
Periodical attenuation of Al(OH)3 particles from seed precipitation inseeded sodium aluminate solution
WU Yu-sheng(吴玉胜)1, ZHANG Di(张 迪)1, LI Ming-chun(李明春)1,
BI Shi-wen(毕诗文)2, YANG Yi-hong(杨毅宏)2
1. School of Materials Science and Engineering, Shenyang University of Technology, Shenyang 110870, China;
2. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 6 July 2009; accepted 25 December 2009
Abstract:
Periodical attenuation of particles, which interferes seriously the normal alumina production, exists in Bayer process. In order to solve this problem, the rule of periodical attenuation of Al(OH) 3 particles was investigated by laboratory experiments under simulated industrial conditions. The results show that at higher temperature the variation period of particle size is shortened, while prolongs with more solid content. Particle size fluctuation amplitude reduces with the temperature rising but increases with the solid content increasing. Particle size distribution becomes more uniform by replenishing fine seeds, enabling the periodical fluctuation of Al(OH)3 particle size to be attenuated. Combining properly the additives with controlling the seed size is able to reduce the amplitude of periodical fluctuation and shorten the attenuation time. With unbalance of particle size distribution, the particles gradually become bigger, even inducing the decrease of the specific surface area of seeds, which is the major reason causing explosive attenuation of Al(OH)3 particles in seed precipitation process.
Key words:
Bayer process; sodium aluminate solution; periodical attenuation; additive; seed precipitation;
The most common industrial process for alumina production from bauxite is the Bayer process. Seed precipitation is the key stage which has a significant effect on the yield and specification of product[1-3]. Supersaturated sodium aluminate (NaAlO2) solution is obtained by dissolving aluminous ore, bauxite, in a hot solution of sodium hydroxide (NaOH). The gibbsite, Al(OH)3, and boehmite, AlOOH, in the bauxite, react with the caustic solution to form soluble aluminate ions, Al(OH)-4. After the removal of the insoluble impurities, the supersaturated solution is cooled to the desired crystallization temperature, and supplied with gibbsite seed particles, so that the dehydroxylation of Al(OH)-4 ions into Al(OH)3 may occur in precipitation. This hydroxide is then calcined or dehydrated to alumina for further processing and production of aluminium[4-7].
Lots of works have been done on precipitation process for enhancing precipitation ratio of sodium aluminate liquor and getting qualified products[8-10]. However, the periodic attenuating of grain size, where the mass fraction of particle with size above 44 μm changes from 50% to 90% and attenuating period changes from 3 to 5 months, inherently exists in the Bayer process in China. During attenuation, the function of erect and flat filters deteriorates and product quality drops. Meanwhile, the electricity consumption and the dust density in the exit of electrofilter increase[11-13]. Although some works have been done to solve this problem, such as using Franch technology and investigating the particle size distribution (PSD) of alumina in some branches of China Aluminum Co., Ltd, the explosive attenuation of alumina particles cannot be avoided. Therefore, solving the problem of periodic attenuation in seeded sodium aluminate solution has great realistic significance for energy saving and eligible sandy alumina producing. Researches on the periodic attenuation of grain size in Bayer process extracting alumina from diasporic bauxite ores have little been reported. In this work, the rule of periodical attenuation of Al(OH)3 particles in precipitation is investigated by simulating the industrial conditions in laboratory.
2 Experimental
Main apparatus for seed precipitation studying included a blade-paddle mixer tank and a Model LB-801 super constant temperature bath. The supersaturated sodium aluminate solutions with industrial concentration and initial gibbsite seed for all experiments were provided by Shandong Branch of China Aluminum Co., Ltd. Cycling experiments were conducted by simulating industrial conditions, and each precipitation period was 40 h. The proportion products of pecipitation act as the seeds for the next circle. After seeds were added, the precipitation commenced by stirring at 260 r/min and the temperature decreased uniformly in the desired ranges.
Solution samples (precipitate suspension) were determined by acid-base titration and complexometric titration methods. Obtained precipitated aluminum trihydroxide samples were washed with hot demonized water and dried at 60 ℃ for 24 h before observations with scanning electron microscope(LEQ-SUPRA35). The PSD and average size were measured by laser diffraction (Malvern Mastersizer 2000).
3 Results and discussion
3.1 Evolution of production particles in different particle sizes
As the main aluminium-bearing phase is diasporic bauxite ores, the alumina producing process is more complex. Moreover, seed size cycling has historically been an inherent feature of the circuit operation performance of the precipitation. An example of the amplitude and frequency of this cycling is shown in Fig.1.
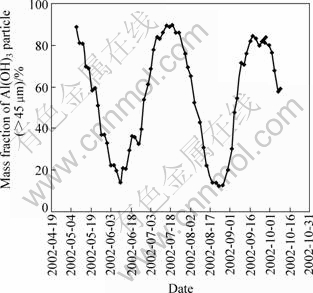
Fig.1 Variation of Al(OH)3 particle size (>45 μm) in precipitation process
To explore the rule of the seed size cycling, the circling precipitation processes were conducted by simulating the industrial conditions in laboratory, and the results are shown in Fig.2 and Fig.3. Fig.2 shows the evolution of number fraction of particle with different sizes, which indicates the variation of the PSD in different size ranges is unbalanced in precipitation. It is found that the number fraction of particles (<5 μm and <15 μm) is 79.89% and 96%, respectively, in the second precipitation, which indicates the nucleation explosions occur. Following cycling precipitation, the particles in different size ranges disappear in turn with similar variation tendency, and more and more coarse particles are produced. Another second nucleation explosion occurs while the number fractions of particles (<25 μm and <30 μm) are less than 0.1% and 10%, respectively.
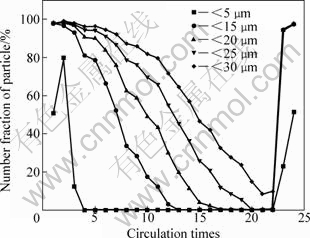
Fig.2 Evolution of different particle size distribution in precipitation
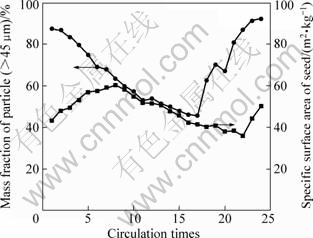
Fig.3 Variations of mass fraction of particle (>45 μm) and specific surface areas of seeds
Fig.3 shows the evolution of mass fraction of particle with size above 45 μm, and specific surface area of seed. It is found that particles (>45 μm) content has an opposite variation tendency with specific surface area. The seed specific surface area increases before the eighth precipitation, and decreases after that. At the 22nd precipitation, the specific surface area touches the bottom at 35.9 m2/kg, followed by the nucleation explosions. By comparing Fig.2 with Fig.3, it can be concluded that in the primary a few precipitation steps, fine particles (<5 μm= disappear quickly by agglomeration, while the particles (>15 μm) increase mainly depending on crystal growth. As we know, the growth rate is normally approximate 1 μm/d, so the mass fraction of particles (>45 μm) presents sinusoidal variation in precipitation. In the last a few precipitation steps, the seed size distribution is so coarse that the reduced seed specific surface area leads to lowered active centers and increased probability of nucleation explosions. The following nucleation explosions increase the fine particles content rapidly. As seen from Fig.4(a), each particle is an aggregate made up of elementary crystallites, and has rougher surface and larger surface area. In the SEM micrographs of products precipitated at different periods in Figs.4(b)-(d), it can be found that the particles exhibit mosaic structure and small specific surface area in seed coarsening period. However, lots of fine particles adhere to the surface of seed, as shown in Fig.4(d).
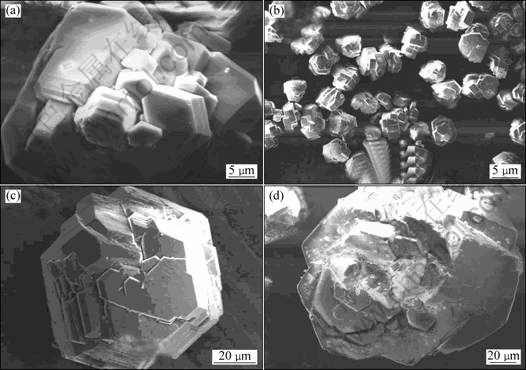
Fig.4 SEM morphologies of particles obtained at different precipitation times: (a) 3; (b), (c) 22; (d) 23
From above analysis, it can be concluded that, unbalance of PSD, where the particles become gradually larger, even induces seeds with smaller specific surface area, which is the major condition causing explosive attenuation of Al(OH)3 particles in precipitation.
3.2 Effect of solid content
Seed loadings of 500 g/L, 600 g/L and 700 g/L were used in precipitation experiments. The results are shown in Fig.5. It is found that increasing seed charge deteriorates the periodical attenuation of produced particle, e.g. with higher seed charge, the refining time becomes longer and fluctuation amplitude grows up. Similar to the evolution tendency of PSD, the result is opposite with low seed charge. In this case, the sodium aluminate liquors in three seed loadings have the same supersaturating degree; that is to say, they have same driving force for seeded precipitation. So, the transformation rate of seed surface area becomes slower, and time to nucleation explosion gets longer in precipitation with high solid content. In this case, nucleation explosions could bring about enormous fine particles, which need more aluminium tri-hydroxide decomposed from sodium aluminate solution and more time to transform into coarse particles. While in the low solid content case, the regular pattern is totally on the opposite.
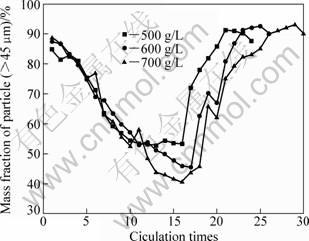
Fig.5 Evolution of particle size (>45 μm) distribution with different solid loadings
3.3 Effect of fine seed amount
The tests with adding fine seeds to precipitation process were carried out by simulating industrial conditions. The fine seeds were gotten by sieving industry gibbsite (provided by Shandong Branch of China Aluminum Co., Ltd) to 30 μm. The evolution of particles size (>45 ?m) distribution is displayed in Fig.6.
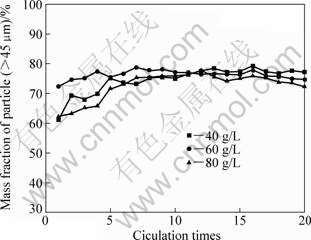
Fig.6 Evolution of particle size (>45 μm) distribution after adding fine seeds
It can be found that fluctuation amplitude of mass fraction of particles (>45 ?m) reduces markedly, from 76% to 60%. It is well known that in order to produce eligible sandy alumina with the minimum residue of 90%, aluminium hydroxide obtained from sodium aluminate solution must have a minimum residue of 92%-94%[14]. So, the production got by adding fine seeds cannot meet the standard of sandy alumina.
3.4 Effect of initial precipitation temperature
In this test, with the initial precipitation temperature changed, the variations of mass fraction of particle (>45 μm) in precipitation are displayed in Fig.7. It is found that higher initial precipitation temperature can prolong attenuation period and reduce fluctuation amplitude of product particles, but cannot eliminate the periodical attenuation. Meanwhile, the precipitation ratio of sodium aluminate solution decreases from 52% to 46%. The effect of temperature on seed precipitation shows two aspects. Lower precipitation temperature increases the precipitation ratio; however, if the seed surface area cannot adapt the supersaturating degree decreasing, the secondary nucleation that refines the size of production aluminium tri-hydroxide will occur. On the other hand, increasing the precipitation temperature will enhance the agglomeration and efficaciously restrain the second nucleation process, decreasing the precipitation ratio.
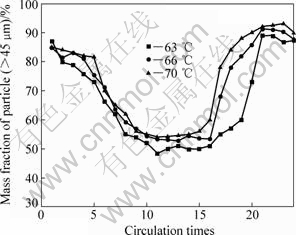
Fig.7 Evolution of particle size (>45 μm) distribution at different initial precipitation temperatures
3.5 Effect of additive
In resent years, numerous studies have focused on addition of surfactants into the sodium aluminate solution before the procedure of precipitation[15], so that the physicochemical properties of the solution are modified, resulting in shortened processing time, enhanced precipitation ratio, and increased crystal size of the product. In this work, the effect of additive on product particles was mainly studied. The adding concentration changes from 10 mg/L to 100 mg/L according to variation of product particles.
Fig.8 shows the variations of mass fraction of production particles (>45 μm) and liquor precipitation ratio. It is found that the amplitude of periodical fluctuation reduces. The mass fraction changes from 72.26% to 99.82%, and variation range decreases by 66% with product particle (>45 μm). The precipitation ratio varies from 47% to 52%. Based on the result above, it can be concluded that adding additives in precipitation process can reduce the fluctuating amplitude and shorten the attenuation time of the product particles. Moreover, it has no harm to precipitation ratio.
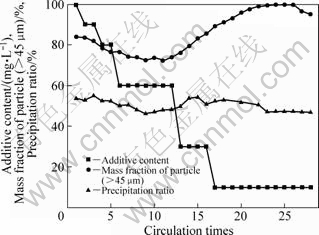
Fig.8 Variety of additive content, mass fraction of production particles (>45 μm) and precipitation ratio with additive
4 Conclusions
1) The precipitation temperature and seed charge have dramatic influence on the variation periods of particle (>45 μm), but have little effect on the varying regularity of particles. Unbalance of PSD, where the particles become gradually bigger, induces the decrease of specific surface area of seeds, which is the major reason causing explosive attenuation of Al(OH)3 particles.
2) The PSD of product becomes more uniform and fluctuation amplitude of product particles (>45 ?m) reduces markedly by adding fine seeds in precipitation process.
3) With additives, the mass fraction of product particle (>45 μm) changes from 72.26% to 99.82%, and amplitude of periodical fluctuation variation range decreases by 66%.
References
[1] CHESTER R, JONES F, LOAN M, OLIVEIRA A, RICHMOND W R. The dissolution behavior of titanium oxide phases in synthetic Bayer liquors at 90 ℃ [J]. Hydrometallurgy, 2009, 96(3): 215-222.
[2] WATLING H, LOH J, GATTER H. Gibbsite crystallization inhibition (1): Effects of sodium gluconate on nucleation, agglomeration and growth [J]. Hydrometallurgy, 2000, 55(3): 275-288.
[3] FARHADI F, BABAHEIDARY M B. Mechanism and estimation of Al(OH)3 crystal growth [J]. Journal of Crystal Growth, 2002, 234(4): 721-730.
[4] DASH B, TRIPATHY B C, BHATTACHARYA I N, DAS S C, MISHRA C R, PANI B S. Effect of temperature and alumina/caustic ratio on precipitation of boehmite in synthetic sodium aluminate liquor [J]. Hydrometallurgy, 2007, 88(1/4): 121-126.
[5] BROWN N. A quantitative study of new crystal formation in seeded caustic aluminate solution [J]. Journal of Crystal Growth, 1975, 29(3): 309-315.
[6] WANG Zhi, BI Shi-wen, YANG Yi-hong, YUANG Zhang-fu. Evolution of particle size and strength of hydrargillite from carbonization in seeded sodium aluminate liquors [J]. Journal of Crystal Growth, 2005, 274(1/2): 218-225.
[7] PAULAIME A M, SEYSSIECQ I, VEESLER S. The influence of organic additives on the crystallization and agglomeration of gibbsite [J]. Powder Technology, 2003, 130(1/3): 345-351.
[8] LI Hui-xin, ADDAI-MENSAH J, THOMAS J C, GERSON A R. The crystallization mechanism of Al(OH)3 from sodium aluminate solutions [J]. Journal of Crystal Growth, 2005, 279(3/4): 508-520.
[9] SEYSSIECQ I, VEESLER S, MANGIN D, KLEIN J P, BOISTELLE R. Modeling gibbsite agglomeration in a constant supersaturation crystallizer [J]. Chemical Engineering Science, 2000, 55(23): 55165-5578.
[10] CHEN Feng, ZHANG Bao-yan, BI Shi-wen, YANG Yi-hong. CHEN Yu-guo. Effect of additive on Al(OH)3 and Al2O3 made by seed precipitation from sodium aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(12): 2054-2059. (in Chinese)
[11] ZHANG Cun-bing, ZHAO Ping. Studied on polarizing particle size of aluminate hydroxide in Bayer process [J]. Light Metals, 1999(9): 17-19. (in Chinese)
[12] ZHANG Guang, YANG Jin-ni. Studied on particle size fluctuating of aluminate hydroxide in Bayer process [J]. Light Metals, 2002(9): 9-12. (in Chinese)
[13] GARNER B, CRISTOL B, SOIRAT A. Precipitation particle size control [J]. Light Metals, 1999: 71-76.
[14] SATAPATHY B K, PADHI T. Determination of grain-size distribution of sandy alumina using electron sensing zone method [J]. Light Metals, 1990: 185-191.
[15] HUNTER T K, MOODY G M, SANKEY S E. Advances with chemical additives for the alumina industry [J]. Light Metals, 1991: 159-162.
Foundation item: Project(50804031) supported by the National Natural Science Foundation of China
Corresponding author: WU Yu-sheng; Tel: +86-24-25497801; E-mail: henanwys@sina.com
DOI: 10.1016/S1003-6326(09)60173-4


