
Influence of TiC catalyst on absorption/desorption behaviors and microstructures of sodium aluminum hydride
CHEN Li-xin, FAN Xiu-lin, XIAO Xue-zhang, XUE Jing-wen, LI Shou-quan, GE Hong-wei, CHEN Chang-pin
Department of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China
Received 10 June 2010; accepted 29 November 2010
Abstract:
TiC-doped NaAlH4 complex hydrides were prepared by hydrogenation of ball-milled NaH/Al mixture with x TiC powder (x = 0, 5%, 8%, 10%, mole fraction). The effects of TiC catalyst content on the absorption/desorption behaviors of the samples were investigated. The results show that TiC can improve the hydriding/dehydriding kinetics of sodium aluminum hydride, the hydriding rate of the sample increases with increasing TiC content. It is found that the TiC-doped NaAlH4 composites have a relatively good cyclic stability. The composite doped with 10% TiC maintains steadily about 4.5% (mass fraction) hydrogen absorption capacity as against about 3.8% (mass fraction) hydrogen desorption capacity over 8 cycles. The particle sizes of the TiC-doped NaAlH4 composites can be reduced to 50–100 nm, which may play an important role in improving the hydriding/dehydriding kinetics.
Key words:
hydrogen storage material; sodium alanate; TiC catalyst; kinetic property; ball milling;
1 Introduction
Regarding the use of hydrogen as fuel for the zero-emission vehicle, one of the main issues to be solved is the storage of hydrogen. After the first stimulating work by BOGDANOVI? and SCHWICKARDI [1], which showed reversible hydrogen storage behavior in the Ti-doped sodium alanate, attempts have been made to develop and modify hydrogen desorption/absorption in terms of its kinetics, reversibility and capacity. Unlike traditional intermetallic hydrides, NaAlH4 releases hydrogen through a series of decomposition reaction described in the following two equations:
3NaAlH4 ![]() Na3AlH6 + 2Al + 3H2 (1)
Na3AlH6 + 2Al + 3H2 (1)
Na3AlH6 ![]() 3NaH + Al + 1.5H2 (2)
3NaH + Al + 1.5H2 (2)
The two combined reactions give a theoretical reversible hydrogen storage capacity of 5.6% (mass fraction). Although many investigations have been focused on the sodium alanate, there is still much room for improving both hydriding/dehydriding kinetics and the less than theoretical reversible capacities.
Heretofore, the most widely used dopants are Ti halides [2-8], although Ti halides significantly improve the hydrogen hydriding/dehydriding kinetics of NaAlH4, their addition will reduce the reversible hydrogen storage capacity of NaAlH4 due to the formation of Na halide as the reaction byproducts between NaH and/or NaAlH4 with Ti halides [2, 7, 9-11]. Because of this, the current state-of-the-art hydrogen storage capacity of NaAlH4 is only about 4.0% (mass fraction) [12-14], much less than the theoretical capacity (5.6%). Therefore, there are persistent efforts to find new effective catalysts that enhance the hydriding/dehydriding kinetics while maintaining its high hydrogen capacity. WANG and JENSEN [15] prepared the Ti-doped NaAlH4 by ball-milling in hydrogen atmosphere with off-the-shelf Ti powder, and got about 3.3% restored hydrogen capacity. SUTTISAWAT et al [16] and LEE et al [17] investigated NaAlH4 catalyzed TiO2, which showed a good catalytic effect, and got hydrogen storage capacities of 3.5% and 4.3%, respectively. LEE et al [17] showed that TiO2 remained stable in the hydrogen desorption/absorption process. It is believed that TiO2 do not react with NaH or/and NaAlH4. In our previous works, we found that doping with 5% (mole fraction) TiC can reduce the decomposition temperature and enhance the dehydriding kinetics of NaAlH4 complex hydride [18]. In this work, we prepared the TiC-doped NaAlH4 complex hydrides by hydrogenating the ball-milled NaH/Al+xTiC composites (x=0, 5%, 8%, 10%, mole fraction), and investigated the effects of TiC catalyst content on the improved absorption/desorption behaviors and microstructures of NaAlH4 system.
2 Experimental
The starting materials were NaH powders (95%, <74 μm), Al powders (99%, <154 μm), TiC powders (99%, <4 μm), which were purchased from Sigma- Aldrich Corp.), and all were used as-received. The powder mixtures of NaH + Al + TiC in a molar ratio of 1:1: x (x = 0, 5, 8%, 10%) were mechanically milled for 48 h by planetary milling at 300 r/min. Steel balls were added at a mass ratio of 30:1 (masses of balls and samples were approximately 90 and 3 g, respectively). The milling was performed under a hydrogen atmosphere, with initial pressure of 0.6 MPa. All operations were performed in an Ar-filled glove box, in which the H2O and O2 levels were below 1 ×10-6.
Hydrogen absorption/desorption behaviors of the samples were monitored with a carefully calibrated Sievert’s apparatus. A typical cycling experiment entailed hydrogenation at 120 °C under an initial hydrogen pressure of 12 MPa and dehydrogenation at 155 °C against 0.1 MPa, respectively. The sample was dehydrided at 155 °C and 0.1 MPa for 40 h before hydriding every time, to make sure that it was completely dehydrogenated for testing in next hydriding/ dehydriding cycle. To minimize the oxygen/moisture contamination, the super pure hydrogen gas (99.9999%) was adopted. Hydrogen capacity was determined with respect to the total mass of sample including the TiC catalyst.
The crystal structures of as-prepared samples were characterized by X-ray diffraction (XRD) using a diffractometer (ARL X’TRA, Thermo Electron Corp.) with Cu Kα radiation. To prevent the reaction with oxygen/moisture during XRD examination, the specimen was covered with a special plastic tape layer, which has a negligible effect on diffraction patterns. The data were collected in the range between 28° and 90° in steps of 0.02°. Scanning electron microscopy (SEM, Hitachi S-4800) was used to study the morphology and microstructure of the samples. Due to the high affinity of the samples to oxygen/moisture, the sample preparation and transfer from the inert gas box to the vacuum chamber were carried out under argon.
3 Results and discussion
3.1 Absorption/desorption behaviors
Figure 1 shows the hydriding curves and hydriding rate of the ball-milled NaH/Al composites doped with x TiC (x = 0, 5%, 8%, 10%, mole fraction, the same below if not mentioned) at 120 °C and the initial hydrogen pressure of 12 MPa. As seen from Fig. 1(a), the undoped sample absorbs little hydrogen of 0.43% (mass fraction) in 9 h. However, doping with 5%–10%, TiC causes a remarkable improvement in the hydriding kinetics of the composite, indicating that TiC surely enhances the hydrogenation process of Na and Al. It can be seen from Fig. 1(b) that, the more TiC is doped in the sample, the higher hydriding rate measured in the first 2 h is got. The 10% TiC-doped composite exhibits the most rapid hydriding rate of 1.406% (mass fraction). It is also found that, after about 5.5 h and 7.5 h, the hydrogen absorbed amounts of 5% and 8% TiC-doped composites exceed that of the 10% TiC-doped composite. This is because though TiC can improve the hydriding kinetics of sodium aluminum hydride, it is at the expense of hydrogen absorption capacity of the system. Since hydrogen absorption capacity is determined with respect to the total mass of the sample including the TiC catalysts, the theoretical effective hydrogen absorption capacity of the sample system decreases as the TiC content increases.
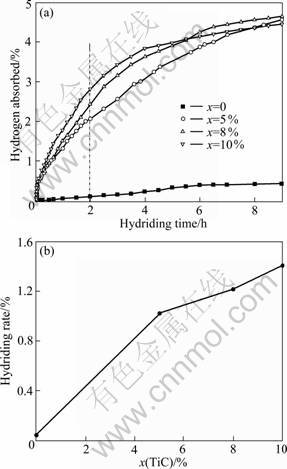
Fig. 1 Hydriding curves (a) and average hydriding rates in the first 2 h (b) for ball-milled NaH/Al composites doped with xTiC (x = 0, 5%, 8%, 10%) at 120 °C and 12 MPa hydrogen pressure
Figure 2 presents the dehydriding curves and average dehydriding rates measured in the first 0.5 h of hydrided NaH/Al composites doped with xTiC (x=0, 5%, 8%, 10%) at 155 °C. It can be seen that, the undoped composite only releases about 0.3% H2 (mass fraction) in 8 h. As x increases from 0 to 8%, the dehydriding rate and hydrogen desorption capacity increase gradually. In the first 0.5 h, the composites doped with 5% and 8% TiC desorbed about 1.55% and 2.31% H2 (mass fraction), respectively. Yet, the difference between samples with 8% TiC and 10% TiC is limited.

Fig. 2 Dehydriding curves (a) and average dehydriding rates measured in the first 0.5 h (b) for hydrided NaH/Al composites doped with xTiC (x = 0, 5%, 8%, 10%) at 155 °C and 0.1 MPa
In order to clarify the change of reversible hydrogen storage capacity and kinetics upon further hydriding- dehydriding cycles, a cycling test was carried out using the sample doped with 10% TiC. The hydrogen absorption of the composite was performed at 120 °C with an initial hydrogen pressure of 12 MPa (see Fig. 3). Figure 3(a) shows the cycling hydriding curves of the NaH/Al+10%TiC composite. It is found that the composite gets the highest hydrogen absorption capacity and the fastest hydriding rate in the first cycle, which can absorb about 4.0% (mass fraction) hydrogen after 3 h and 4.6% (mass fraction) hydrogen after 9 h. This indicates that TiC-doped NaH/Al composite does not have to be activated by a cycling procedure to achieve a high reversible and optimum kinetics, which is quite similar to NaAlH4 doped with other catalysts [19-21]. The hydrogen absorption capacities and hydriding kinetics in the following cycles are very close; however, all of these deteriorate compared with those in the first cycle. Figure 3(b) presents the hydrogen capacities within 9 h. It can be seen that the composite absorbs about 4.6% (mass fraction) hydrogen in the first cycle. In the following cycles, its hydrogen absorption capacities maintain about 4.5% (mass fraction) steadily, and there is no significant change in the absorption behavior.

Fig. 3 Cycling hydriding curves (a) and hydrogen absorbed capacities (b) of NaH/Al composite doped with 10% TiC at 120 °C and 12 MPa of hydrogen pressure
The cycling dehydriding curves of hydrided NaH/Al composite doped with 10% TiC are shown in Fig. 4. The dehydriding behaviors of the hydrided composite were measured at 155 °C and 0.1 MPa. Before dehydrogenation each time, the sample was first hydrided at 120 °C for 10 h with an initial hydrogen pressure of 12 MPa. As seen from Fig. 4(a), the dehydriding kinetics of TiC-doped NaAlH4 in different cycles is very similar. The amount of hydrogen desorbed after 8 h in different cycles maintains about 3.8% (mass fraction) steadily (Fig. 4(b)). Moreover, the desorption capacity can be a maximum value of about 4.5% (mass fraction) after 40 h (Fig. 4(c)), which agrees well with the desorption capacity obtained in Fig. 3(b).

Fig. 4 Cycling dehydriding curves for 8 h (a), hydrogen desorbed capacities (b) and cycling dehydriding for 40 h (c) of hydrided NaH/Al composite doped with 10% TiC at 155 °C and 0.1 MPa
3.2 Microstructures
Figure 5 presents the XRD patterns of the NaH/Al composite doped with 10% TiC in different hydrogenation stages. Na3AlH6 diffraction peaks can be observed in the ball-milled sample before the first hydrogenation; however, its main phases are still Al and NaH. After the first hydrogenation, the main phase of the sample turns to NaAlH4, yet a few phases of Na3AlH6 and Al can still be found, which suggests that the hydrogenation reaction is incomplete. In the XRD pattern of the sample after dehydriding for 8 h, the NaAlH4 phase disappears, while residual Na3AlH6 can be observed. This indicates that neither hydrogenation reaction nor dehydrogenation reaction is complete, which is in line with result reported by BOGDANOVI? et al [22] and can explain why the hydrogen absorption capacity is less than theoretical capacity, and its hydrogen desorption capacity is less than hydrogen absorption value. The mechanism for the incomplete hydrogenation and dehydrogenation reactions is uncertain. It may be due to an inhomogeneous distribution of the catalysts or impurity effects [23]. From Fig. 5, we also find that the peaks of the ball-milled composite before the first hydrogenation are much broader than those of the sample after dehydrogenation of the first cycle, indicating that the crystal grain of the composite grows quickly during the first hydriding-dehydriding cycle. However, the patterns of the sample after dehydrogenation in different cycles are quite similar, as shown in Figs. 5(c) and (d). It has been proved that the bulk diffusion of Al species is the rate-limiting step in the dehydrogenation of Ti-doped NaAlH4 [24], grain boundaries may accelerate the diffusing, thus cause the difference of hydriding kinetics between the first cycle and the following cycles. Because TiC powders are thermodynamically more stable than alkali hydride in the hydrogenation/dehydrogenation processes, they are not reduced and kept in its initial phase. So it does not produce the “dead mass” of byproduct such as Na halides, and the sodium alanate system can maintain relatively high hydrogen storage capacities. It is believed that TiC is stable in the whole process of hydrogenation/dehydrogenation, and may serve as a surface catalyst affecting the hydriding/dehydriding reactions, such as other catalysts [25-26].
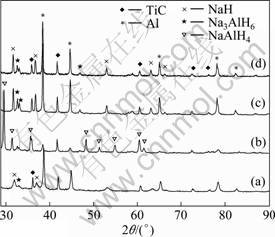
Fig. 5 XRD patterns of ball-milled NaH/Al composite doped with 10% TiC: (a) Before the first hydrogenation; (b) After hydrogenation of the first cycle; (c) After dehydrogenation of the first cycle at 155 °C; (d) After dehydrogenation of the 7th cycle at 155 °C
The SEM micrographs of the NaH/Al mixture doped with 10% TiC at different stages are shown in Fig. 6. The initial sodium hydride particles exhibit strip of typically 30 μm in size, while aluminium particles are irregular with size in the range of 10–20 μm (Fig. 6(a)). The particle size and morphology of the samples after the ball-milling and the first dehydrogenation are shown in Figs. 6(b) and (c). After ball-milling, the composite particles become much finer and exhibit spherical form, with size ranging from about 50 nm to over 100 nm. However, due to the resolution limit of SEM, it is hard to conclude that these nano-particles are made of one crystal or several smaller nanocrystal. It has been proved that the particle size will affect the hydriding/ dehydriding kinetics greatly [27-29]. This may play a partial role in improving the hydriding/dehydriding kinetics of the present NaAlH4 system. After the first dehydrogenation, some larger particles can be observed, presumably formed by agglomeration of very small primary particles. Moreover, it is believed that if the particle size of the catalyst is reduced to below several nanometers, the catalysis can be greatly improved [17]. Such further investigation on the nano-TiC catalyst is necessary.

Fig. 6 SEM images of NaH/Al mixture doped with 10% TiC: (a) Before ball-milling; (b) After ball-milling; (c) After the first dehydrogenation at 155 °C for 8 h
4 Conclusions
1) The effects of TiC catalyst content on the absorption/desorption behaviors of the ball-milled NaH/Al composites doped with x TiC powder (x = 0, 5%, 8%, 10%) were investigated.
2) TiC can improve the hydriding/dehydriding kinetics of sodium aluminum hydride, and the hydriding rate of the sample increases with increasing TiC content.
3) The composite doped with 10% TiC has a hydrogen absorption capacity of 4.6% (mass fraction) after 9 h at 120 °C in the first cycle, and a relatively good cycling stability, maintaining above 4.5% (mass fraction) hydrogen cycling storage capacity.
4) The particle sizes of the TiC-doped composites can be reduced to 50-100 nm by dry ball-milling, which may play a partial role in improving the hydriding/dehydriding kinetics.
References
[1] BOGDANOVI? B, SCHWICKARDI M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials [J]. J Alloys Compds, 1997, 253-254: 1-9.
[2] SANDROCK G, GROSS K, THOMAS G. Effect of Ti-catalyst content on the reversible hydrogen storage properties of sodium alanate [J]. J Alloys Compds, 2002, 339: 299-308.
[3] ANTON D L. Hydrogen desorption kinetics in transition metal modified NaAlH4 [J]. J Alloys Compds, 2003, 356-357: 400-404.
[4] MAJZOUB E H, GROSS K J. Titanium-halide catalyst-precursors in sodium aluminum hydrides [J]. J Alloy Compds, 2003, 356-357: 363-367.
[5] BRINKS H W, SULIC M, JENSEN C M, HAUBACK B C. TiCl3-enhanced NaAlH4: impact of excess Al and development of the Al1-yTiy phase during cycling [J]. J Phys Chem B, 2006, 110: 2740-2745.
[6] LEON A, KIRCHER O, ROTHE J, FICHTNER M. Chemical state and local structure around titanium atoms in NaAlH4 doped with TiCl3 using X-ray absorption spectroscopy [J]. J Phys Chem B, 2004, 108: 16372-16376.
[7] SINGH S, EIJT S W H, HUOT J, KOCKELMANN W A, WAGEMAKER M, MULDER F M. The TiCl3 catalyst in NaAlH4 for hydrogen storage induces grain refinement and impacts on hydrogen vacancy formation [J]. Acta Mater, 2007, 55: 5549-5557.
[8] EIGEN N, GOSCH F, DORNHEIM M, KLASSEN T, BORMANN R. Improved hydrogen sorption of sodium alanate by optimized processing [J]. J Alloy Compds, 2008, 465: 310-316.
[9] GROSS K J, SANDROCK G, THOMAS G J. Dynamic in situ X-ray diffraction of catalyzed alanates [J]. J Alloy Compds, 2002, 330: 691-695.
[10] BRINKS H W, JENSEN C M, SRINIVASAN S S, HAUBACK B C, BLANCHARD D, MURPHY K. Synchrotron X-ray and neutron diffraction studies of NaAlH4 containing Ti additives [J]. J Alloy Compds, 2004, 376: 215-221.
[11] TERMTANUN M, RANGSUNVIGIT P, KITIYANAN B, KULPRATHIPANJA S, TANTHAPANICHAKOON W. Effect of metal type and loading on hydrogen storage on NaAlH4 [J]. Sci Technol Adv Mater, 2005, 6: 348-351.
[12] GROSS K J, MAJZOUB E H, SPANGLER S W. The effects of titanium precursors on hydriding properties of alanates [J]. J Alloy Compds, 2003, 356-357: 423-428.
[13] von BELLOSTA C J M, FELDERHOFF M, BOGDANOVI? B, SCH?TH F, WEIDENTHALER C. One-step direct synthesis of a Ti-doped sodium alanate hydrogen storage material [J]. Chem Commun, 2005, 37: 4732-4734.
[14] ONKAWA M, ZHANG S, TAKESHITA H T, KURIYAMA N, KIYOBAYASHI T. Dehydrogenation kinetics of Ti-doped NaAlH4—Influence of Ti precursors and preparation methods [J]. Int J Hydrogen Energy, 2008, 33: 718-721.
[15] WANG P, JENSEN C M. Preparation of Ti-doped sodium aluminum hydride from mechanical milling of NaH/Al with off-the-shelf Ti powder [J]. J Phys Chem B, 2004, 108: 15827-15829.
[16] SUTTISAWAT Y, JANNATISIN V, RANGSUNVIGIT P, KITIYANAN B, MUANGSIN N, KULPRATHIPANJA S. Understanding the effect of TiO2, VCl3, and HfCl4 on hydrogen desorption/absorption of NaAlH4 [J]. J Power Sources, 2007, 163: 997-1002.
[17] LEE G J, SHIM J H, CHO Y W, LEE K S. Improvement in desorption kinetics of NaAlH4 catalyzed with TiO2 nanopowder [J]. Int J Hydrogen Energy, 2008, 33: 3748-3753.
[18] FAN X L, XIAO X Z, HOU J C, ZHANG Z, LIU Y B, WU Z, CHEN C P, WANG Q D, CHEN L X. Reversible hydrogen storage behaviors and microstructure of TiC-doped sodium aluminum hydride [J]. J Materials Science, 2009, 44: 4700-4704.
[19] EIGEN N, KUNOWSKY M, KLASSEN T, BORMANN R. Synthesis of NaAlH4-based hydrogen storage material using milling under low pressure hydrogen atmosphere [J]. J Alloy Compds, 2007, 430: 350-355.
[20] SUTTISAWAT Y, RANGSUNVIGIT P, KITIYANAN B, MUANGSIN N, KULPRATHIPANJA S. Catalytic effect of Zr and Hf on hydrogen desorption/absorption of NaAlH4 and LiAlH4 [J]. Int J Hydrogen Energy, 2007, 32: 1277-1285.
[21] GUPTA B K, SRIVASTAVA O N. Investigations on the structural, microstructural and dehydrogenation/ rehydrogenation behavior of Ti doped sodium aluminum hydride materials [J]. Int J Hydrogen Energy, 2007, 32: 1080-1085.
[22] BOGDANOVI? B, FELDERHOFF M, GERMANN M, HARTAL M, POMMERIN A, SCH?TH F, WEIDENTHALER C, ZIBROWIUS B. Investigation of hydrogen discharging and recharging processes of Ti-doped NaAlH4 by X-ray diffraction analysis (XRD) and solid-state NMR spectroscopy [J]. J Alloy Compds, 2003, 350: 246-255.
[23] GROSS K J, THOMAS G J, JENSEN C M. Catalyzed alanates for hydrogen storage [J]. J Alloy Compds, 2002, 330-330: 683-690.
[24] GUNAYDIN H, HOUK K N, OZOLINS V. Vacancy-mediated dehydrogenation of sodium alanate [J]. Proc Natl Acad Sci USA, 2008, 105: 3673-3677.
[25] WANG P, KANG X D, CHENG H M. Exploration of the nature of active Ti species in metallic Ti-doped NaAlH4 [J]. J Phys Chem B, 2005, 109: 20131-20136.
[26] GRAETZ J, REILLY J J, JOHNSON J, IGNATOV A Y, TYSON T A. X-ray absorption study of Ti-activated sodium aluminum hydride [J]. Appl Phys Lett, 2004, 85: 500-502.
[27] BALDE C P, HEREIJGERS B P C, BITTER J H, JONG K P. Facilitated hydrogen storage in NaAlH4 supported on carbon nanofibers [J]. Angew Chem Int Ed, 2006, 45: 3501-3503.
[28] ZHENG S Y, FANG F, ZHOU G Y, CHEN G R, OUYANG L Z, ZHU M, SUN D L. Hydrogen storage properties of space-confined NaAlH4 nanoparticles in ordered mesoporous silica [J]. Chem Mater, 2008, 20: 3954-3958.
[29] BALDE C P, HEREIJGERS B P C, BITTER J H, JONG K P. Sodium alanate nanoparticles-linking size to hydrogen storage properties [J]. J Am Chem Soc, 2008, 130: 6761-6765.
TiC催化剂对铝氢化钠吸放氢行为和微观结构的影响
陈立新, 范修林, 肖学章, 薛晶文, 李寿权, 葛红卫, 陈长聘
浙江大学 材料科学与工程学系,杭州310027
摘 要:采用氢化NaH/Al + x TiC (x = 0, 5%, 8%, 10%,摩尔分数)混合物的方法制备TiC掺NaAlH4配位氢化物,系统研究TiC催化剂含量对样品吸放氢行为的影响。结果表明:TiC能有效改善铝氢化钠的吸放氢动力学性能,样品的加氢速率随着TiC含量的增加而提高;TiC掺NaAlH4复合物具有良好的吸放氢循环稳定性,掺杂10% TiC(摩尔分数)的NaAlH4复合物经过8次吸放氢循环后,其吸、放氢容量仍可稳定保持为4.5%和3.8%(质量分数);TiC掺NaAlH4复合物的颗粒尺寸可降低到50–100 nm,这对改善体系吸放氢反应动力学起到主要作用。
关键词:储氢材料;铝氢化钠;TiC催化剂;动力学性能;球磨
(Edited by LI Xiang-qun)
Foundation item: Project (2010CB631300) supported by the National Basic Research Program of China; Projects (50871099, 51001090) supported by the National Natural Science Foundation of China; Projects (20080440196, 200902622) supported by the China Postdoctoral Science Foundation; Project (20090101110050) supported by the University Doctoral Foundation of the Ministry of Education, China
Corresponding author: XIAO Xue-zhang; Tel: +86-571-87951876; Fax: +86-571-87951152; E-mail: xzxiao@zju.edu.cn
DOI: 10.1016/S1003-6326(11)60856-X


