1, HUANG You-lin1, OUYANG Yi-fang2
(1. School of Materials science and Engineering, Nanchang Hangkong University, Nanchang 330063, China;
2. School of Physical Science and Technology, Guangxi University, Nanning 530004, China)
Abstract: Spinel ferrites, such as CoFe2O4, can be applied in varied fields such as electronic equipment, microwave equipment and high density magnetic storage due to their good electromagnetic properties. The crystal structure, electronic structure and magnetic properties of Ni doped CoFe2O4 were investigated within the density functional theory in the generalized gradient approximation with Hubbard U correction (GGA+U). The results show that Ni ions tend to occupy the octahedral site and with the increase of Ni doping content, the lattice constant decreases. The electronic structure analysis shows that the band gap changes with the change of Ni doping content, and generally it is an insulator and there is a strong 3d-2p orbital hybridization. Magnetic properties depend on the Ni doping content, and with the increase of the Ni doping content, the lattice atomic magnetic moments of change is not obvious, but the total magnetic moment shows linear decreasing trend. It is mainly attributed to the different numbers of the unpaired electrons between Ni-3d orbitals and Co-3d orbitals, thus affects the magnetic properties obviously.
Key words: cobalt ferrite; first-principle calculation; dopping; electronic structure
Foundation item: Projects(11304146, 51401103) supported by the National Natural Science Foundation of China; Projects(20151BAB212005, 20151BAB216003) supported by the Natural Science Foundation of Jiangxi Province, China; Project(2014ZF56017) supported by the Aeronautical Science Foundation of China
Received date: 2016-05-26; Accepted date: 2016-10-28
Corresponding author: HOU Yu-hua; +86-791-86453203; E-mail: hyhhyl@163.com
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(11304146,51401103);江西省自然科学基金资助项目(20151BAB212005,20151BAB216003);航空科学基金资助项目(2014ZF56017)
收稿日期:2016-05-26;修订日期:2016-10-28
通信作者:侯育花,讲师,博士;电话:0791-86453203;E-mail:hyhhyl@163.com
摘 要:尖晶石型钴铁氧体因具有良好的电磁性质,广泛应用于电子装备、微波装备及高密度磁存储等领域。采用基于密度泛函理论(DFT)的第一性原理平面波赝势法,结合广义梯度近似(GGA+U),系统研究不同Ni掺杂量对CoFe2O4晶体结构、电子结构和磁性能的影响。结果表明:Ni离子倾向于占据八面体位置且随着Ni掺杂量的增加,晶格常数呈递减趋势。通过电子结构分析表明,带隙随着Ni掺杂量的变化而变化,大部分都呈绝缘体,且存在强烈的3d-2p轨道杂化。磁性能依赖于Ni掺杂量,随着Ni掺杂量的增加,晶格中的原子磁矩变化不明显,但总磁矩呈线性递减趋势,这主要是因为Ni离子最外层的3d未配对电子数与Co离子最外层3d未配对电子数不同,因而对磁性能的影响较大。
[1] 高海涛, 王建江, 赵志宁, 蔡旭东, 侯永伸. 铁氧体吸波材料吸波性能影响因素研究进展[J]. 磁性材料及器件, 2014, 45(1): 68-73.
[3] 刘 渊, 刘祥萱, 王煊军, 张泽洋, 李 茸, 郭 磊. 离子取代镍基铁氧体的性能及吸波涂层优化设计[J]. 中国有色金属学报, 2013, 23(1): 168-174.
[16] 李武会, 任凤章, 马战红, 贾淑果, 田保红, 郑茂盛. 电子理论在材料科学中的应用[J]. 中国有色金属学报, 2008, 18(3): 494-504.
[21] 侯育花, 黄有林, 刘仲武, 曾德长. 稀土掺杂对钴铁氧体电子结构和磁性能影响的理论研究[J]. 物理学报, 2015, 64(3): 037501(1-7).


