Trans. Nonferrous Met. Soc. China 23(2013) 484-493
Heavy metal contamination, physico-chemical and microbial evaluation of water samples collected from chromite mine environment of Sukinda, India
S. DAS1, S. C. PATNAIK1, H. K. SAHU2, A. CHAKRABORTY3, M. SUDARSHAN3, H. N. THATOI1
1. Department of Biotechnology, College of Engineering and Technology, Biju Patnaik University of Technology, Bhubaneswar, Odisha-751003, India;
2. Department of Zoology, North Orissa University, Baripada, Odisha-757003, India;
3. University Grants Commission-Department of Atomic Energy, Center for Scientific Research, Kolkata Centre, Bidhan Nagar, Kolkata, West Bengal-700098, India
Received 7 March 2012; accepted 6 June 2012
Abstract:
Water samples from chromite mine quarry of Sukinda and its adjacent areas were analyzed for their heavy metal contamination along with physico-chemical and microbial contents. The chromite mine water samples possessed high concentrations of heavy metals in the order of Cr>Fe>Zn>Ni>Co>Mn while ground water did not show any heavy metal contamination except Fe. Physico-chemical parameters of mine water samples showed deviation from those of normal water. Mine water harboured low microbial populations of bacteria, fungi and actinomycetes in comparison with mine adjacent water samples. The correlation of data between metals with physico-chemical parameters showed both positive and negative responses while that of metal and microbial population exhibited negative correlation. Bacterial strains isolated from chromite mine water exhibited high tolerance towards chromium and other heavy metals as well as antibiotics which could be used as an indicator of heavy metal pollution.
Key words:
bioremediation; hexavalent chromium; physico-chemical properties; mine water pollution; microbial population; chromium tolerant bacteria;
1 Introduction
Environmental pollution due to toxic metal ions is one of the major concerns in mining areas around the world. Heavy metals are stable and persistent environmental contaminants since they cannot be degraded nor destroyed [1]. They produce adverse effects on health of human and other living beings in terrestrial and aquatic environment and also affect the food chain. Chromium is one of the chief minerals occurring in Sukinda valley of Jajpur district of Odisha, the richest chromiferous field mass of India accounting 97% chromite deposits in the county [2]. Extensive mining of chromite in this area in the past several years poses serious threat to the environment through pollution of toxic hexavalent chromium in soil and water bodies. As a result of heavy mining, a large volume of over burdens (OB), comprising of chromite ores and waste rock material generate which are dumped in the open ground without considering its adverse impact on the environment. Leaching of heavy metals from these dumps is possible during the rainy season which contaminate the surface water as well as ground water bodies [3]. Besides, water naturally occurring from mine quarries due to excavation is also invariably contaminated with Cr(VI) and other heavy metal ions which are usually discharged into surrounding environment without any pre-treatment. Beside, the accumulated seepage water in the chromite mine quarries of Sukinda is evacuated by pumping that ultimately finds its way into the stream water Damsala Nala, the principal drainage channel of the valley. Running off from overburden dumps during monsoon also results in the pollution of surface water body. The inert chromites in serpentine rocks generate hexavalent chromium through natural oxidation facilitated by weathering process, chemical and microbial action, and are mobilized into the nearby water bodies [4].
Water contains chromium compounds in different valence states of -2 to +4; however, Cr(VI) and Cr(III) are most common species in aquatic environment. Cr(VI) is mobile in the environment and is highly toxic to all forms of living systems including microorganisms, which causes oxidative stress in organisms [5]. It can easily penetrate the cell wall and all biological membranes [6]. Moreover, Cr(VI) is also mutagenic, carcinogenic and teratogenic and has been recognized as a priority pollutant [7]. According to the World Health Organization (WHO) drinking water guidelines, the maximum permissible limit for total chromium is 0.05 mg/L. On the other hand, Cr(III) is considered a trace element and is essential for the proper functioning of living organisms at a lower concentration [8]; it is poisonous only at high concentrations. Cr(III) is nearly insoluble at neutral pH [9] and is precipitated out. Chromium ions are not biodegradable and the conventional treatment methods viz, solvent extraction or precipitation, reduction are not completely satisfactory because these processes have many disadvantages like finite aqueous solubility of extractants and dilutions. On the contrary, microbial reduction of toxic Cr(VI) to relatively non-toxic Cr(III) form has been identified as a cost effective strategy for detoxification and removal of Cr(VI) [9]. The microorganisms have the potency to resist and reduce (detoxify) Cr(VI) and other heavy metals [9]. This could be a promising approach for bioremediation of Cr(VI) in a wide range of environments. Thus, heavy metal resistant micro- organisms have a significant role in waste water treatment system. The detoxifying ability of these resistant microorganisms can be exploited for bioremediation of heavy metals from waste water and effluents having heavy metals. Thus, the metal tolerant microorganisms naturally occurring in heavy metal contaminated environment can be used for bioremediation programme. In view of the above, the present study is aimed at evaluating metal contamination and its effect on physico-chemical parameters and microbial population with a view to assess the status of the water pollution due to mining activities. Further attempt was also made to screen out metal and antibiotics tolerance potential of the isolated bacteria for their possible use in bioremediation of hexavalent chromium from the metal contaminated environments.
2 Materials and methods
2.1 Study site
Sukinda lies between latitude 21° 1′ to 21° 4′ N and longitude 85° 45′ to 85° 48′ E and is a part of Sukinda valley, Jajpur district, Odisha. Sukinda, ultramafic belt of Odisha forms an E-W trending V-shaped valley bounded by the Daitari and Mahagiri hill ranges of Precambrian quartzites and banded iron formation. Damsala Nala (stream), the principal drainage channel of the valley, flows from east to west and, after a 20 km stretch of westerly flow, turns south to meet the major river, Brahmani. In the southern part, Mahagiri hill ranges lie with an altitude of 300 m above mean sea level, whereas Daitari hill ranges in northern part of the valley have attained an altitude of 200 m above mean sea level [3]. Most of the mines are located in the central part of the valley and are leased to various companies. The ultramafic rocks of Sukinda carry large reserves of chromite ores in the form of thick seams, lenses and pods are extensively altered and laterized (oxidized). As many as ten chromite seams, each several metals in thickness are known in the area and the mineral is being extensively exploited by opencast mining. Several such chromite quarries are being operated by different agencies in these regions. The giant mining companies are Orissa Mines Corporation (OMC), Indian Metals and Ferro Alloy, TISCO, etc [3]. The chromite ores occur as bands within the ultramafic body at six stratigraphic levels. The thickness of chromite ore body ranges from 0.3 to 20 m and from 100 m to 7 km in length. The thickest and longest bands occur in Kaliapani- Bhimtangar tract, whereas, in the western sectors the ores occur as dissemination or discontinuous bands. Damsala Nala in mine area which is fed from rain water and water discharged (pumping) from mine quarries. The main source of potable water in the area is ground water, which is tapped both by dug wells as well as tube wells. The variation in the depth of these wells varies from 3 m to 10.5 m below ground level.
2.2 Collection of water samples
Five water samples were collected from different locations of the mine area in screw capped sterilized bottles and designated as chromite water (CW-1-5). CW-1 and CW-2 were collected from seepage water and storage water from mine quarry, CW-3 from upstream of Damsala Nala, CW-4 and CW-5 were collected from dug well and tube well respectively from the mine adjacent areas and were treated as control samples. The water samples were collected during the month of February 2010 in winter season following the methods in Ref. [10]. Water temperature, pH, and electrical conductance (EC) were recorded at the time of sample collection by using a thermometer, pocket digital pH meter and conductivity meter. Samples were immediately transported aseptically and stored at 4 °C for further analysis.
2.3 Physico-chemical analysis of water samples
The physico-chemical characteristics of water were analyzed to determine the quality of mine water. The physical variables such as temperature, pH and EC were analyzed at the site and the other parameters such as dissolved oxygen (DO), total hardness (TH), calcium, chloride and phosphate were analyzed in the laboratory [11]. Dissolved oxygen was determined by titration against sodium thiosulphate, total hardness (TH) was analyzed by titration using a standard EDTA solution, with eriochrome black T as an indicator. Calcium content was evaluated by titration with EDTA using murexide as an indicator. Chloride (Cl-) content was measured using potassium chromate as an indicator relatively to silver nitrate. Phosphate ( ) was determined using the turbidimetric method, comparing the results with the standard graph using stannous chloride as reagents.
) was determined using the turbidimetric method, comparing the results with the standard graph using stannous chloride as reagents.
2.4 Metals analysis by atomic absorption spectro- photometer (AAS)
Metal contents of the water samples were analyzed by AAS (Model: ECILTM AAS-4141) following the method of APHA [11]. For the determination of heavy metals, the water samples were digested with 20 mL aqua-regia (HCl/HNO3 3:1, volume ratio) in a beaker (open beaker digestion) on a thermostatically controlled hot plate. Then 5.0 mL hydrogen peroxide was added to the sample to complete the digestion and the resulting mixture was heated again to near dryness in a fume cupboard and filtered by Whatman no. 42 filter paper and the volume was made up to 50 mL by double distilled water.
2.5 Microbial populations
Microbial populations such as bacteria, fungi and actinomycetes were carried out for different water samples following standard dilution plate technique [12]. In this method, 1 mL water sample was taken and volume was made up 100 mL with sterile water which was further serially diluted to get 10-4 dilution. From these diluted samples, 1 mL water sample was dispensed over each of three replicates and then media for growth of different microorganisms were added nutrient agar used for isolation of bacteria while potato dextrose agar and ammonium chloride-starch agar medium were used for fungi and actinomycetes respectively, the Petri plates were incubated at 35 °C for 48 h for bacteria, 25 °C for 72 h for fungi and 30 °C for 120 h for actinomycetes. The microbial populations were enumerated as colony- forming units (CFU) from a serial dilution of soil suspensions. The microbial colonies were counted in the three replica plates and the average values were calculated. The populations of microorganisms were considered from the number of microbes multiplied by the dilution factor for each sample.
2.6 Isolation of chromium tolerant bacteria and evaluation for their heavy metal tolerance
Isolation of chromium tolerant bacteria was done by enrichment culture technique, using potassium dichromate (K2Cr2O7) as hexavalent chromium (100×10-6) supplement in nutrient agar (NA) medium. Serially diluted water samples were inoculated into the Cr(VI) enriched agar plate, and incubated at 35 °C for 48 h. Twenty-two morphologically district colonies were picked up and then pure culture was prepared by repeated streaking and was preserved in NA slants under refrigerated (4 °C) conditions for further use. These isolates were designated as CWB-1-22. For estimation of chromium tolerance, molten NA medium was supplemented with Cr (VI) with final concentration ranging from 100×10-6 to 900×10-6 and then the isolates were streaked onto the NA plates and incubated at 35 °C for 48 h. This process was repeated successively with higher concentrations of Cr(VI) until the minimum inhibitory concentration (MIC) of bacterial isolate was obtained. Similarly, tolerance towards different metals was also carried out by varying the concentration in (100-1000)×10-6 of each metal like Co2+, Ni2+, Cu2+, Mn2+ and SeO3- [12] for the same 22 bacterial isolates.
2.7 Antibiotics disk sensitivity test
Antibiotic susceptibility of eight high metal tolerant bacterial isolates (CWB-5, CWB-7, CWB-9, CWB-10, CWB-13, CWB-17, CWB-19 and CWB-21) was determined by the disk diffusion method, using the antibiotic disks: ampicillin, streptomycin, trimenthoprim, chloramphenicol, tetracycline, gentamicin, sulpha- furazole, penicillin, clotrimazole, methicillin at 30 μg/disc concentration. Based on the production of inhibition zones in NA plates after 24 h of incubation at 35 °C, isolates were classified as sensitive, intermediate behavior or resistance, according to French guidelines [13].
2.8 Statistical analysis
Statistical analysis was performed with the Pearson methods. Correlation analysis and principal components analysis of metals with physico-chemical parameters and microbial parameters were performed by SPSS, version 18 for Window (SPSS Inc, IBM Corporation, USA).
3 Results and discussion
3.1 Physico-chemical analysis of water samples
The physico-chemical characteristics of water samples from mine and its adjacent area are presented in Table 1. The pH of water samples from mine quarry (CW-1, CW-2) ranged from 5.4 to 5.8 whereas the water from Damsala Nala (CW-3), dug well (CW-4) and tube well (CW-5) ranged from 7.1 to 7.2. pH of mine water is less than 7, therefore mine water can be characterized as acidic water. However, this is not evident from analysis of water from stream water and ground water. Mine water comes in contact with metal ions from minerals and vergin rockmass in the presence of atmospheric air and acid mine waters and heavy metals were formed [14]. The pH of water generally influences the concentration of many metals by altering their availability and toxicity [15]. Metal pollution is caused in mine water through leaching when metals contained in excavated rock or exposed mine come in contact with such water. Although metals can become mobile in neutral conditions, leaching is particularly accelerated in the low pH conditions, which are created by acid mine drainage [15]. Acidic water is a matter of primary concern since it can directly be injurious to aquatic organisms. It also facilitates leaching of toxic metals into the water, which is hazardous to aquatic life directly or can disturb the habitat after precipitation [15]. Similarly acidic mine water (pH 5.9 to 6.5) has been reported from Boula-Nuasahi chromite mine area [16]. Besides, DEY and PAUL [17] have also reported acidic seepage mine water with pH 5.5-7.2 and 5.1-5.5 from chromite mine quarries of Sukinda and Nuasahi-Boula respectively, which could be attributed to the generation of hazardous hexavalent chromium from the inert chromite ore bodies and its distribution in accumulated seepage water in chromite mine quarries. The EC in the study area varies from 0.54 to 0.86 μS/cm. The EC of mine water samples lies between 0.73 (CW-2) and 0.86 μS/cm (CW-1), whereas that of Damsala Nala was found to be 0.78 μS/cm. On the contrary, EC of ground water (dug well and tube well) was low (0.54 μS/cm and 0.61 μS/cm). EC of the water is the sum of ionic conductance of all the ionic constituents. It depends upon the dissolved nutrients and micronutrients of the water samples. The more the dissolved solids in the water are, the more the EC is [18]. High EC content in mine water indicates dissolution of minerals due to mining of ores in quarries. Dissolved oxygen (DO) values of the water samples, CW-1, CW-2 and CW-3 were 2.10, 2.40 and 3.20 mg/L respectively, whereas the DO values of CW-4 and CW-5 were 4.20 and 2.4 mg/L respectively. The dissolved oxygen of water less than 3 mg/L is stressful to most aquatic organisms. It’s levels at least 5-6 mg/L are usually required for growth of aquatic organisms [18]. According to WHO’s guideline for drinking water the minimum level of dissolve oxygen is 4.0 mg/L. Dissolved oxygen in water is essential for sustaining higher forms of life in water bodies. It is an important parameter to assess water quality. Low DO may be due to metal contamination in mine water, which can cause serious environmental problem [16]. The calcium concentrations of the samples CW-1, CW-2 and CW-3 were 29.00, 24.00 and 19.00 mg/L respectively and those of ground water samples, CW-4 and CW-5 were 21.00 and 52.00 mg/L respectively. The chloride contents of the samples CW-1, CW-2 and CW-3 were 205-275.00 mg/L and CW-4 and CW-5 were 217.00 mg/L and 282.00 mg/L, respectively. The magnesium concentrations of CW-1, CW-2 and CW-3 ranged between 6.42 and 17.50 mg/L and those of ground water samples CW-4 and CW-5 were 1.31 and 0.085 mg/L, respectively. The water hardness (CaCO3) depends on anions such as carbonate, bicarbonate, sulphate and chloride and major cations, such as calcium and magnesium [19], which are all within the permissible limits. The total hardness values (CaCO3) of the samples CW-1, CW-2 and CW-3 varied from 275.00 to 297.00 mg/L while that of CW-4 and CW-5 were 308.00 and 256.00 mg/L, respectively. The water hardness has no known adverse effects in the environment [16] but high hardness creates problems for daily human uses. The phosphate values of chromite mine water are similar to those reported by DHAL et al [16] from Boula-Nuasahi chromite mine area. The phosphate values of mine waters, CW-1, CW-2 and CW-3 ranged between 0.39 and 0.46 and mg/L, while the ground water samples, CW-4 and CW-5 were 0.37 and 0.78 mg/L respectively. Phosphate, which is critical nutrient for the growth of microorganisms like algae in water [19] and can cause eutophication, was very low.
Table 1 Physico-chemical parameters of water samples from chromite mine and its adjacent areas of Sukinda, Odisha
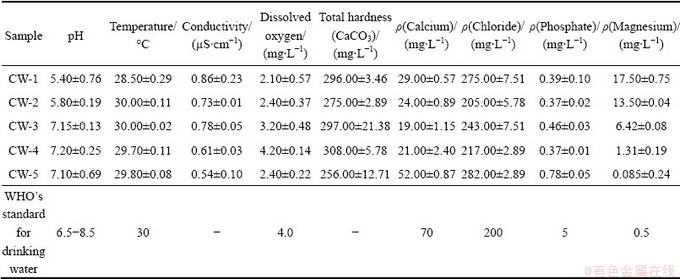
Analyses of metal content of chromite mine and its adjacent area are presented in Table 2. Water samples from mine quarry (CW-1, CW-2), and Damsala Nala (CW-3) showed contamination with the metals tested while ground water samples from mine adjacent areas CW-4 and CW-5 (dug well and tube well) did not contain these metals except iron. Metals content in mine water samples were in the order of Cr>Fe>Zn> Ni>Co>Mn. The total Cr (0.74-3.12) mg/L and Cr(VI) (0.347-2.15 mg/L) in mine water samples were quite high. The total chromium and Cr(VI) were absent in ground water samples. Mining of chromite ore provides obvious source of contamination of different metals including chromium. Different metals usually occur along with chromium in chromite mine soil and water [20]. The concentration of metals (Cr, Ni, Fe, Co, Mn and Zn) in mine water samples was strikingly higher than that of the permissible range. Elevated levels of heavy metals can be found in and around chromite mine water due to discharge and dispersion of heavy metals into water sources and eventually possess health risk [18]. There has been reported that the heavy metal pollution of water is a significant environmental problem and different heavy metals (Cr, Ni, Mn, Cd, Co, Fe, Pb) contaminated water coming from different mining sources have toxic effects on human and environment. Among the different heavy metals, cadmium is nonessential but poisonous heavy metal for plants, animals and humans, and leads a major heavy metal pollutant that is found in soil, water and air is highly toxic to human, animals, plants and microbes [7]. Similarly, nickel has been implicated as an embryotoxin and teratogen [7]. Chromium and mercury are toxic, carcinogenic, cytotoxic, and mutagenic to animals and human beings [7]. Metals can be dissolved from mining sites through the action of acid runoff or can be washed into streams as sediment [15]. Most of the water bodies in the coal mining area of Jaintia Hills have been found containing high concentration of metals. These metals can be toxic to fish and other aquatic organisms when present in dissolved condition with high concentrations [15].
3.2 Spatial variation of microbial assemblages
The microbial population of the mine and adjacent area water samples was studied using dilution plate technique and presented in Table 3. Among the five samples, CW-1 collected from mine quarry had the lowest population of bacteria (29.66), fungus (0.33), and actinomycetes (0). On the other hand water samples from the ground water, CW-5 (tube well) contained the highest number of microbial colonies (142.66 bacteria, 6.66 fungi) except actinomycetes (0). Water samples from Damsala Nala (CW-3) and dug well (CW-4) contained actinomycetes populations 6.0 and 3.0, respectively. It is apparent from the present finding that the number of heterotrophic bacteria in the water was affected by the introduced metal contaminants [21].
Table 2 Metal concentration analysis of water samples from chromite mine and its adjacent area Sukinda, Odisha by AAS

Table 3 Microbial population of water samples from chromite mine and its adjacent area Sukinda, Odisha

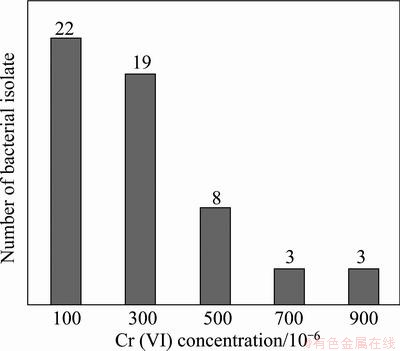
Fig. 1 Screening of bacterial isolates towards different concentrations of Cr(VI) isolated from water samples, Sukinda, Odisha
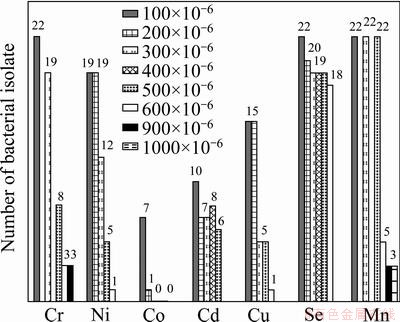
Fig. 2 Screening of chromium resistance bacteria towards different heavy metals with different concentrations
3.3 Evaluation and screening of chromium and other metals tolerance bacteria
In search for metal tolerant bacteria, 22 bacteria isolated from chromite mine water samples were screened for their tolerance towards increasing concentrations of Cr(VI) and other metals as shown in Fig. 1 and Fig. 2. While all the 22 bacterial isolates showed tolerance up to 200×10-6 of Cr(VI), 19 bacteria up to 300×10-6, 8 bacteria upto 500×10-6 and 3 bacteria only upto 900×10-6 of Cr(VI). There are earlier reports suggesting that more than 74% bacteria isolated from chromite mine water from Sukinda could tolerate more than 100×10-6 Cr (VI) [17]. Screening of all the 22 bacterial isolates towards other metals (Co2+, Ni2+, Cu2+, Mn2+ and SeO3-) showed that most of the bacterial isolates tolerated high concentrations of SeO3- and Mn2+. Comparatively less bacterial isolates were tolerance towards Co2+, Ni2+, Cd+2 (400×10-6). Very few isolates were tolerant to Cu+2 (300×10-6).
Bacteria continuously exposed to heavy metal contaminations of the environment may develop genetically determined resistance system against heavy metal toxicity [8], as shown in Table 4, which is frequently coded by the plasmids present in bacteria [8]. Multiple heavy metal resistance determinants, namely the Cd, Co, Zn genes (czc), the Co, Ni, Cr genes (cnr, chr) and the Hg (mer) have been isolated from plasmids [8]. The combined resistance to different heavy metals suggests that the metal resistances of the bacteria were interrelated to each other. The combined resistance to Ni, Cr and Zn was reported to support that the metal resistances of the bacteria were interrelated each other [8].
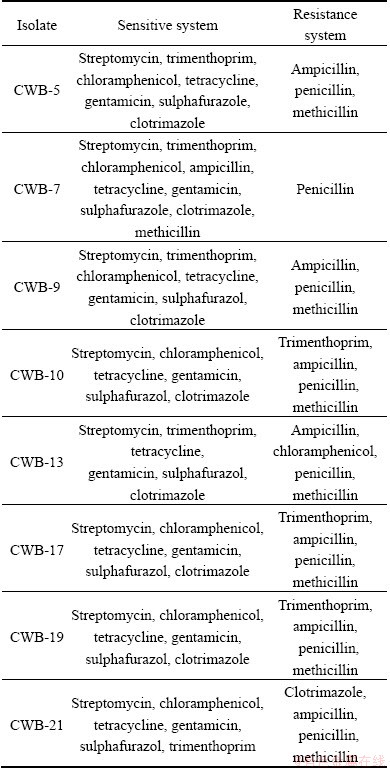
Table 4 Antibiotic sensitivity test of bacterial isolates from chromite mine and its adjacent area Sukinda, Odisha (Concentration of antibiotics= 30 mg/disc)
3.4 Statistical analysis
The principal component analysis and the correlation matrix of metals and physico-chemicals parameters of the water samples were calculated and relations between them were studied (Fig. 3 and Tables 5, 6). The principal component analysis includes loading for the rotated component matrix, eigen values for each component, percent of variance and cumulative percent of variance explained by each component indicating the portion of variance of each variable controlled by the set of components. The eigen values of the four principal components were 6.079, 4.492, 2.679 and 1.310 respectively. The total variance of the four principal components was 90.99%. The temperature, dissolved oxygen and total hardness show positive correlation towards most of the metal components. The correlation analysis of metals and physico-chemical study revealed that the total chromium exhibited a significant positive correlation with pH, temperature, conductivity, dissolve oxygen, PO4 and negative correlates with total hardness, calcium, Mg, Cl. Cr(VI) also showed the similar results with total chromium (Table 6 and Fig. 3). KAR et al [22] found the similar results, while studying in Ganga river water polluted by different heavy metals. The pH of the water samples is found to be acidic, the EC and DO contents (except tube well) are also found to be high, while the total hardness and calcium were found below level of prescribed standards. Generally, heavy metals create toxic effect by forming complexes with organic compounds. It is said that the toxic effects of metals can change according to the structure of metals. The solubility of the metals primarily depends on the pH, dissolved oxygen, and hardness [23]. The results of the principal component analysis and correlation analysis between the metal contents and microbial population in the five water samples collected from chromite mine area of Sukinda are shown in Tables 7 and 8 and Fig. 4. The eigen values of the three principal components were 4.833, 2.099 and 1.348, respectively. The total cumulative variance is 82.803% and KMO adequacy value is 0.455. The results showed that the bacterial, fungal and actinomycetes population were negatively correlated with all the metals. Thus, it is a negative influence of metal concentration on the growth of the microbial population. High concentration of toxic heavy metals can reduce the population of the microorganisms [24]. Many heavy metals are detrimental to microorganisms even at the low concentrations present in natural waters [25]. Besides, there are also reports that microorganisms adapt to heavy metals [26]. This phenomenon has important implications for microbial ecology in polluted ecosystems.
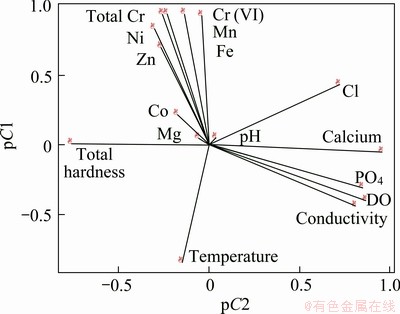
Fig. 3 Correlation analysis of metal contents with physico- chemical parameters of water samples from chromite mine and its adjacent area Sukinda, Odisha
Table 5 Correlation matrix of metal contents with physico-chemical parameter of water samples from chromite mine and its adjacent area Sukinda, Odisha
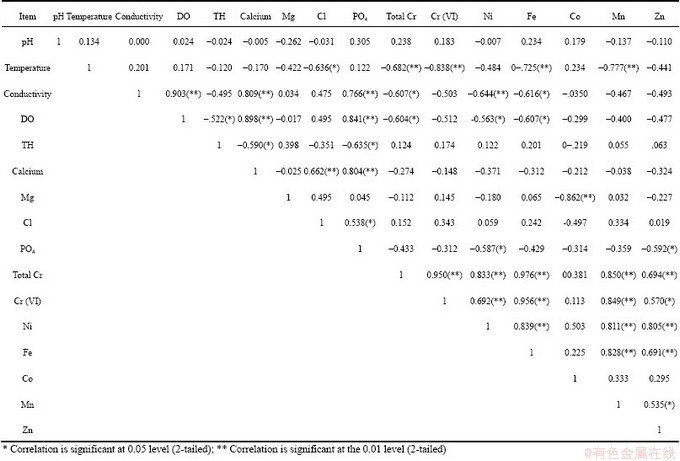
Table 6 Rotated component matrix (a) of water samples from chromite mine and its adjacent area Sukinda, Odisha
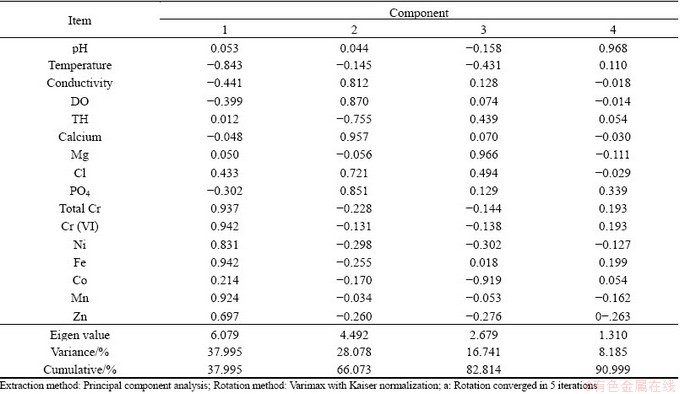
Table 7 Correlation matrix analysis between metal contents and microbial population of water from chromite mine and its adjacent area Sukinda, Odisha
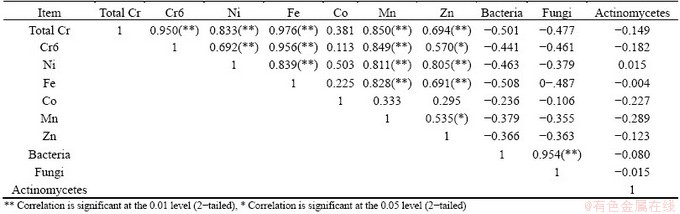
Table 8 Rotated component matrix (a) of water samples from chromite mine and its adjacent area Sukinda, Odisha

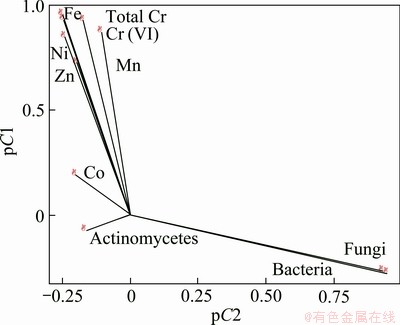
Fig. 4 Correlation analysis of metal contents with microbial populations of water samples from chromite mine and its adjacent area Sukinda, Odisha
3. 5 Antibiotic sensitivity test of bacterial isolates
Among the 22 bacterial isolates, 8 selected chromium tolerant bacteria which showed high tolerance towards different metals also showed resistance towards different antibiotics (Table 4). Among the 8 isolates, 7 isolates were resistant to ampicillin, penicillin, methicillin, chloramphenicol and trimethoprin, and CWB-7 was resistant to penicillin only. No strain could show resistance towards streptomycin, trimethoprin, tetracycline, gentamicin and sulphafurazol. A large number of chromate tolerant microorganisms capable of multiple antibiotic resistances have been reported early [8]. A substantial number of reports suggest that metal contamination in natural environments could have an important role in the maintenance of antibiotic resistance [27]. Resistance to antibiotics can be conferred by chromosomal or mobile genetic elements (e.g. plasmids) and achieved using four main strategies: reduction of membrane permeability to antibiotics, drug inactivation, rapid efflux of the antibiotics and mutation of the cellular target [28]. In addition, antibiotics sequestration has also been known for several decades that metal and antibiotic resistance genes are linked, particularly on plasmids [28].
These Cr(VI) resistant isolates growing under Cr-stress condition may have developed mechanism of Cr-reduction as an effective tool for detoxification of toxic Cr(VI) and can be exploited for reduction of Cr(VI). Thus, chromium resistant microorganisms will play a significant role in wastewater treatment system.
4 Conclusions
Chromite mine water exhibited poor water quality contaminated with toxic metal ions and was affected by imbalanced pH and physico-chemical and low survival of microbial population, which is a great concern for the mine environment. Water from Damsala Nala, the principal water channel is also found to be contaminated with toxic hexavalent chromium which poses threat to the aquatic environment. However, the ground water (dug well and tube well) is not affected by metal contaminations expect low iron contents. The present study also reveals that metal ions not only have direct effect on survival of microorganism but have the partial effect on physico-chemical parameters. Bacteria which were found to be tolerate towards metals including Cr(VI) as well as different antibiotics will have great scope for their application in bioremediation of toxic Cr(VI) from contaminated environments.
Acknowledgments
Financial support of the UGC-DAE, Center for Scientific Research, Kolkata Centre is thankfully acknowledged. Authors thank the authorities of College of Engineering and Technology, Biju Patnaik University of Technology, Bhubaneswar for providing the laboratory faculties.
References
[1] SEVGI E, CORAL G, GIZIR A M, SAGUN M K. Investigation of heavy metal resistance in some bacterial strains isolated from industrial soils [J]. Turk Journal of Biology, 2009, 34: 423-431.
[2] MISHRA B K, NAYAK C R. Environmental implication of chromite mining in Sukinda valley [C]// Proceeding of National Seminar on Recent Trends in Monitoring and Bioremediation of Mine and Industrial Environment. Odisha, India: North Orissa University, 2009.
[3] DHAKATE R, SINGH V S. Heavy metal contamination in groundwater due to mining activities in Sukinda valley, Odisha—A case study [J]. Journal Geography and Regional Planning, 2008, 1(4): 58-67.
[4] GODGUL G, SAHU K C. Chromium contamination from chromium mine [J]. Environmental Geology, 1995, 25(4): 251-257.
[5] ACKERLEY D F, BARAK Y, LYNCH S V, CURTIN J, MATIN A. Effect of chromate stress on Escherichia coli K-12 [J]. Journal of Bacteriology, 2006, 88: 3371-3381.
[6] BARNOWSHKI C, JAKUBOWSKI N D, STUEWER J A C. Speciation of chromium by direct coupling of ion exchange chromatography with inductively coupled plasma mass speciation [J]. Journal of Analytical Atomic Spectrometry, 1997, 12(10): 1155-1161.
[7] CHEN J M, HAO O J. Microbial chromium (VI) reduction critical reviews [J]. Environmental Science and Technology, 1998, 28(3): 219-251.
[8] SUNDAR K, VIDYA R, MUKJHERJEE A, CHANDRASEKARAN N. High chromium tolerant bacteria strains from Palar river basin: Impact of tannery pollution [J]. Research Journal of Environmental Earth Science, 2010, 2(2): 112-117.
[9] DIEDERIK J O. The mechanism of chromate reduction reduction by Thermus scotoductus SA-01 [D]. Bloemfontein, South Africa: Department of Microbiology, Biochemical and Food Biotechnology, University of the Free State. 2008.
[10] ZOBELL C E. Action of micro-organism on hydrocarbons [J]. Bacteriology Review, 1946, 10: 1-49.
[11] APHA. Standard methods for the examination of water and waste water [S]. Washington DC: American Public Health Association, 2005.
[12] MEGHARAJ M, AVUDAINAYAGAM S, NAIDA R. Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste [J]. Current Microbiology, 2003, 47: 51-54.
[13] COMITE D. Committee communicates the susceptibility of the French scoiete Microbiology [J]. Bul Soc Fr Microbiology, 1998, 13: 243-258.
[14] NAVARRO-TORRES V F, SINGH R N. Assessment of water quality due to wolfram mining in Portugal [J]. Journal of Mining Environment, 2010, 2: 21-28.
[15] SWER S, SINGH P. Status of water quality in coal mining areas of Meghaloya, India [C]// Proceeding of National Seminar on Environmental Engineering with Special Emphasis on Mining Environment, NSEEME, 2004.
[16] DHAL B, DAS N N, PANDEY B D, THATOI H N. Environmental quality of the Boula-Nuasahi chromite mine area in india [J]. Mine Water and the Environment, 2011, 30(3): 191-196.
[17] DEY S, PAUL K A. Occurrence and evaluation of cfhromium reducing bacteria in seepage water from chromite mine quarries of Odisha, India [J]. Journal of Water Research Protection, 2010, 2: 380-388.
[18] TEKADE P V, MOHABANSI N P, PATIL V B. Study of physico-chemical properties of effluents from soap industry in Wardha [J]. Rasayan Journal of Chemistry, 2011, 4(2): 461-465.
[19] TRIVEDI R K, GOEL P K. Chemical and biological methods for water pollution studies [J]. Karad Environmental Publication, 1984, 1-251.
[20] MOHANTY J K, RAO D S, PAUL A K, KHAOASH S. Characterization of high magnesium rocks for suitability as flux in iron and steel industry [J]. Journal of Geology and Mining Research, 2009, 1(7): 149-155.
[21] LULI G W, TALNAGI J W, STROHL W R, PFISTER R M. Hexavalent chromium resistance bacteria isolated from river sediments [J]. Applied Environmental Microbiology, 1983, 46(4): 846-854.
[22] KAR D, SUR P, MANDAL S K, SAHU T, KOLE R K. Assessment of heavy metal pollution in surface water [J]. International Journal of Environmental Science and Technology, 2008, 5(1): 119-124.
[23] BARLAS N. A pilot study of heavy metal concentration in various environments and fishes in the upper Sakarya river basin [J]. Environmental Toxicology, 1999, 14(3): 367-373.
[24] KIM S J. Effect of heavy metals on natural populations of bacteria from surface microlayers and subsurface water [J]. Marine Ecology Progress Series, 1985, 26: 203-206.
[25] MILLS A L, COLWELL R R. Microbiological effects of metal ions in Chesapeake Bay water and sediment [J]. Bulletin of Environmental Contamination and Toxicology, 1977, 18: 99-103.
[26] AZAM F, VACCARO R F, GILLESPIE P A, MOUSSALLI E I, HODSON R E. Controlled ecosystem pollution experiment: Effect of mercury on enclosed water columns. Marine bacterioplankton [J]. Marine Science Communities, 1977, 3: 313-329.
[27] OHTAKE H, CERVANTES C, SILVER S. Decreased chromate uptake in Pseudomonas fluorescence carrying a chromate resistance plasmid [J]. Journal of Bacteriology, 1987, 169: 3853-3856.
[28] PIMENTEL B E, MORENO-SANCHEZ R, CERVANTES C. Efflux of chromate by Pseudomonas aeruginosa cells expressing the ChrA protein [J]. FEMS Microbiology Letter, 2002, 212: 249-254.
印度Sukinda铬铁矿区水样的重金属污染、理化性质和微生物的评价
S. DAS1, S.C. PATNAIK1, H. K. SAHU2, A. CHAKRABORTY3, M. SUDARSHAN3, H. N. THATOI1
1. Department of Biotechnology, College of Engineering and Technology, Biju Patnaik University of Technology, Bhubaneswar, Odisha-751003, India;
2. Department of Zoology, North Orissa University, Baripada, Odisha-757003, India;
3. University Grants Commission-Department of Atomic Energy, Center for Scientific Research, Kolkata Centre, Bidhan Nagar, Kolkata, West Bengal-700098, India
摘 要:分析了Sukinda铬铁矿采石场及其邻近区域水样的重金属污染及理化性质和微生物含量。铬铁矿的水样含有高浓度重金属,其浓度顺序为Cr>Fe>Zn>Ni>Co>Mn,然而该地区的地下水除了Fe以外并没有受到重金属污染。矿井水样的理化参数与正常水的有差别。与相邻矿水样相比,矿井水样含有一些低浓度的微生物种群,包括细菌、真菌和放线菌。金属浓度与相关的理化参数显示了他们之间有正、负响应,而金属浓度和微生物种群之间表现出了负的相关性。从铬铁矿废水中纯化出来的菌株对铬和其他的重金属以及抗生素表现出高的耐受性,可作为重金属污染的指示剂。
关键词:生物降解;Cr(VI);理化性质;矿水污染;微生物种群;耐铬细菌
(Edited by Xiang-qun LI)
Corresponding author: H. N. THATOI; Tel: +91-674-2386182, E-mail: hn_thatoi@rediffmail.com
DOI: 10.1016/S1003-6326(13)62489-9
Abstract: Water samples from chromite mine quarry of Sukinda and its adjacent areas were analyzed for their heavy metal contamination along with physico-chemical and microbial contents. The chromite mine water samples possessed high concentrations of heavy metals in the order of Cr>Fe>Zn>Ni>Co>Mn while ground water did not show any heavy metal contamination except Fe. Physico-chemical parameters of mine water samples showed deviation from those of normal water. Mine water harboured low microbial populations of bacteria, fungi and actinomycetes in comparison with mine adjacent water samples. The correlation of data between metals with physico-chemical parameters showed both positive and negative responses while that of metal and microbial population exhibited negative correlation. Bacterial strains isolated from chromite mine water exhibited high tolerance towards chromium and other heavy metals as well as antibiotics which could be used as an indicator of heavy metal pollution.


