
Growth and corrosion behavior of rare earth film on hot-dip galvanized steel
LU Jin-tang(卢锦堂), WU Hai-jiang(吴海江),
KONG Gang(孔 纲), CHE Chun-shan(车淳山), XU Qiao-yu(许乔瑜)
College of Materials Science and Engineering, South China University of Technology,
Guangzhou 510640, China
Received 18 February 2006; accepted 17 July 2006
Abstract:
Hot-dip galvanized(HDG) steel sheets were treated for 30 s-24 h by the rare earth aqueous solution containing 20 g/L Ce(NO3)3?6H2O, and the growth behavior and corrosion resistance of the rare earth film were investigated by SEM, EDS, AES and NSS. The results reveal that the rare earth film becomes thick while the mass gain of the samples does not distinctly change due to the zinc dissolution with the increase of treatment time. The film grows up more quickly and is apt to cracking in the vicinity of zinc grain boundaries, and eventually the film partly warps and flakes off with increasing film thickness. The NSS results show that the corrosion resistance of the film is dominated by both the film thickness and the cracks. With increasing treatment time, the corrosion resistance of the film increases within 1 h due to the increased film thickness and decreases after 1 h because the cracking and flaking off gradually become dominant factor.
Key words:
rare earth film; hot dip galvanizing; corrosion resistance;
1 Introduction
For a long time, chromate compounds Cr(VI) have been used as effective and inexpensive corrosion inhibitors for zinc and zinc coating. However, with the advent of increasing environmental awareness, the toxicity and carcinogenic nature of Cr(VI) base treatment have made its use undesirable[1]. There- fore, new alternative and more environmentally friendly corrosion inhibitors or passivators need to be developed.
In the last decade, several researchers focused on the rare earth elements. They have demonstrated that the treatments with aqueous solutions of rare earth salts, especially cerium salts, effectively inhibited the zinc coating corrosion. The first research on the rare earth salts-based treatments was made by HINTON et al[2]. They found that cerium was effective in reducing the corrosion rate of metallic substrates by inhibiting the cathodic reaction. The mechanism of corrosion inhibition on zinc treated with cerium salts was also investigated by ARAMAKI[3-5], who reported the formation of a hydrated or hydroxylated Ce-rich layer that was constructed by adsorption on the hydroxylated zinc surface. This process leads to the formation of a Ce2O3 framework on the zinc surface, which suppresses the cathodic reactions.
However, most of the researches were based on aluminum alloy[6-10], zinc[2-5] and electroplated zinc [2,11-13], only few works were reported on hot dip galvanized(HDG) coating[14]. Among these rare earth salts, cerium nitrate is considered to be environmentally benign. In this paper, the cerium nitrate solution treatment for hot dip galvanized steel was studied, the growth characteristic, chemical composition and corrosion resistance of the rare earth film were investigated.
2 Experimental
Q235 steel sheets (40 mm×30 mm×2 mm) were degreased, pickled, fluxed, dried and dipped in a 10 kg molten zinc bath in graphite crucible in an electric furnace at 450 ℃ for 1 min, withdrawn slowly, cooled in air for zinc freezing and immediately quenched in water. The thickness of each galvanized coating, which was measured by STH-1 pachometer, was about 50 μm of which it was composed of 30 μm Fe-Zn alloy layer and 20 μm free zinc layer.
After the zinc coatings were applied, the galvanized samples were immersed in the rare earth solution at room temperature for different time from 30 s to 24 h, then dried in air at room temperature. The rare earth solution contained 20 g/L Ce(NO3)3?6H2O, 20 mL/L H2O2 and a few additives and had a pH value of 3.5.
The mass gains of the samples were weighed by an analytical balance with a precision of 0.1 mg. Surface morphology of the films was observed by a LEO 1530 VP scanning electron microscope(SEM). The chemical composition of the films was analyzed by an Oxford Inca 300 energy dispersive X-ray spectrometer(EDS). The distribution of the elements in the films was analyzed by Auger electron spectroscopy(AES) using a Perkin-Elmer PHI-550 ESCA/SAM electron spectrometer. The neutral salt spray test(NSS) was carried out in accordance with ISO 3768 (Metallic coatings-Neutral salt spray test).
3 Results3.1 Appearance of rare earth film and mass gain of sample
For the sample treated for less than 5 min, its appearance does not change and still keeps bright white metallic luster of zinc coating. When the treatment time is increased to 10 min, the film color is light yellow luster. After treated for 20 min, the film color gradually changes to golden yellow luster. Obviously, the rare earth film grows with the increase of the treatment time.
The test of the mass gain shows that the sample treated for 30 s has the little mass gain, however, the mass gain does not distinctly change with the increase of the treatment time (Fig.1).
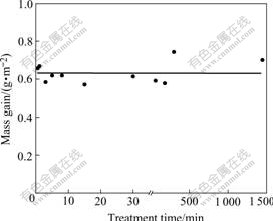
Fig. 1 Effect of treatment time on mass gain of sample
3.2 Surface morphology and chemical composition of rare earth film
The SEM observation with low magnification shows that the sample treated for less than 10 min does not obviously change compared with the untreated sample, and the zinc grain boundaries appear on the surface (Fig.2(a)). After treated for 10-20 min, the local film in the vicinity of grain boundaries is cracked and warped (Fig.2(b)). The observation with high magnification shows that on the surface of the sample treated for 10 min the local initial cracks and warps appear (Fig.2(c)). With increasing treatment time, the film cracks and warps seriously and the film flaks off obviously (Figs.2(d) and (e)).
The EDS results for samples treated for less than 10 min show that the content of Ce and O increases with increasing treatment time while the content of Ce and O on the grain boundaries is higher than that inside the grain, as shown in Fig.3. Since the thickness of the rare earth film might be less than the depth of the composition area that EDS data can report, especially for the shorter treatment time, these data cannot be the actual composition of the film, but they can indicate the thickness change of the films.
The EDS results of the samples treated for longer time (20-30 min) are listed in Table 1. The contents of Ce and O on the intact and warped area increase with increasing treatment time while the contents of Ce and O on the warped area are the highest for the same sample. The EDS data reported are the average composition of the whole warped film for warped area while it might be the composition containing some zinc substrate for unwarped area. The content of Ce on the area where the film has flaked off is less, which is resulted from the phenomenon that the surface layer containing more Ce has flaked off and the thinner rare earth film joining zinc substrate remained.
Fig.4(a) shows the AES spectrum of the rare earth film treated for 5 min. The AES depth profile of major elements in the rare earth film is illustrated in Fig.4(b). From Figs.4(a) and (b), it can be seen that the film contains the elements of O, Ce, Zn and C. From very small distance below the surface, the contents of O, Ce and Zn distribute along concentration gradient. The profile shows that the Ce and O contents are higher on the surface and decrease gradually along the depth direc-
Table 1 EDS results of rare earth film treated for different time (mole fraction, %)
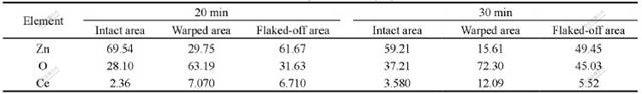

Fig.2 Surface morphologies of sample treated for 1 min(a), 10 min(c), 20 min((b, d)) and 30 min(e)
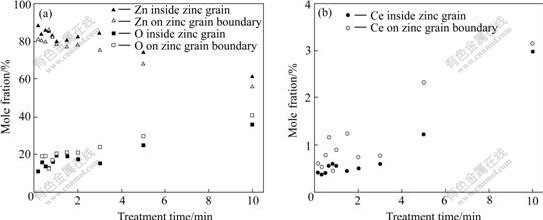
Fig.3 EDS results of rare earth film inside zinc grain and on zinc grain boundary after different treatment time
tion of the film, while the zinc content is lower on the surface and increases gradually along the depth direction of the film. The signal of C appears on the surface slightly, which may result from the slight contamination or the basic zinc carbonate generated on the sample surface, which usually appears on the surface of zinc coating exposed in atmosphere[15]. According to the rate and the time of argon ion sputtering, the thickness of the film is within the range from 50 to 100 nm.
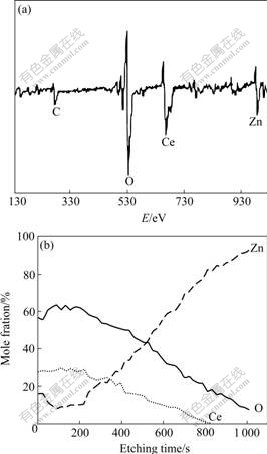
Fig.4 AES spectrum(a) and AES composition depth profile curves(b) of rare earth film after 5 min treatment
3.3 Corrosion test
The results of NSS are summarized in Fig.5. After being sprayed for 2 h, the corroded area of HDG sample is up to 75%. Moreover, after being sprayed for exceeding 8 h, the HDG sample is corroded seriously and entirely. However, the formation of the white rust is remarkably delayed after HDG samples are treated in rare earth aqueous solution. The corrosion resistance of the film initially increases and then decreases with the increase of treatment time. Between 30 min and 1 h, the corrosion resistance of the film is the optimum, but the corrosion resistance changes badly if the treatment time exceeds 1 h.
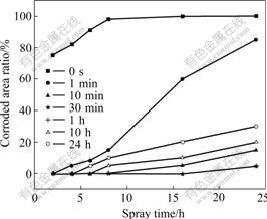
Fig.5 Results of NSS test of hot dip galvanized steel treated for different time
4 Discussion
Generally, when immersing HDG steel in the Ce(NO3)3 aqueous solution, the anodic reaction (zinc dissolution) and the cathodic reaction (oxygen reduction and OH- generate) simultaneously occur on the surface. The production of alkaline conditions in the cathodic areas due to oxygen reduction leads to precipitation of Ce(OH)3 and Zn(OH)2, eventually the rare earth film is formed on the zinc surface[2-5,13]. The results show that the thickness of the film increases with the increase of treatment time, but the mass gain of the samples is little changed, which means that some zinc are dissolved in the solution.
For HDG coating, there exists apparent wave and there are a lot of lattice defects and high impurity concentrations near zinc grain boundaries, where the reactivity is higher than inside grain and the film grows up more quickly[16]. The high Ce and O concentration determined by EDS verifies this phenomenon. When the thickness of the film increases to a certain extent, the cracks will occur on the surface of the film in the vicinity of zinc grain boundaries. Because the film observed by SEM is only the dried film, it is not determined whether the film cracks in the growth process or in the dry process, but it is sure that the necessary condition of the film cracking is sufficient film thickness and certain Ce and O contents. The cracking of the film has something to do with internal stress produced in the growth process and/or tensile stress produced in the dry process due to dehydration and contraction.
The rare earth film is a layer in which the concentrations of O, Ce and Zn change continuously and gradually and there is not a distinct interface between the film and zinc substrate, and the thickness of the film increases with the increase of treatment time. Moreover, the characteristic of this kind of continuous gradual change does not alter with increasing treating time[2, 14]. According to the above results and analyses, a growth and cracking model of cerium film on hot dip galvanized coating is put forward according to the above experimental results, as shown in Fig.6. The concentration change of Ce and O is represented by depth of color.
An apparent characteristic of the film is that it does not flake off by entire film. For thick film cracking, the warped part is only the exterior surface Ce-rich layer on the film surface. After this layer flakes off, there are considerable Ce film below it. An important factor that affects the corrosion resistance of the film is its thickness. With less than 10 min treatment, the corrosion resistance of the film also increases with increasing film thickness and treatment time. However, after 10 min treatment, the film thickness continuously increases, but the corrosion resistance of the film is not obviously changed because of the local cracking of the film. After 1 h, the cracking and flaking off gradually become dominant factor. The corrosion resistance of the film decreases with the increase of treatment time. However, the film that flakes off is only the little part on the film surface, so its corrosion resistance is better than that of the film treated for 1-10 min.
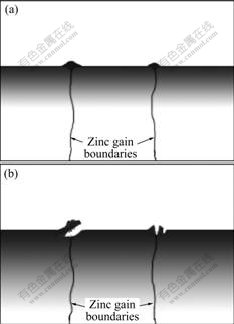
Fig.6 Schematic diagrams of growth and cracking model of cerium film on hot dip galvanized coating: (a) Faster growth in vicinity of zinc grain boundaries; (b) Cracking and flaking off in vicinity of zinc grain boundaries
5 Conclusions
1) The rare earth films on hot dip galvanized steel sheets are obtained by simple immersion in a cerium nitrate aqueous solution.
2) The thickness of the film increases with the increase of treatment time, but the mass gain of the film does not distinctly change.
3) The film grows up more quickly in the vicinity of zinc grain boundaries and is apt to cracking, and eventually the film partly warps and flakes off with increasing film thickness.
4) The corrosion resistance of the film is dominated by the film thickness and the cracks. It initially increases and then decreases with the increase of treatment time.
References[1] EICHINGER E, OSBORNE J, CLEAVE T V. Hexavalent chromium elimination: an aerospace industry progress report [J]. Metal Finishing, 1997, 95(3): 36-41.
[2] HINTON B R W, WILSON L. The corrosion inhibition of zinc with cerous chloride [J]. Corrosion Science, 1989, 29(8): 967-985.
[3] ARAMAKI K. The inhibition effects of cation inhibitors on corrosion of zinc in aerated 0.5 M NaCl [J]. Corrosion Science, 2001, 43(8): 1573-1588.
[4] ARAMAKI K. Treatment of zinc surface with cerium(III) nitrate to prevent zinc corrosion in aerated 0.5 M NaCl solution [J]. Corrosion Science, 2001, 43(11): 2201-2215.
[5] ARAMAKI K. A self-healing protective film prepared on zinc by treatment in a Ce(NO3)3 solution and modification with Ce(NO3)3 [J]. Corrosion Science, 2005, 47(5): 1285-1298.
[6] DABALA M, ARMELAO L, BUCHBERGER A, CALLIARI I. Cerium-based conversion layers on aluminium alloys [J]. Applied Surface Science, 2001, 172(3/4): 312-322.
[7] BETHENCOURT M, BOTANA F J, CANO M J, MARCOS M. High protective, environmental friendly and short-time developed conversion coatings for aluminium alloys [J]. Applied Surface Science, 2002, 189(1/2): 162-173.
[8] BETHENCOURT M, BOTANA F J, CANO M J, MARCOS M. Advanced generation of green conversion coatings for aluminium alloys [J]. Applied Surface Science, 2004, 238(1-4) : 278-281.
[9] CAMPESTRINI P, TERRYN H, HOVESTAD A, de WIT J H W. Formation of a cerium-based conversion coating on AA2024: relationship with the microstructure [J]. Surface Coatings and Technology, 2004, 176(3): 365-381.
[10] YU Xing-wen, ZHOU De-rui, YIN Zhong-da, ZHOU Yu-hong. Rare earth metal conversion coating on aluminum 2024 [J]. The Chinese Journal of Nonferrous Metals, 1999, 9(1): 73-78. (in Chinese)
[11] SHOJI H, SAKASHITA M. Surface treated metallic materials with corrosion resistance and surface treatment used therefore [P]. Japan WO28291, 1996.
[12] ROMAN L, BLIDARIU M, CRISTESCU C. Study of conversion coating on zinc deposition obtained from low pollution solutions [J]. Trans IMF, 1997, 75(5): 171-174.
[13] MOTTE C, MAURY N, B, OLIVIER M G, PETITJEAN P J, WILLEM F J. Cerium treatments for temporary protection of electroplated steel [J]. Surface Coatings and Technology, 2005, 26(10): 2366-2375.
[14] MONTEMOR M F, SIM?ES A M, FERREIRA M G S. Composition and behaviour of cerium films on galvanised steel [J]. Progress in Organic Coatings, 2001, 43(4): 274-281.
[15] DARYL E T. Metals handbook [M]. Vol.5, 9th edition. Ohio: AMS, 1982: 232-332.
[16] LU Jin-tang, KONG Gang, CHEN Jin-hong, XU Qian-yu, SUI Run-zhou. Growth and corrosion behavior of molybdate passivation film on hot dip galvanized steel [J]. Trans Nonferrous Met Soc China, 2003, 13(1): 145-148.
(Edited by LI Xiang-qun)
Corresponding author: LU Jin-tang; Tel: +86-20-85511540; E-mail: mcjtlu@scut.edu.cn


