Coupling leaching of sphalerite concentrate
PENG Peng(彭 鹏)1, 2, XIE Hui-qin(谢惠琴)1, LU Li-zhu(卢立柱)1
(1. Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100080, China;
2. Engineering Command Institute of Chemical Defence, Beijing 102205, China)
Abstract:
Coupling process of sphalerite concentrate leaching in H2SO4-HNO3 and tetrachloroethylene extracting of sulfur was investigated. Effects of leaching temperature, leaching time, mass ratio of liquid to solid and tetrachloroethylene addition on zinc leaching processes were examined separately. SEM images of sphalerite concentrate and residues were performed by using JEM-6700F field emission scanning electron microscope. The relationship between the number of recycling and extraction ratio of zinc was studied. The results indicate that 99.6% zinc is obtained after leaching for 3h at 85℃ and pressure of 0.1MPa O2, with 20g sphalerite concentrate in 200mL leaching solution containing 2.0mol/L H2SO4 and 0.2mol/L HNO3, in the presence of 10mL C2Cl4. The leaching time of zinc is 50% shorter than that in the common leaching. The coupling effect is distinct. The recycled C2Cl4 exerts little influence on extraction ratio of zinc.
Key words:
sphalerite; H2SO4-HNO3; coupling leaching CLC number: TF111.31;
Document code: A
1 INTRODUCTION
With diminishing mineral resources and more stringent policy on environmental protection, pyrometallurgy has in many respects been giving way to hydrometallurgical processes especially for low-grade and complicated sulfide ores. In hydrometallurgy, sulfur in the ore is converted to elemental sulfur, which remains in the solid residue, rather than sulfur dioxide in the gaseous effluent to pollute the atmosphere. However, the sulfur formed during leaching usually covers the ore particles, thus preventing effective contacting of the ore particles with the leaching solution, and reducing the leaching rate of the metal being extracted[1-7]. To enhance the leaching ratio, increasing the intensity of stirring, controlling the temperature of leaching or adding catalytic agents has been resorted[8-15], but the results are by no means ideal. In this paper, the leaching of sphalerite concentrate in H2SO4-HNO3 is coupled to the extraction of the sulfur layer on the ore particles by the addition of tetrachloroethylene.
2 EXPERIMENTAL
2.1 Experimental materials
The sphalerite concentrate having the chemical composition shown in Table 1 was first dried and then ground in a ball mill to less than 74μm. Sulphuric acid, nitric acid and tetrachloroethylene were all analytical grade.
Table 1 Composition of sphalerite concentrate(mass fraction, %)

2.2 Principle of coupling leaching
The coupling of the leaching of sphalerite concentrate and the extractive separation of the sulfur formed enables the simultaneous completion of these two processes in the same equipment. This operation was difficult to be realized in traditional technology.
The reactions involved in coupling leaching processes can be described by the following stoichimetric relationships as
3ZnS+3H2SO4+2HNO3= 3ZnSO4+2NO↑+3S0+4H2O(1)
2NO+O2=2NO2(2)
3NO2+H2O=2HNO3+NO(3)
The overall reactions can be given as
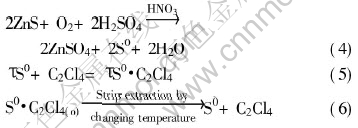
According to reactions (1) to (4), the NO formed in the reaction (1) is oxidized into NO2 by O2, and HNO3 formed in reaction (3) returns to the leaching solution, thus maintaining the concentration of HNO3 constant, that is, HNO3 is used as a catalyst. The elemental sulfur formed in reaction (1) is extracted by tetrachloroethylene in time as shown in reaction (5), thus reducing the resistance of sulfur layer on the ore particles to leaching process. Tetrachloroethylene is chosen as the organic solvent for sulfur because of its high solubility for sulfur and its stability in the leaching pulp thus facilitating recycling as shown in reaction (6). The operational schematics of the coupled process is shown in Fig.1.

Fig.1 Operational schematics of coupling process
2.3 Leaching procedure
The leaching procedure was followed throughout the study. All experiments were carried out in a 500mL titanium lining autoclave, which was equipped with control of temperature, agitation frequency and gas injection. The reactor was charged with 200mL leaching solution containing 2.0mol/L H2SO4 and 0.2mol/L HNO3, and introduced sphalerite concentrate varying from 10g to 200g and C2Cl4 varying from zero to 30mL, then heated to the desired temperature varying from 45℃ to 85℃. Then seal the autoclave, open the stirrer and temperature-controlling equipment. The oxygen was admitted into the autoclave by means of a specially designed injection unit when the pulp was heated to the desired temperature. The oxygen pressure was controlled about 0.1MPa through a gas flow meter. Take a sample to analyze once every half an hour. The zinc contents in the raw material, leaching liquor and residue were analyzed by EDTA titration method.
3 RESULTS AND DISCUSSION
3.1 Effect of leaching temperature on zinc extraction
Leaching experiments were carried out with 20g sphalerite concentrate in 200mL solution containing 2.0mol/L H2SO4 and 0.2mol/L HNO3 at 0.1MPa O2 and temperature varying from 45℃ to 85℃ for 2h. The relationship between the leaching temperature and extraction ratio of zinc is shown in Fig.2. The extraction ratio of zinc increased proportionally with the increase in the leaching temperature. The results indicate that the enhancement of leaching temperature accelerates not only the rate of leaching solution diffusing to the surface of ore particles, but also the leaching reaction.

Fig.2 Effect of leaching temperature on zinc extraction
3.2 Effect of mass ratio of liquid to solid on zinc extraction
These series of experiments were carried out in the solution containing 2.0mol/L H2SO4 and 0.2mol/L HNO3 at 85℃ and 0.1MPa O2, leach- ing time 2h. The relationship between the mass ratio of liquid to solid and extraction ratio of zinc is shown in Fig.3. It indicates that the mass ratio of

Fig.3 Effect of mass ratio of liquid to solid on zinc extraction
liquid to solid exerts obvious influence on zinc extraction. When the mass ratio of liquid to solid is too small, the material has poor mobility, the viscosity of leaching solution increased, the motion rate of molecular goes down, which affects the rate of leaching reaction. When the mass ratio of liquid to solid is too large, the rate of zinc extraction increases a little. But the system loading increases evidently, the energy consumption goes up, the productivity comes down. So it is important to choose an appropriate ratio of liquid to solid. In this experiments, the mass ratio of liquid to solid is 10∶1.
3.3 Effect of leaching time on zinc extraction
These experiments were carried out in the solution containing 2.0mol/L H2SO4 and 0.2mol/L HNO3 at 85℃ and 0.1MPa O2, the mass ratio of liquid to solid being 10∶1. Fig.4 shows the relationship between the leaching time and extraction ratio of zinc. The extraction ratio of zinc increases as the increasing leaching time. After the sphalerite concentrate was leached 2h, the leaching rate of zinc increases slowly. It increases from 86.1% to 99.2% as the leaching time increases from 2h to 6h. The elemental sulfur layer formed on the surface of ore particles became thicker and thicker as the leaching process ran. Thus the resistance of the leaching solution diffusing to the surface of ore particles increases, leading to the result that the rate of leaching in later stage is much slower than that in earlier stage.

Fig.4 Effect of leaching time on zinc extraction
3.4 Effect of tetrachloroethylene on zinc extraction
These experiments were carried out with 20g sphalerite concentrate in 200mL leaching solution containing 2.0mol/L H2SO4 and 0.2mol/L HNO3 at 85℃, 0.1MPa O2, in the presence of zero to 30mL C2Cl4, leaching time being 1h. Fig.5 shows the relationship between the adding volume of C2Cl4 and extraction ratio of zinc. The zinc extraction increases as the adding volume of C2Cl4 increases. It indicates that C2Cl4 extracts and separates the elemental sulfur layer covering the ore particles and the new surface of ore particles is then exposed, which increases the contact chance of the ore particle surface and leaching solution, thus accelerating the leaching reaction. The leaching rate of zinc is enhanced evidently. When the adding volume of C2Cl4 in 200mL leaching solution was more than 10mL, the zinc extraction increases slowly. It increases from 88.1% to 89.9% as the adding volume of C2Cl4 increases from 10 to 20mL. While the adding volume of C2Cl4 in 200mL leaching solution was more than 20mL, the extraction ratio of zinc began to reduce because the viscosity of C2Cl4 is large, which increases the resistance of the leaching solution diffusing to the surface of ore particles and reduces the leaching reaction.

Fig.5 Effect of C2Cl4 addition volume on zinc extraction
3.5 Coupling leaching of sphalerite concentrate
Coupling leaching experiments were investigated at 85℃, 0.1MPa O2, in 200mL solution of 2.0mol/L H2SO4 and 0.2mol/L HNO3, in the presence of 10mL C2Cl4, the ratio of liquid and solid being 10∶1. Fig.6 shows the comparison of effect on sphalerite concentrate leaching with or without C2Cl4. From Fig.6, the extraction ratio of zinc increases evidently after adding 10mL C2Cl4. The leaching ratio of zinc amounted to 88.2% after leaching 1h. However, under the same condition when without C2Cl4, the extraction ratio of zinc is only 70.1%. After leaching 3h under the coupling conditions, the extraction ratio of zinc reaches 99.6%. But the leaching rate of zinc is 99.2% after leaching 6h under the common leaching conditions. As a result, the coupling leaching time of zinc is 50% shorter than that of the common leaching. The coupling effect is distinct. When the leaching of sphalerite concentrate and the extracting of elemental sulfur were coupled, the new surface of ore particles exposed which increased the contact chance of the sphalerite surface and leaching solution, thus reducing the resistance of the leaching solution diffusing to the surface of ore particles, and accelerating the leaching reaction. The leaching rate of zinc is enhanced evidently, and the leaching time is reduced enormously.

Fig.6 Leaching effect of C2Cl4 on sphalerite concentrate with and without C2Cl4
3.6 Morphology of sphalerite concentrate surface
In order to analyze the effect of C2Cl4 during the leaching of sphalerite concentrate, the SEM images of sphalerite concentrate and residues were performed by using JEM-6700F field emission scanning electron microscope. The results shown in Fig.7 indicates that a sulfur layer is formed on sphalerite concentrate particles during the leaching without C2Cl4 (shown in Fig.7(b)) and the sulfur layer is dissolved and removed from ore particles surface obviously by adding C2Cl4(shown in Fig.7(c)). The disappearance of the sulfur layer reduces the leaching resistance and accelerates the leaching rate notably.
3.7 Recycling and loss of C2Cl4
The organic phase containing sulfur was obtained by separating the organic and aqueous phase from the leaching solution in the hot. High quality sulfur and the recycled C2Cl4 were recovered by cooling the organic phase. The recovery rate of sulfur was 86.4%. Table 2 shows the relationship between the number of recycling and extraction ra-[CM(22]tio of zinc. The results indicates that the recycled C2Cl4 exerts little influence on extraction ratio of zinc.
Table 2 Effect of recycled C2Cl4 on zinc extraction


Fig.7 SEM images of sphalerite concentrate surface
During the leaching reaction, C2Cl4 adhered to the surface of ore particles while it extracted and separated the sulfur layer on the ore particles. Stirring the leaching solution might separate the greater part of C2Cl4 from ore particles, but there was still a little C2Cl4 on the surface of ore particles which resulted in the loss of 15% C2Cl4. The loss percentage of C2Cl4 has been reduced to 5% by adding a small amount of surfactant sodium lingo sulphonate which reduced surface tension of leaching solution and altered the hydrophilicity of ore particles, resulting in well-dispersion of ore particles in the leaching solution.
4 CONCLUSIONS
1) The leaching rate of zinc was considerably enhanced while coupling the leaching of sphalerite concentrate in H2SO4-HNO3 to the extracting of elemental sulfur formed during extraction, result- ing in a leaching fraction of zinc as high as 88.2% in 1h, as compared to 18.1% for conventional leaching.
2) The appropriate conditions for leaching are as follows: concentration of H2SO4 2.0mol/L, concentration of HNO3 0.2mol/L, ratio of leaching solution to sphalerite concentrate 10∶1, temperature of leaching 85℃, process pressure of oxygen 0.1MPa, ratio of organic solvent to leaching solution 1∶20.
3) It is convenient to operate the coupled leaching technology and sampling during reaction is easy.
REFERENCES
[1]Warren G W, Henein H, Jin Z M. Reaction kinetics of the ferric chloride leaching of sphalerite—an experimental study[J]. Metallurgical transactions B,1984,15B: 5-12.
[2]Majima H. The leaching of chalcopyrite in ferric chloride and ferric sulfate solution[J]. Can Metall Quar, 1989 , 24(4): 283-291.
[3]Dutrizac J E, Macdonald R J I. The dissolution of sphalerite in ferric chloride solution[J]. Metallurgical Transactions B, 1978, 9B: 543-551.
[4]Dutrizac J E. Elemental sulfur formation during the ferric chloride leaching of chalcopyrite[J] . Hydrometallurgy, 1990, 23: 153-176.
[5]Dutrizac J E, Chen T T. Effect of the elemental sulfur reaction production on the leaching of galena in ferric chloride media[J]. Metallurgical Transactions B, 1990, 21: 935-993.
[6]Lotens J P, Wesker E. The behaviour of sulphur in the oxidative leaching of sulphide minerals[J]. Hydrometallurgy, 1987, 18(1): 39-54.
[7]Venkataswamy Y, Khangaonkar P R. Ferric chloride leaching sphalerite in the presence of an organic solvent for sulphur[J]. Hydrometallurgy, 1981, 7(1-2): 1-5.
[8]Neou-Singouna P, Fourlaris G. A kinetic study of the ferric chloride leaching of an iron-actived bulk sulfide concentrate[J]. Hydrometallurgy, 1990, 23: 203-220.
[9]Bobeck G E, Su H. The kinetics of dissolution of sphalerite in ferric chloride solution[J]. Metallurgical Transactions B, 1985, 16B: 413-424.
[10]Crundwell F K. Kinetics and mechanism of the oxidative dissolution of a zinc sulphide concentrate in ferric sulphate solution[J]. Hydrometallurgy, 1987, 19: 227-242.
[11]Dutrizac J E. Elemental sulfur formation during the ferric sulphate leaching of chalcopyrite[J]. Can Metall Quar, 1989, 28(4): 337-344.
[12]Balaz P, Ebert I. Oxidative leaching of mechanically activated sphalerite[J]. Hydrometallurgy, 1991, 27(2): 141-150.
[13]Lochmann J, Pedlik M. Kinetic anomalies of dissolution of sphalerite in ferric sulfate solution[J]. Hydrometallurgy, 1995, 37: 89-96.
[14]Maurice D, Hawk J A. Ferric chloride leaching of mechanical activated chalcopyrite[J]. Hydrometallurgy, 1998, 49(1-2): 103-123.
[15]Maurice D, Hawk J A. Simultaneous milling and ferric chloride leaching of chalcopyrite[J]. Hydrometallurgy, 1999, 51(3): 371-377.
Foundation item: Project(20276074) supported by the National Natural Science Foundation of China
Received date: 2004-06-08; Accepted date: 2004-09-11
Correspondence: PENG Peng, PhD; Tel: +86-10-86200817; E-mail: pengpeng@home.ipe.ac.cn


