DOI: 10.11817/j.issn.1672-7207.2020.12.001
废旧锂离子电池的湿法回收研究进展
杨健,秦吉涛,李芳成,蒋良兴,赖延清,刘芳洋,贾明
(中南大学 冶金与环境学院,湖南长沙,410083)
摘 要:
快速更新换代和动力汽车的飞速发展,产生了越来越多的废旧锂离子电池。废旧锂离子电池中含有的大量有毒有害物质,会对环境和人类健康产生严重危害,此外,废旧锂离子电池中含有丰富有价金属,可作为重要的二次资源,因此,废旧锂离子电池的回收已成为全球关注的热点。湿法冶金过程被认为是低能耗、低成本、低污染以及更适合规模化应用的废旧离子电池回收技术。湿法冶金回收过程包括电池预处理、有价金属浸出以及高附加值产品回收。本文对采用湿法冶金技术回收废旧锂离子电池中有价金属的研究现状进行综述,对比分析各个步骤中不同处理技术之间的优劣,提出当前湿法冶金回收过程中存在的问题,并对湿法冶金技术回收废旧锂离子电池发展方向进行展望。
关键词:
中图分类号:TF813 文献标志码:A
文章编号:1672-7207(2020)12-3261-18
Review of hydrometallurgical processes for recycling spent lithium-ion batteries
YANG Jian, QIN Jitao, LI Fangcheng, JIANG Liangxing, LAI Yanqing, LIU Fangyang, JIA Ming
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: The amount of spent lithium-ion batteries has grown dramatically in recent years with the rapid development of electronic products and the rapid development of power vehicles. On the one hand, a large amount of toxic and harmful substances contained in spent lithium-ion batteries will pose a threat to the environment and human health, and on the other hand, the rich valuable metals contained in spent lithium-ion batteries can be used as an important secondary resources, and therefore, the recycling of spent lithium-ion batteries has become a global hotspot. The hydrometallurgical process was considered to be the most suitable method for the recycling of spent lithium-ion batteries. A series of hydrometallurgical procedures include pretreatment of the spent lithium-ion batteries, leaching process and separation of valuable metals from leaching solution. The current status of hydrometallurgical recycling technologies of spent lithium-ion batteries was reviewed in this paper. And then the advantages and problems of different recyding technologies were analyzed. Finally, the prospects and direction of the recycling of spent lithium-ion batteries were put forward.
Key words: spent lithium ion batteries; recycling; hydrometallurgical process; prospects
与铅酸电池、镍镉电池、镍氢电池等二次电池相比,锂离子电池具有能量密度高、储存寿命长、体积小、质量小、自放电效率低和无记忆效应等优点,自1991年被索尼公司引入市场以来,已越来越广泛地应用于便携式电子设备和电动汽车领域[1-3]。据统计,中国锂离子电池的保有量从2016年的78.4亿只快速增长到2019年的150亿只以上[4]。然而,便携式电子设备中锂离子电池的生命周期一般为3~5 a,车用动力锂离子电池的生命周期一般为5~8 a[5]。随着便携式电子设备的快速更新换代以及电动汽车的大力推广,近年来产生了大量的废旧锂离子电池,预计到2025年,中国动力锂离子电池的报废量将超过50万t[6],如图1所示。因此,如何合理处置废旧锂离子电池已成为全球关注的热点。
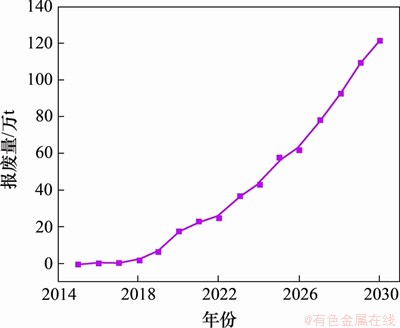
图1 中国锂离子动力电池现有报废量及预测增长量
Fig. 1 Current scrap quantity and forecast growth of lithium ion power battery in China
锂离子电池通常由正极、负极、电解质和隔膜等组成。其中,负极由石墨、黏结剂、导电剂和集流体铜箔组成,正极由活性物质粉末、黏结剂以及导电剂涂覆在集流体铝箔上制成。当前,正极活性物质粉末主要有LiCoO2,LiNiO2,LiMnO2,LiFePO4以及LiNixCoyMn1-x-yO2等[7]。隔膜的主要成分聚丙烯(PP)或聚乙烯(PE)等在自然界难以降解[5]。电解质分为固态电解质和液态电解质,当前商用锂离子电池使用的电解质多数为LiPF6液态有机电解质,其暴露在潮湿的空气中会生成极具危害的HF [8]。据报道,4 000 t废旧锂离子电池中包含1 100 t重金属以及200 t以上有毒电解质[2]。一方面,若只采用简单的掩埋或焚烧处理,则会对环境和人类健康构成严重威胁;另一方面,废旧锂离子电池包含5%~20%(质量分数,下同)的钴,5%~10%的镍,5%~7%的锂和5%~10%的其他金属(铜、铝、铁等),其中一些金属的质量分数远超其在自然矿物中的质量分数[2,9],可成为一种重要的二次资源,若对其中有价成分进行回收处理,将会产生可观的经济效益。据估计,2020年中国市场的废旧锂离子电池回收总经济效益将超过150亿元[10]。因此,非常有必要对废旧锂离子电池进行回收,以促进整个产业的绿色可持续发展。
当前,已经报道的废旧锂离子电池回收技术可概括为火法冶金和湿法冶金两大类。典型的火法冶金过程包括电池的还原焙烧、造渣以及合金的熔炼和精炼。为实现废旧锂离子电池中有价金属的高效回收利用,火法冶金过程通常与湿法冶金过程相结合。例如,优美科公司首先对废旧锂离子电池进行还原熔炼获得Co-Ni-Cu-Fe合金,然后,通过湿法冶金的方式得到高纯的单一金属和化合物[11]。火法冶金过程具有工艺流程短、设备要求低以及可操作性强等优势,但同时也具有一些无可避免的缺陷,如能耗高、环境污染大、产品纯度低等。此外,锂和铝的还原活性强,导致在还原熔炼过程中难以回收而随着造渣剂进入渣相中,需进一步处理[12]。湿法冶金回收工艺具有金属综合回收率高、产品纯度高、能耗低以及环境污染小等优势,被认为是更加高效的废旧锂离子电池回收工艺[13]。
废旧锂离子电池湿法冶金回收过程主要包括预处理、活性物质浸出以及产品回收过程,其工艺如图2所示。本文对各回收过程当前的发展现状进行综述,并对相关发展方向进行展望。
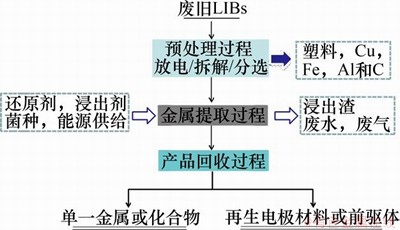
图2 废旧LIBs湿法冶金过程主体工艺流程图
Fig. 2 Main flow chart of spent LIBs hydrometallurgical recovery process
1 预处理过程
锂离子电池结构复杂,由外壳、隔膜、正极、负极等多个部件组成,有必要通过一系列方法使其不同部件分离。废旧锂离子电池通常存在一定的残余电压,若不进行适当处理,会容易引起自燃和爆炸,进而威胁操作人员的安全。基于此,废旧锂离子电池的预处理过程通常包括预放电、电池的粉碎和拆解以及集流体的脱附,如图3所示。
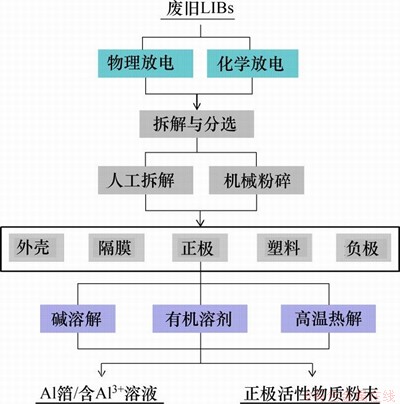
图3 预处理流程图
Fig. 3 Flow chart of pretreatment process
1.1 放电
废旧锂电池的放电方式按照放电过程中有无电子得失可分为物理放电和化学放电[14],其中,物理放电包括强制手段放电和短路放电。早期,美国Umicore和Toxco公司采用液氮对废旧锂电池进行低温(-198 ℃)处理后强制穿孔放电,但这种方法对设备要求高,经济适用性低,已逐渐被摒弃。当前对废旧锂离子电池物理放电方法研究较多的是将废旧锂离子电池埋没于导电粉末中,造成电池正负极之间短路而逐渐消耗残余电量。基于经济性和易获取性,导电粉末通常使用金属铜粉和石墨粉[15-17]。物理放电方法具有对电池外壳无破坏、操作简单且放电过程中无废水和废气产生等优势,但在放电过程中会产生大量热量,需要额外的散热装置。此外,采用物理放电方法时,放电速率往往比较慢且放电效果也不太理想,对于部分残压高的废旧锂离子,电池放电周期将大大延长,安全隐患较大。
化学放电是指在将电池浸泡于导电盐溶液中释放电池残余电量,其原理是基于正负极发生的氧化还原反应来逐渐消耗电池残余的电量。当前报道的导电盐溶液有NaCl溶液[18-20]、Na2SO4溶液[21]、MnSO4溶液[22]以及FeSO4溶液[23]等。其中,使用报道最多的是质量分数为5%~20% NaCl溶液,如LI等[24]研究了NaCl溶液浓度和放电时间对废旧LIBs放电效率的影响,结果表明,在质量分数为10%的NaCl溶液中浸泡6 h后,LIBs的残余电量可降为初始的30%左右,达到安全拆解的要求。由于Cl-的腐蚀性,在放电过程中会腐蚀电池外壳,使电解液LiPF6泄露进而与水发生反应生成HF(见反应(1))[25],易造成二次环境污染。为了克服这一问题,宋秀玲等[22]利用抗坏血酸的酸性、还原性及稳定性构建了化学性质相对温和的硫酸盐溶液放电体系,确定最佳放电条件为:0.8 mol/L MnSO4,2 g/L抗坏血酸,pH=2.78,放电时间8 h。在此最佳放电条件下,最终电池残压降至0.54 V,满足绿色高效放电的要求。

与物理放电方法相比,化学放电方法具有放电效率高、周期短的优势,更加有利于大规模应用。尽管采用性质温和的放电盐溶液体系可以减弱这一现象,但在放电过程中可能仍会产生有害气体,造成二次环境污染,且放电后需要进行进一步处理。基于此,可以采用物理方法和化学方法相结合的放电体系,如在低浓度的盐溶液中加入密度相近的固体导电粉末可能会大幅度减弱放电过程中产生的二次环境污染。此外,在放电过程中,可以施加辅助手段如超声或外加磁场也有可能提高放电效率。
1.2 电池的拆解
废旧锂离子电池放电完成后需对电池进行拆解与分选,即通过人工或机械的方式分选出电池的各个部件。其中,人工拆解是在安全防护下借助工具先将废电池的外壳移除得到电池卷芯,然后将电池卷芯手动分离得到正极、负极以及有机隔膜。人工拆解方式可以实现废旧锂离子电池各个组件的全分离。但传统消费类锂电池尺寸较小且数量巨大,导致人工拆解效率低,工作强度大,再加上对人身健康会产生损害等,导致其不适合大规模使用。
与人工拆解相比,机械处理可实现大规模作业且成本较低,表现出更加优越的经济适用性和更广阔的工业应用前景[26-27]。机械拆分通过电池的破碎、筛分及分选等工序得到较粗颗粒的外壳、集流体碎屑以及细颗粒的正极粉末和负极碳粉的混合组分。WANG等[28]采用机械处理过程获得LiCoO2活性物质粉末(品位>95%)的回收率为80%左右。BI等[29]以LiFePO4为例,提出电分选和磁力浮选相结合的方式分离电池破碎颗粒中的不同组分,在转速800 r/min下,铜箔碎屑和铝箔碎屑之间的分离效率达到85%以上。尽管通过机械破碎分选方法可以实现废旧电池不同组分颗粒之间的分离,但由于电池组分复杂,在机械处理过程中相互干扰,不可能实现各个组分的完全分离。SHIN等[9]对废旧LiCoO2电池放电后通过控制破碎粒径筛分得到不同粒径范围的碎屑,对这些碎屑成分进行分析得出Fe和Al的含量随着碎屑粒径降低而降低,而Co的含量呈现出相反的变化趋势。
在机械破碎分选过程中,为了提高活性物质粉末的回收率,必须通过2次甚至3次破碎降低碎屑粒径的方式实现,但这样势必会增大分选难度,降低分离组分的回收品位。单一的机械破碎分选难以兼顾活性物质回收率和回收品位,因此,通过其他的技术手段使活性物质粉末从集流体脱附显得尤为重要。
1.3 电极粉末的脱附
正极粉末是通过黏结剂的作用涂覆碾压在集流体铝箔上。要实现正极粉末与集流体的高效分离,可以从2方面入手:一是破坏集流体铝箔,使活性物质失去承载的对象;二是破坏黏结剂使之结构发生破坏进而失去黏结的作用。
1.3.1 破坏集流体铝箔
根据金属铝可以溶解在碱性溶液中的特性,将正极卷芯浸泡在碱性溶液中可以达到正极粉末与集流体分离的目的。王洪彩[30]研究了NaOH浓度、碱液温度以及浸没时间对正极粉末脱附率的影响,发现在1.5 mol/L NaOH,NaOH与铝箔质量比为2.5:1.0,反应时间为15 min时,可在室温下实现集流体铝箔的完全溶解。相似地,NAYL等[31]利用NH4OH作为碱溶液溶解集流体铝箔和铜箔,在NH4OH浓度为4.0 mol/L,液固质量比为15:1,溶解温度为60 ℃,反应时间为60 min条件下,获得Al的溶出率为97.8%,铜溶出率为64.7%。破坏集流体铝箔的方法具有能耗低、操作性强等优势,但集流体铝箔以离子的形式进入溶液中,需要进一步进行回收处理。此外,该过程需要大量的碱溶液,为防止碱液产生二次污染,需要进行中和处理,这样将需要额外的成本开销。由于碱溶解过程中黏结剂并没有被破坏,过滤烘干得到的活性物质仍然是粒径较大的黏聚体,不利于后续有价金属的浸出,且为避免引入的碱液对粉料产生污染,在过滤过程中,要对脱附活性物质进行充分冲洗或酸中和。
1.3.2 破坏黏结剂
当前使用的黏结剂主要为聚偏二氟乙烯(PVDF)和聚四氟乙烯(PTEE)[32-33],通过溶解黏结剂或者破坏黏结剂的结构使之失活也可以达到电极粉末脱附的目的,且集流体铝箔可以固体的形式得到回收。
根据“相似相容”原理,采用有机溶剂可以溶解黏结剂PVDF,目前报道的有机溶剂主要有:N-甲基吡咯烷酮(NMP)[34-35];N,N二甲基乙酰胺(DMAC)[36];N,N-二甲基甲酰胺(DMF)[37]和二甲基亚砜(DMSO)[38]等。HE等[39]对比了NMP,DMAC,DMF以及DMSO等有机溶剂对正极粉末脱附率的影响,结果表明NMP具有最大的PVDF溶出率,正极粉末的脱附率达99%。此外,还发现采用超声辅助溶解可使正极粉末的脱附率大幅度提升,且溶解完全后使用过的NMP等有机溶剂可以通过减压蒸馏再生实现重复利用,而铝箔以金属Al的形式回收。虽然有机溶剂溶解法具有上述优势,但有机溶剂价格通常昂贵,不太适合大规模工业应用,且有机溶剂并不能完全溶解PVDF,得到的正极粉末是粒径较大的黏聚体,不利于后续浸出。此外,有机溶剂溶解并不适合分离所有类型的黏接剂,例如,当以锂离子电池中使用的黏结剂为PTEE时,NMP等有机溶剂的溶解能力可以忽略不计[40]。
从破坏黏结剂的结构入手是当前分离集流体铝箔的主要研究方法,以离子液体为介质或者于空气中直接加热到特定温度可以使黏结剂失活以达到分离集流体铝箔的目的。ZENG等[41]利用离子液体1-丁基-3-甲基咪唑鎓四氟硼酸酯([BMIm][BF4])为介质,采用油浴的方式进行加热,在185 ℃下保温25 min,正极粉末的脱附率超过99%。WANG等[42]以胆碱氯化物-甘油共溶体系与集流体共热实现了活性物质粉末的脱附,其原理是利用共热过程中胆碱氯化物中的碱性官能团对PVDF中的酸性氢原子进行攻击,使PVDF失活。在190 ℃下共热15 min,活性物质的脱附率达到99.8%。以离子液体作为加热介质,可以在低于黏结剂的分解温度下对黏结剂的结构产生破坏,使之失活,但在处理过程中需要使用价格昂贵的离子液体,且离子液体难以回收再利用,也不适合大规模的工业应用。
将正极集流体置于马弗炉中加热到PVDF或PTEE分解温度之上,使黏结剂分解失活是当前最广泛采用的分离集流体方法。CHEN等[43]研究了热解温度和时间对正极粉末脱附率的影响,结果表明,在热解温度550 ℃下保温处理2 h可实现正极粉末的高效脱附;在热解过程中,黏结剂和导电剂石墨在高温下逐渐分解燃烧,通过筛分后可以得到细颗粒的正极活性物质粉末。此外,正极粉末在高温处理过程中层状结构被破坏,生成少量Co3O4,这对后续浸出过程有利。然而,热解过程通常需要较高温度,且黏结剂和有机电解质在高温热解过程中会分解产生HF等有毒有害的气体,需要配备额外的尾气净化处理装置[4]。为了克服这一问题,WANG等[44]采用CaO辅助热解实现了正极粉末的低温脱附,大大降低了热解过程中的能耗,且热解过程中产生的有害气体HF可以被CaO原位吸收生成CaF2,实现无害化处理。在焙烧温度为300 ℃,CaO与集流体的质量比为8:1,保温时间为30 min时,正极粉末的脱附率达到97%以上。
2 浸出过程
浸出是指将获得的正极活性物质粉末通过湿法冶金的方法使其中的有价金属由氧化物态转变为水溶性的离子态,得到富集金属离子(Li+,Ni2+,Co2+和Mn2+等)的浸出液。其中,浸出过程是整个湿法冶金回收废旧锂离子电池中有价金属元素的关键步骤。综合相关文献报道,正极粉末的浸出按浸出剂和浸出方法的不同可分为无机酸浸出、有机酸浸出、氨浸出以及生物浸出。此外,采用辅助措施如机械化学方法、超声以及电场可显著强化有价金属元素的浸出过程。总的浸出过程技术路线如图4所示。
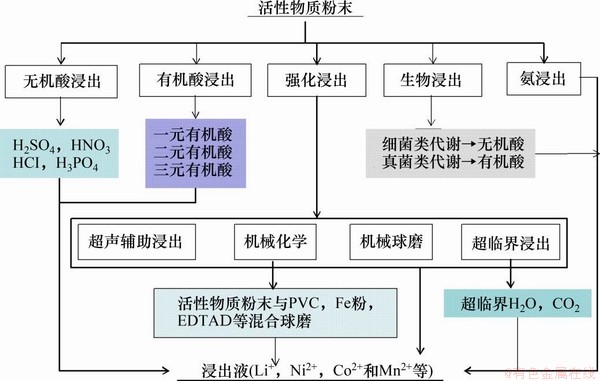
图4 浸出过程工艺技术路线
Fig. 4 Technological route of leaching process
2.1 无机酸浸出
无机酸如H2SO4,HCl,HNO3和H3PO4是正极粉末浸出常用的浸出剂。由于正极材料中钴和锰的化学价为+3和+4价,根据Ni-Co-Mn-H2O的电位E-pH图可知[45],高价态的Co和Mn化合物无法以离子的形态在水溶液中存在。因此,在浸出过程中,往往要添加还原剂将Co和Mn从高价氧化物相还原成易溶于水的低价态Co2+和Mn2+,以提高它们的浸出率。由于Cl-具有还原性,可以将Co或Mn从高价态还原为低价态,以HCl作为浸出剂,浸出率较高[46-47],但在浸出过程中会有Cl2生成(以LiCoO2为例,反应如式(2)所示),这不仅会恶化操作环境,而且需要专用的防腐设备,从而增加了回收成本。

由于SO42-,NO3-和PO43-不具备Cl-的还原能力,以HNO3,H3PO4和H2SO4作为浸出剂时需要添加还原剂以提升活性物质粉末的浸出率。当前报道的还原剂可分为无机还原剂和有机质还原剂,其中,无机还原剂有H2O2[48-50],Na2SO3[51],NaHSO3[52]和Na2S2O5[53]等;有机质还原剂有葡萄糖[54]、淀粉[55]、抗坏血酸[56]等。当前使用最多的还原剂是H2O2。以LiCoO2为例,当使用H2O2作为还原剂时,反应如下:
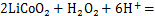

GAO等[57]对比了添加还原剂H2O2前后金属元素浸出率变化,并从浸出动力学角度对其机理进行了解释,结果表明:当添加体积分数6%的H2O2时,在同等条件下,Ni,Co和Mn的浸出率由不加还原剂时的30%左右提升至97%以上;当存在还原剂时,高价态的Co和Mn被还原成更易溶解的Co2+以及Mn2+,使镍钴锰的浸出表观活化能分别由未添加还原剂时的41.52,41.64和41.16 kJ/mol降至37.17,39.38和38.74 kJ/mol,从而使金属元素的浸出率显著提高。
与H2O2相比,有机质还原剂具有价格低、易存储以及易获取的优势被研究者广泛关注[58]。MENG等[54]以H3PO4作为浸出剂,葡萄糖作为还原剂从LiCoO2中浸出有价金属,在优化条件下,Li和Co的浸出率分别达到98%和99%。通过对葡萄糖在浸出过程中的行为进行研究,发现其首先被氧化成葡萄糖酸,随后继续被氧化成短链酸如乳酸、醋酸和甲酸等,这些短链酸最终被氧化成CO2和H2O。类似地,赖延清等[55]提出了H2SO4+淀粉的浸出体系,在优化条件下,超过97%的Li,Ni及Co被浸出,超过92%的Mn被浸出。Mn浸出率较其他金属浸出率稍低的原因是Mn2+在温度较高的水溶液中容易被氧化成MnO2或Mn2O3重新进入渣相中。但以有机质作为浸出还原剂,在浸出结束时不可避免地有一些有机质残留在浸出液中,对正极材料的再生产生不利影响。
2.2 有机酸浸出
以无机酸作为浸出剂,在还原剂辅助下,可获得较高的有价金属元素浸出率。但无机酸多数属于中强酸,腐蚀性强,对浸出设备要求较高。其次,在浸出过程中会产生一些有害气体如Cl2,SO3以及NOx等[59-60],恶化操作环境,需要气体收集及净化装置,增大环保压力。与无机酸相比,有机酸大多属于弱酸,对浸出设备的要求低,且有机酸可自然降解,不易造成二次污染。此外,多数有机酸都有一定的还原能力,如草酸、柠檬酸以及抗坏血酸表现出较强的还原特性,作为浸出剂可促进高价金属氧化态还原为低价态。基于有机酸的以上特性,研究者试图用有机酸替代无机酸作为废旧锂离子电池浸出剂。
CHEN等[61]以柠檬酸作为浸出剂,葡萄糖作为还原剂用于LiCoO2的浸出,在最佳条件下,可使Li和Co的浸出率超过98%。ZENG等[62]以草酸作为浸出剂,用于LiCoO2浸出,在浸出温度为95 ℃,浸出时间为150 min,固液比为15 g/L,转速为400 r/min下,超过98%的Li和97%的Co被浸出。在加入H2O2时,金属元素的浸出率并没有明显变化,这是因为在浸出过程中,草酸本身也可作为还原剂(见下式)。

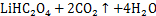
一方面,有机酸大多属于弱酸,在水溶液不能完全电离,造成浸出溶液中自由的H+浓度低。即使添加足够的还原剂H2O2,有机酸也不可能与正极活性物质充分反应,导致浸出速率较慢,且在保证金属浸出率的同时只能选取低的浸出固液比,这又导致整个浸出过程的活性物质的处理能力大大降低。另一方面,有机酸通常价格昂贵,且大多数有机酸相对分子质量较大,会增大整个浸出过程中酸的添加质量,从而使成本大大增加,不太适合大规模工业应用。
2.3 生物浸出
与无机酸和有机酸浸出相比,生物浸出对环境友好、成本较低且易于工业化应用,是一种较好的替代方法。生物浸出的机理是利用微生物活动产生的无机酸或有机酸来浸出废旧锂离子电池中的有价金属元素[6]。迄今为止,人们采用生物浸出提取有价金属[63-65]。
为了探究活性物质中有价金属生物浸出过程的机制,XIN等[63]分别采用硫杆菌和硫铁杆菌浸出废旧三元NCM电池中的有价金属元素,结果显示Li主要是由硫杆菌代谢活动中产生的硫酸溶出,而Ni,Co以及Mn的浸出是酸与Fe2+共同作用的结果。MISHRA等[64]引入酸性氧化铁硫杆菌用于从废旧LiCoO2电池中浸出Co和Li,在培养液初始pH=2.5,固液比为10 g/L,Fe2+初始质量浓度为3 g/L,S质量分数为1%,浸出25 d时,Co和Li的浸出率分别为65%和10%左右。为了提升金属的浸出率,ZENG等[65]以酸性硫氧化铁杆菌为菌种,提出了一种铜催化生物浸出工艺从废旧LiCoO2中浸出Li和Co,发现在Cu2+质量浓度为0.75 g/L的环境下浸出6 d后,超过99%的Co被浸出,而在没有Cu2+时,Co的浸出率仅为43.1%。
相对于细菌而言,简青霉、产黄青霉以及黑曲霉等真菌具有更强的生存能力和更快的浸出速率。HOREH等[66]采用黑曲霉作为菌种用于浸出废旧锂离子电池中的有价金属,在矿浆质量分数为1%,浸出14 d时,接近100%的Cu和95%的Li被浸出,Mn的浸出率也超过了70%,而Al,Ni以及Co的浸出率只有40%左右。经分析发现,黑曲霉代谢活动中产生的有机酸主要包括柠檬酸、苹果酸、草酸和葡萄糖酸等,其中柠檬酸对金属的浸出率贡献最大。
尽管通过一些离子的催化作用或者改变菌种体系可以使一些金属的浸出率显著提高,但生物浸出方法仍然难以在工业中大规模应用,其原因是:一方面,菌种难培养不易存活,对生长环境要求高;另一方面,生物浸出往往只有在极低矿浆密度下才能表现出良好的浸出效果,且浸出周期长,极大地限制了整个浸出过程的处理能力[66-67]。
2.4 氨浸出
无机酸、有机酸以及生物浸出的原理都是基于在酸性介质中氢离子与正极活性粉末之间的反映。酸浸处理后得到的浸出液中残酸浓度往往较高,以回收前驱体为例,镍、钴以及锰氢氧化物完全沉淀的pH均在10以上[52],故需要大量的碱中和浸出液中的残酸,这会导致额外开销。不同于上述浸出方法,氨浸出是基于强碱性环境下氨根离子与金属离子之间的相互络合作用。目前,已有大量从废旧离子电池中通过氨浸出有价金属元素的研究报道[68-70]。
ZHENG等[69]以NH3·H2O-(NH3)2SO4-Na2SO3浸出体系从LiNixCoyMn1-x-yO2正极活性物质中浸出有价金属,其中(NH3)2SO4充当pH缓冲剂,Na2SO3作为浸出还原剂。在最佳浸出条件下,超过98%的Ni以及Co被浸出,而Mn的浸出率在2%以下。其原因是Ni2+和Co2+与NH3反应可以形成稳定的Ni(NH3)n2+和Co(NH3)n2+络合物进入浸出液中,而Mn在浸出过程中首先形成Mn(NH3)x2+,最终以(NH4)2Mn(SO3)·H2O沉淀的形式进入浸出渣中。Mn浸出率低有利于浸出液中金属离子间后续分离。氨浸出工艺避免了酸浸工艺中所得浸出液残酸浓度高的问题,通过调整浸出剂的组成,可以实现Co,Ni以及Cu的高效浸出,而Mn和Al基本不被浸出。
2.5 辅助强化浸出
一般地,酸浸出和氨浸出都需要较长的浸出时间和较高的浸出温度。为了提高金属的浸出效率,可采用一些辅助方法来增强浸出。常见的辅助浸出手段有超声辅助以及机械化学辅助[71-72]。
JIANG等[71]以H2SO4作为浸出剂,H2O2作为还原剂从废旧LiCoO2中浸出Li和Co。在浸出温度为30 ℃和浸出时间为30 min时,只有70%的Co和Li被浸出;当施加超声辅助时,Co和Li的浸出率均超过95%。与常规酸浸出相比,超声辅助浸出可以显著降低浸出所需温度和时间,其原因是:一方面,在浸出过程中,超声可以促进离子的传质作用;另一方面,基于超声的“空穴效应”,可在活性物质粉末颗粒与浸出剂的接触界面上产生大量的非稳态微小气泡,这些非稳态气泡在液-固接触界面处急速收缩扩张,产生大量能量,进而降低了激活浸出反应所需的初始活化能[72-73]。
对矿物预先进行活化预处理后可显著提升金属的浸出率,基于此,ZHANG等[74]采用LiCoO2与PVC粉末混合球磨的方式从废旧锂离子电池中提取有价金属,其机理是利用机械能诱导引发物质发生物理化学变化,在共混球磨36 h后取出进行水浸出,结果显示超过90%的Co和99%的Li被浸出。相似地,WANG等[75]将LiCoO2分别与PVC,FeCl3,Na2-EDTA以及EDTA等物质混合球磨,发现在最佳条件下与EDTA混合球磨,Li和Co的浸出率最高,分别达到99%和98%。对其机理进行研究发现,在混合球磨过程中Li和Co分别与EDTA作用形成了水溶性金属螯合物Li-EDTA和Co-EDTA。机械化学强化浸出回收工艺避免了酸或碱的使用,可有效避免二次污染,但处理效率和处理量有待提升。
此外,也有报道在某些极端环境下浸出废旧锂离子电池活性物质中有价金属。BERTUOL等[76]以超临界CO2为萃取剂,以H2SO4和H2O2作为助溶剂从废旧锂离子电池中萃取Co,结果表明该方法与传统湿法相比,浸出过程极大缩短了浸出时间,在体积分数为4%的H2O2作用下,Co的萃取率在 5 min内即可达到98%以上。基于聚氯乙烯(PVC)中Cl-在高温下易于脱出的特性,LIU等[77]在亚临界水中共处理PVC和LiCoO2粉末,在最佳条件即温度为350 ℃,PVC与LiCoO2质量比为3:1,浸出时间为30 min,固液比为16 g/L下,超过95%的Li以及Co被浸出。其浸出机理是在超临界水下PVC脱氯并产生HCl,实现了对Li及Co的浸出。此法同时实现了PVC脱氯无害化处理,避免了直接热解PVC过程中有毒气体的产生[78],可以达到绿色处理废旧锂离子电池和废PVC的目的。但以上方法都需要在高压环境下操作实现,对设备要求高且安全隐患巨大,不利于大规模推广应用。
MENG等[79]提出了一种电化学辅助浸出LiCoO2中有价金属的方法,其中以苹果酸作为浸出剂,以铂电极作为正极,LiCoO2粉末制成的电极作为负极,并施加8 V的电压。在最佳条件下,超过95%的Li和90%的Co被浸出。对其机理进行研究发现,在浸出过程中,在铂电极上发生析氧反应,负极LiCoO2粉末在电场作用下与浸出剂中游离的H+反应被还原成Co2+,其转变过程反应方程式见式(5)和式(6)。


Co首先在电极颗粒表面在电场的作用下生成Co(OH)2,然后在酸性溶液中变成Co2+。该方法避免了还原剂的使用,但其本质依旧是基于酸性介质中氢离子与LiCoO2粉末之间的反应。
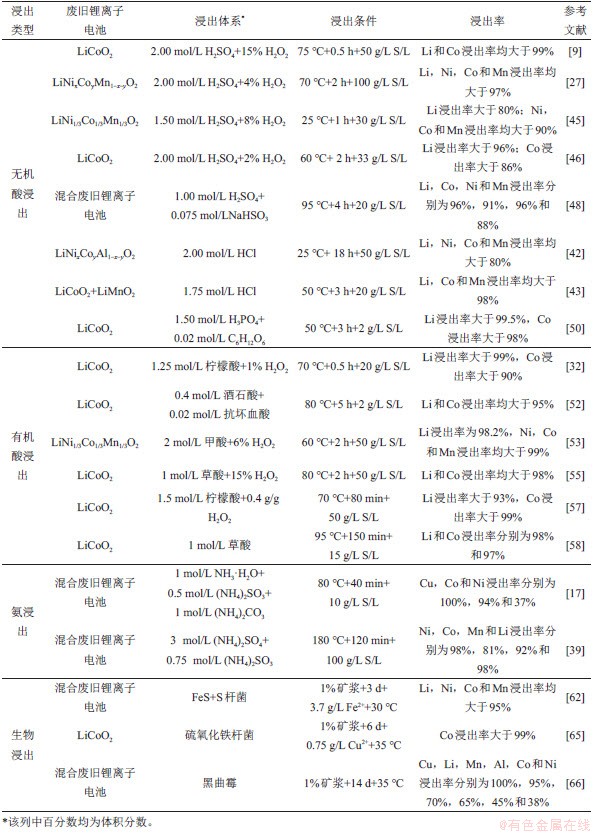
活性物质中有价金属的浸出率受浸出时间、浸出温度、浸出剂浓度等因素影响,各典型的浸出体系下金属在最佳条件下的浸出率如表1所示。
表1 不同浸出体系下废旧LIBs中有价金属浸出率
Table 1 Leaching rate of valuable metals from spent LIBs under different leaching systems
2.6 选择性浸出
由于电极材料种类的多样性以及成分的复杂性使所得浸出液中含有多种有价金属离子,这将增加从浸出液中分离出某一金属化合物或者前驱体的难度以及回收成本。为了降低浸出液中有价金属化合物的分离和回收难度,并为了选择性地回收附加值高的金属成分,一些研究者根据不同金属在正极活性物质中的行为特性提出了选择性浸出的方法。
由于LiCoO2,LiNiO2和LiNi1/3Co1/3Mn1/3O2等活性电极粉末的晶型结构均为α-NaFeO2层状结构,其中Li以离子的形式自由地存在于层状结构中,通过电池的充放电即可实现自由脱嵌,因此,Li应当比其他金属更易被浸出。基于此,CHEN等[80]提出了一种从正极活性物质选择性浸出锂的方法,在实验中采用中性酸H3PO4作为浸出剂,分别对不同类型的正极活性物质进行浸出,结果表明,在最佳条件下,LiCoO2,Li2MnO2,LiNi1/3Co1/3Mn1/3O2以及LiFePO4中锂的浸出率均可达到92%以上,而其他金属(Co,Ni,Mn和Fe等)的浸出率均在20%以下。对浸出过程的动力学进行研究发现,Li的浸出过程受扩散控制,且浸出表观活化能远比其他金属的低,这证实了Li与其他金属相比易于被浸出。PENG等[81]提出了一种选择性沉淀Cu的浸出工艺,以硫酸作为浸出剂,以柠檬酸作为还原剂,从废旧LiCoO2中浸出有价金属,发现加入柠檬酸后,Cu的浸出率大大降低,而Co和Li的浸出率显著提升。其原因是:一方面,Cu2+与柠檬酸反应使溶液中的Cu2+重新被还原成Cu粉进入渣相中;另一方面,柠檬酸本身具有还原性,促进了LiCoO2中高价Co(Ⅳ)转变为Co2+,进而提升了Co和Li的浸出率。该工艺在浸出Co以及Li的同时选择性地沉淀Cu,这有助于降低浸出液净化除杂的工作量。
由于MC2O4(M为Ni,Co和Mn)不溶于水,而Li2C2O4易溶于水,因此,H2C2O4既可作为废旧正极活性物质的浸出剂,又可作为金属离子的选择性沉淀剂。ZENG等[62]以草酸作为浸出剂,H2O2作为还原剂,从LiCoO2粉末中浸出有价金属,发现在最佳条件即浸出温度为95 ℃,浸出时间为150 min,固液比为15 g/L和转速为400 r/min时,超过97%的Co以CoC2O4·2H2O的沉淀形式回收,而Li则以Li2C2O4的形式保留在溶液中,实现了Li和Co在浸出阶段被分离的目的。
选择性浸出方法是一种高效环保的废旧正极粉末中有价金属浸出的方法,其中,选择性浸出Li改变了常规浸出方法最后回收Li的思路,有助于提高Li的综合回收率。选择性沉淀Co实现了在浸出阶段Co和Li的分离,大大缩减了后续沉淀分离的工序,但该方法可能适合于处理单一且纯度高的废旧正极活性物质。由于Ni,Co以及Mn的化学性质相近,对于成分复杂的混合活性物质粉末在浸出的同时,难以实现某一金属离子的选择性沉淀,且得到的产物很容易混入杂质,需要进行进一步纯化处理。
3 分离提纯及产品回收过程
通过不同浸出方法和体系得到含有有价金属离子的浸出液,需要进一步处理,以得到最终的目标产物。该过程包括浸出液的净化除杂和最终产品的分离回收。
3.1 浸出液净化除杂
在工业生产中,机械破碎分选技术是实现废旧锂离子电池各组分之间分离的主要方法。虽然通过技术参数的调整优化,当前废旧锂离子电池各个组分之间的分离效率大幅度提升,但仍然会有少量(约10%)的Fe屑、Cu屑和Al屑夹杂进入活性物质粉末中。在浸出过程中,这些夹杂碎屑与浸出剂反应以离子的形式进入浸出液中。此外,在废旧锂离子电池破碎处理中,电解液如LiPF6回收成本高,往往不对其进行回收处理,这也会导致浸出液中氟、磷等非金属杂质离子被引入。虽然绝大多数关于废旧锂离子电池回收的文献报道没有提及这些金属和非金属杂质离子,但是,为了避免这些杂质离子对回收产品的纯度和性能造成影响,必须对浸出液进行净化除杂。
目前,在工业生产中,从浸出液除铁的方法主要为中和水解法,包括针铁矿法、黄钠铁矾法以及Fe(OH)3水解法[82]。理论上,Fe3+易水解成Fe(OH)3沉淀,调节浸出液的pH至3.53,在25 ℃时浸出液中的Fe3+浓度即可至1 μmol/L,但Fe(OH)3往往以无定型的胶体形态存在于浸出液中使其过滤困难,且在沉淀过程中,生成的Fe(OH)3胶体会吸附浸出液中的其他金属离子如Ni2+和Co2+等,造成目标金属离子以共沉淀的形式损失。基于此,研究者们研发了针铁矿法和黄钠铁矾法除铁工艺。与黄钠铁矾法相比,针铁矿法具有除铁渣晶粒大、夹带有价金属少等优点,但在陈铁过程中需要严格控制Fe3+的质量浓度在1 g/L以下,且必须事先向待除铁液中加入针铁矿晶种[83]。黄钠铁矾法除铁的机理为

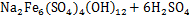
黄钠铁矾法虽然渣量较大,但废旧锂离子电池浸出液中Fe3+的质量浓度较低,一般为2~3 g/L,且工业生产中一般以H2SO4作为浸出剂使浸出液中含有大量的SO42-,用NaOH调节浸出液pH,无需额外添加NaSO4。值得注意的是,用黄钠铁矾法除铁会有H+产生,所以,需要定时添加NaOH,调节溶液的pH稳定在1.5~1.8,保持反应温度为85~90 ℃,反应时间为4~6 h,在此条件下,Fe的去除率在99.5%以上[84]。
对于杂质离子Al3+可通过简单地调节pH去除:当调节浸出液的pH>5时,溶液中的Al3+以Al(OH)3的形式析出,浸出液残留Al3+浓度可降至1 μmol/L[85]。但废旧锂离子电池浸出液Ni2+在pH为5.1时开始沉淀,所以,控制浸出液的pH在4.5~5.0之间可使Al3+的去除率超过98%[86]。去除浸出液中Cu2+的主要方法有萃取除铜[87]、Fe粉置换除铜[88]以及Na2S2O4除铜[89]等。萃取铜常用的萃取剂为M5640,李学鹏等[87]利用M5640从含有Ni2+和Co2+等离子的溶液中萃取除铜,在最佳条件即pH=3,相比为1:1,萃取剂体积分数为15%,震荡时间为5 min下,铜离子的萃取率高于99.8%。萃取除铜具有产品纯度高等优点,但萃取剂价格昂贵,且萃取剂本身具有一定的毒性,可能会造成二次环境污染。基于此,LI等[88]采用Fe粉置换从含有Ni,Co以及Mn等离子的废旧锂离子电池浸出液中除铜,在最佳条件即温度为30 ℃,Fe与Cu质量比为1.5,反应时间为30 min时,浸出液中残留的Cu2+质量分数最低可降至0.175×10-6。Fe粉置换除铜避免了有机物萃取剂的引入,但除铜后液中又引入了Fe2+,需进一步氧化成Fe3+后除去,这增加了净化除杂的工作量。与以上2种除Cu方法相比,Na2S2O4除铜法更具有工业大规模应用的潜力,其原理是Na2S2O4与Cu2+在中性或酸性环境下,可以与Cu2+形成络合,将溶液加热,Cu2+以Cu2S的形式沉淀析出,即

其他离子如Ni2+,Co2+和Mn2+等不具备这种能力,从而不能分离Cu2+。胡宝兰[89]利用Na2S2O4从含Ni2+浸出液中除铜,在最佳条件下,Cu的去除率在98%以上,而Ni的损失率在1%以下。
对于浸出液中的非金属杂质离子F和P等的去除尚未发现相关报道,但凭借从锌电解液中除F的经验,可以先将浸出液的pH调至5~7,然后加入不溶于水的碱金属氧化物或稀土碳酸盐(La和Ce等)可以达到深度除F的目的[90]。由于AlPO4与FePO4均属于沉淀物,通过控制浸出液的pH可以达到同时去除Al,Fe和P的目的。张萌等[91]采用Fe3+去除浸出液中的P,在Fe与P质量比为3,pH为1.5~2.0条件下,搅拌反应为15 min后再静置30 min,溶液中残留的P质量分数可降至15×10-6以下。基于以上分析,由于浸出液中F和P等离子的质量分数较低,可在除Fe3+和Al3+的过程中以共沉淀的形式同时去除。
3.2 产品回收过程
浸出液经过净化除杂后,对净化后液中的有价金属离子进行进一步回收。根据处理方式及最终产品形态的不同,产品回收方法可以概括为化学沉淀法、溶剂萃取法、电沉积法以及溶胶-凝胶法。
3.2.1 化学沉淀法
化学沉淀法是常用的从溶液中沉淀回收金属化合物的方法,其原理是:在特定的pH下,向除杂后液中加入OH-,CO32-以及C2O42-等沉淀剂,使溶液中的金属离子以金属化合物的形式析出。
PANT等[92]采用H2C2O4和饱和Na2CO3溶液分别沉淀废旧锂离子电池浸出液中Li,Ni,Co以及Mn离子。首先,在浸出液中加入H2C2O4调节浸出液的pH为1.5,以CoC2O4·2H2O的形式回收Co;其次,加入饱和Na2CO3溶液调节过滤液的pH至7.5,以MnCO3的形式回收Mn;继续向沉锰后液中加入饱和Na2CO3调节溶液pH至9,以NiCO3的形式回收Ni;最后调节Li富集液的pH至14,以Li2CO3的形式回收Li。类似地,CONTESTABILE等[93]向含Li+和Co2+的浸出液中加入4 mol/L NaOH,最后以Co(OH)2的形式回收Co。
除常规沉淀剂外,还有一些其他从浸出液中沉淀回收金属化合物的方法,如HUANG等[94]从含有Li+,Mn2+和Fe3+的废旧锂离子电池浸出液中回收有价金属。首先采用离子液体浮选剂[Hbet][Tf2N]将浸出液中的Fe3+以[Fe(bet)n][(Tf2N)3]的形式选择性沉淀,随后用HCl进行反溶,最终以FeCl3的形式回收Fe。随后,向沉Fe后液中添加饱和KMnO4溶液氧化过滤液中的Mn2+并以MnO2/Mn2O3的形式回收。最后,向沉锰后液中加入Na3PO4溶液沉淀Li+以Li3PO4的形式回收。在最佳条件下,超过81%的Li,85%的Fe以及81%的Mn被回收,所得Li3PO4,FeCl3和MnO2/Mn2O3化合物的纯度均在98%以上。
由于废旧锂离子电池种类繁多,所用的浸出剂也各不相同,所得的浸出液往往含有复杂的金属离子如Cu,Li,Ni,Co以及Mn等。为了避免分步回收单一金属化合物时繁杂的步骤以及降低回收成本,直接以前驱体的形式回收镍钴锰更具有优势。当前报道的镍钴锰前驱体主要有NixCoyMn1-x-y(OH)2[95-96],NixCoyMn1-x-yCO3[97]以及NixCoyMn1-x-yC2O4[98]。ZOU等[96]首先使用H2SO4+H2O2的浸出体系浸出废旧锂离子电池得到含有Li,Ni,Co,Mn,Fe和Al等离子的浸出液,随后用4 mol/L NaOH溶液调节浸出液pH至4.5左右,沉淀Al(OH)3和Fe(OH)3;随后,调节过滤后液中Ni,Co以及Mn离子的物质的量比为1:1:1,继续添加NaOH调节pH至11,得到Ni1/3Co1/3Mn1/3(OH)2前驱体,最后通过加入饱和Na2CO3溶液以Li2CO3的形式回收Li。此外,HE等[97]以及LI等[98]分别用类似的方法从废旧锂离子电池浸出液中直接回收制备出Ni1/3Co1/3Mn1/3CO3和Co0.13Ni0.13Mn0.54C2O4前驱体,并通过与Li2CO3混合煅烧制备再生活性物质。
使用化学沉淀方法从浸出液回收得到的产品形态各不相同,目前,文献中报道的Li,Ni,Co以及Mn的产品的主要回收形式如图5所示。
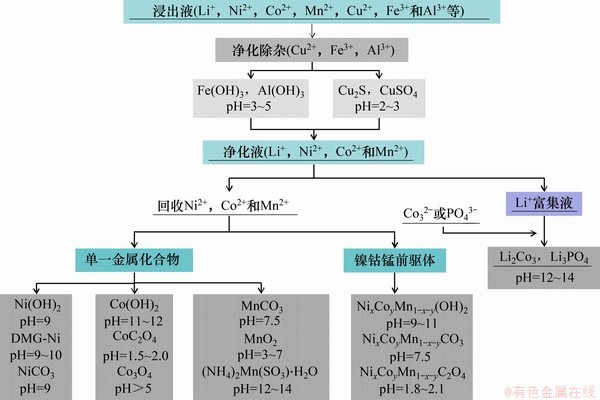
图5 一些典型的产品回收过程
Fig. 5 Some typical product recycling processes
3.2.2 溶剂萃取法
溶剂萃取法是另一种常用的从溶液中分离金属离子的方法,其原理是利用化合物在2种不同液体中的溶解度不同而实现各自分离。尽管采用此法分离具有相似萃取性能离子的效率较低,但此法仍然是一种高效可行的方法,已有多种萃取剂如Cyanex 272,D2EHPA,Acorga M5640,三辛胺(TOA)和PC-88A等用于从废旧锂离子电池浸出液中分离回收Ni,Co和Cu等。ZHAO等[99]从含有Li,Co和Mn等离子的废旧电池浸出液中分离回收单一金属化合,首先采用Cyanex 272+PC-88A协同萃取体系在pH为5.2~5.4时萃取Co以及Mn,用H2SO4反萃有机相得到含有Co2+以及Mn2+的硫酸盐溶液;随后,调节硫酸盐溶液的pH至5,用EDTA从硫酸盐溶液萃取Mn2+,分离出Co2+。最后,用0.01 mol/L的H2SO4对Mn负载有机相进行反萃得到高纯的Mn硫酸盐溶液。
如前面所述,废旧锂离子电池浸出液中包含种类繁多的金属离子,只采用单纯的溶剂萃取法分离各种离子面临许多困难,且萃取分离具有相似官能团的化学物效果差。为了尽可能避免此类问题,采用化学沉淀和萃取分离相结合的方法显得更具优势。CHEN等[100]从含有Li+,Ni2+,Co2+以及Mn2+的浸出液中分离回收各金属离子。首先,采用调节浸出液pH至5,采用丁二酮肟(C4H8N2O2)沉淀Ni2+;然后,调节pH至6,保持溶液温度为55 ℃,加入(NH4)2C2O4以CoC2O4的形式沉淀Co;用萃取剂D2EHPA在pH=5,水相与油相体积比为0.5,时间为300 s条件下萃取Mn。最后,采用0.5 mol/L Na3PO4以Li3PO4的形式沉淀Li。通过该回收过程,超过98%的Ni,Co以及Mn分别以C4H8N2O2-Ni,CoC2O4·2H2O和MnSO4的形式回收,超过89%的Li以Li3PO4的形式回收。
溶剂萃取法具有能耗低、分离效果好、所得产品纯度高等优点。但萃取剂价格普遍昂贵,这增加了回收处理过程的成本。
3.2.3 其他方法
除了常规的化学沉淀法和溶剂萃取法外,还有一些其他方法如电化学沉积法和溶胶-凝胶法等被用于从废旧锂离子电池浸出净化液中回收金属或金属化合物。
电化学沉积法是在电场的作用下从浸出液中以金属或金属氢氧化物的形式回收有价金属的方法。RABAHARAN等[101]调节含Li+,Co2+以及Mn2+浸出液的pH至2.0~2.5,在电流密度为200 A/m2时电沉积分离Co和Mn,其中,浸出液中Co2+在阴极沉积金属Co(式(9)),Mn2+在阳极以MnO2的形式回收(式(10))。



在整个回收过程中,Co和Mn的回收率均在95 %以上。类似地,LUPI等[102]使用电沉积技术从含Ni2+和Co2+的废旧锂离子电池浸出液分离回收Ni,在电流密度为250 A/m2,浸出温度为50 ℃,浸出液pH为3.0~3.2,Ni2+和H3BO3质量浓度分别为50 g/L和20 g/L时,Ni沉积的电流效率和能耗分别约为87%和2.96 kW·h/kg,且处理后的浸出液中Ni2+质量分数低于100×10-6。与化学沉淀法和溶剂萃取法相比,采用电化学沉积法分离浸出液中的有价金属离子无需额外的添加剂,且得到的金属或金属化合物纯度高,但此过程需要施加电场,可能会带来额外的能耗。
此外,也有研究报道采用溶胶-凝胶的方式从浸出液直接制备再生正极活性物质。YAO等[103]开发了一种从废旧锂离子电池浸出液中直接再生LiNi1/3Co1/3Mn1/3O2正极材料的方法:首先,采用柠檬酸+H2O2的浸出体系浸出废旧三元锂离子电池;然后,调节浸出液中锂、镍、钴、锰离子的物质的量比为3.05:1.00:1.00:1.00,用氨水调节pH至8,通过水浴加热浸出液得到湿凝胶干燥后在750 ℃下煅烧12 h得到再生的正极材料。经测试,再生的正极材料表现出良好的电化学性能。类似地,LI等[104]用柠檬酸和乳酸以溶胶-凝胶的方法合成了再生电极材料,发现以柠檬酸为浸出体系合成的再生正极材料表现出较好的电化学性能。溶胶-凝胶法是一种从废旧锂离子电池浸出液中回收有价金属的处理方式,具有环保无污染的特点,其中有机酸作为螯合剂,氨水作为络合剂,得到的再生电极材料表现出良好的电化学性能。然而,该方法对浸出液的纯度要求高,如前面所述浸出液中含有多类杂质离子,不进行除杂净化而直接采用该方法,则再生的电极材料性能可能达不到要求。
综合对比上述方法,采用化学沉淀剂分离浸出液中的各种金属离子是一种较低能耗和成本的方法,特别是用共沉淀的方式直接合成镍钴锰前驱体避免了复杂的金属离子分离程序。但化学沉淀法存在一些不足,如浸出液中还含有目标金属离子以外的金属或非金属离子,在目标金属离子沉淀过程可能会夹杂其他离子,导致回收产品的纯度降低。此外,采用共沉淀法回收镍钴锰前驱体时,需要调节浸出液中金属离子的物质的量比,且沉淀过程中可能会夹杂锂。因此,开发各种回收方法相结合的回收方式似乎更高效可行。
4 结论与展望
1) 目前,电池放电多采用不同浓度的NaCl溶液作为放电介质,由于Cl-的腐蚀特性容易造成电池外壳破损,导致电解液的泄露产生二次污染,因此,开发一种条件温和高效的放电体系是当前需要解决的问题。
2) 机械粉碎分选比人工拆解更高效适合大规模应用,但机械粉碎分选很难实现废旧锂离子电池中不同组分间的高效分离,因此,如何实现废旧锂离子电池中不同组分间的高效分离应是今后研究的重点之一。
3) 在活性物质从集流体脱附的过程中多采用热处理的方式,此过程需要高温条件且在此过程中会产生HF和PF5等有害气体。为了减少或避免有毒气体的产生,开发低温焙烧工艺势在必行。
4) 当前电极活性物质中有价金属离子的浸出方法有无机酸浸出、有机酸浸出、生物浸出以及氨浸出,其中,H2SO4浸出实现了工业化应用。但在浸出过程中,酸的利用率低且浸出液中残酸浓度较高,因此,如何提升浸出过程中的酸利用率、增大浸出过程的处理量、避免或减弱浸出过程中可能带来的二次污染应是今后研究的关注点之一。
5) 当前对废旧锂离子电池的回收关注点多集中在较高附加值的正极活性物质上,较低附加值的负极石墨回收也应是今后的关注点。
6) 化学沉淀法和溶剂萃取法是从浸出液中回收分离有价金属的2种主要方法。由于浸出液中含有多种金属离子,为了获得良好的分离效果和较高的回收产品纯度,这2种方法往往结合起来使用。但单一的金属化合物产品回收过程复杂,操作繁杂,且由于Ni,Co和Mn等离子化学特性相似,不能保证单一金属化合物的纯度。为了避免上述问题,采用共沉淀或者溶胶-凝胶的方式直接从浸出液中合成镍钴锰前驱体是比较好的方法,但回收方法仍然存在一些问题,如合成前驱体之前需要对浸出液进行深度除杂。因此,开发一种高效环保的共沉淀直接制备前驱体的生产工艺势在必行。
参考文献:
[1] 吴越, 裴锋, 贾蕗路,等 . 废旧锂离子电池中有价金属的回收技术进展[J]. 稀有金属, 2013, 37(2): 320-329.
WU Yue, PEI Feng, JIA Lulu, et al. Overview of recovery technique of valuable metals from spent lithium ion batteries[J]. Chinese Journal of Rare Metals, 2013, 37(2): 320-329.
[2] ORDOEZ J, GAGO E J, GIRARD A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries[J]. Renewable and Sustainable Energy Reviews, 2016, 60: 195-205.
[3] LU Weiguang, WANG Zhonghang, CAO Hongbin, et al. A critical review and analysis on the recycling of spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering,2018, 6: 1504-1521.
[4] YAO Yonglin, ZHU Meiying, ZHAO Zhuo, et al. Hydrometallurgical processes for recycling spent lithium-ion batteries:a critical review[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 13611-13627.
[5] 李建波, 徐政, 纪仲光, 等. 废旧锂离子动力电池回收的研究现状[J]. 稀有金属, 2019, 43(2): 201-212.
LI Jianbo, XU Zheng, JI Zhongguang, et al. Overview on current technologies of recycling spent lithium-ion batteries[J]. Chinese Journal of Rare Metals, 2019, 43(2): 201-212.
[6] ZENG Xianlai, LI Jinhui, SINGH N. Recycling of spent lithium-ion battery: a critical review[J]. Critical Reviews in Environmental Science and Technology, 2014, 44(10): 1129-1165.
[7] 尹文艳, 魏致慧. 废旧锂离子电池中有价金属回收利用技术研究进展[J]. 金属材料与冶金工程, 2018(2): 56-60.
YI Wenyan, WEI Zhihui. Progress of recovery and recycling of valuable metals from spent lithium-ion battery[J]. Metal Materials and Metallurgy Engineering, 2018(2): 56-60.
[8] GRANATA G, PAGNANELLI F, MOSCARDINI E, et al. Simultaneous recycling of nickel metal hydride, lithium ion and primary lithium batteries: accomplishment of European guidelines by optimizing mechanical pre-treatment and solvent extraction operations[J]. Journal of Power Sources, 2012, 212: 205-211.
[9] SHIN S M, KIM N H, SOHN J S, et al. Development of a metal recovery process from Li-ion battery wastes[J]. Hydrometallurgy, 2005, 79(3/4): 172-181.
[10] 吴越, 裴锋, 贾蕗路, 等. 从废旧磷酸铁锂电池中回收铝、铁和锂[J]. 电源技术, 2014, 38(4): 629-631.
WU Yue, FEI Feng, JIA Lulu, et al. Recovery of aluminum, iron and lithium from spent lithium iron phosphate batteries[J]. Chinese Journal of Power Sources, 2014, 38(4): 629-631.
[11] BARIK S P, PRABAHARAN G, KUMAR B. An innovative approach to recover the metal values from spent lithium-ion batteries[J]. Waste Management, 2016, 51: 222-226.
[12] GEORGI-MASCHLER T, FRIEDRICH B, WEYHE R, et al. Development of a recycling process for Li-ion batteries[J]. Journal of Power Sources, 2012, 207: 173-182.
[13] CHAGNES A, POSPIECH B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries[J]. Journal of Chemical Technology & Biotechnology, 2013, 88(7): 1191-1199.
[14] WANG Guangxu, LI Jia, XU Zhenming. Recycling valuable metals from spent lithium ion discharge batteries[J]. Materials Review, 29, 113-123.
[15] 汤依伟, 黄家奇, 彭灿. 一种废旧锂离子电池的回收方法[P]. CN: 201810894917.9. [2018-08-08].
TANG Yiwei, HUANG Jiaqi, PENG Can. A method for recycling spent lithium ion battery[P]. CN:201810894917.9. [2018-08-08].
[16] 张湘红. 一种废旧锂离子电池的回收方法[P]. CN: 201910571441.X. [2019-06-28].
ZHANG Xianghong. A method for recycling spent lithium ion battery[P]. CN: 201910571441.X. [2019-06-28].
[17] 史红彩. 废旧锂离子动力电池中镍钴锰酸锂正极材料的回收及再利用[D]. 郑州: 郑州大学化学学院, 2017: 1-2.
SHI Caihong. Recovery and reuse of LiNixCoyMn1-x-yO2 cathode material from spent lithium ion power batteries[D]. Zhengzhou: Zhengzhou University. College of Chemistry, 2017: 1-2.
[18] HE Yaqun, ZHANG Tao, WANG Fangfang, et al. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by Fenton reagent-assisted flotation[J]. Journal of Cleaner Production, 2017, 143: 319-325.
[19] LU Mi, ZHANG Houan, WANG Bingchen, et al. The re-synthesis of LiCoO2 fro m spent lithium ion batteries separated by vacuum-assisted heat-treating method[J]. International Journal of Electrochemical Science, 2013, 8(6): 8201-8209.
[20] KU H, JUNG Y, JO M, et al. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching[J]. Journal of Hazardous Materials, 2016, 313: 138-146.
[21] NIE Hehe, XU Long, SONG Dawei, et al. LiCoO2: recycling from spent batteries and regeneration with solid state synthesis[J]. Green Chemistry, 2015, 17(2): 1276-1280.
[22] 宋秀玲, 戴书琪, 徐永胜, 等. 废旧锂离子电池放电的实验研究[J]. 应用化工, 2015(4): 594-597.
SONG Xiuling, DAI Shuqi, XU Yongsheng, et al. Experimental study on the discharge of the waste lithium ion battery[J]. Applied Chemical Industry, 2015(4): 594-597.
[23] YAO Linpeng, ZENG Qi, QI Ting, et al. An environmentally friendly discharge technology to pretreat spent lithium-ion batteries[J]. Journal of Cleaner Production, 2020, 245: 118820.
[24] LI Jia, WANG Guangxu, XU Zhenming. Generation and detection of metal ions and volatile organic compounds(VOCs) emissions from the pretreatment processes for recycling spent lithium-ion batteries[J]. Waste Management, 2016, 52: 221-227.
[25] 范汇吉, 孙虎元, 孙立娟, 等. 电解液的浓度和温度对铝空气电池负极性能的影响[J]. 腐蚀科学与防护技术, 2012, 24(2): 149-152.
FAN Huiji, SUN Huyuan, SUN Lijuan, et al. Effects of chloride ion concentration and temperature on anode performance of aluminum/air batteries[J]. Corrosion Science and Protection Technology, 2012, 24(2): 149-152.
[26] WANG Fangfang, ZHANG Tao, HE Yaqun, et al. Recovery of valuable materials from spent lithium-ion batteries by mechanical separation and thermal treatment[J]. Journal of Cleaner Production, 2018, 185: 646-652.
[27] ZHANG Guangwen, HE Yaqun, WANG Haifeng, et al. Application of mechanical crushing combined with pyrolysis-enhanced flotation technology to recover graphite and LiCoO2 from spent lithium-ion batteries[J]. Journal of Cleaner Production, 2019, 231: 1418-1427.
[28] WANG Haifeng, LIU Jiangshan, BAI Xuejie, et al. Separation of the cathode materials from the Al foil in spent lithium-ion batteries by cryogenic grinding[J]. Waste Management, 2019, 91: 89-98.
[29] BI Haijun, ZHU Huabing, ZU Lei, et al. A new model of trajectory in eddy current separation for recovering spent lithium iron phosphate batteries[J]. Waste Management, 2019, 100: 1-9.
[30] 王洪彩. 含钴废旧锂离子电池回收技术及中试工艺研究[D]. 哈尔滨: 哈尔滨工业大学化学与化工学院, 2013: 1-2.
WANG Caihong. Research on recovery technology and pilot test process of cobalt-containing spent lithium ion batteries[D]. Harbin: Harbin Institute of Technology, School of Chemistry and Chemical Engineering, 2013: 1-2.
[31] NAYL A A, ELKHASHAB R A, BADAWY S M, et al. Acid leaching of mixed spent Li-ion batteries[J]. Arabian Journal of Chemistry, 2017, 10: S3632-S3639.
[32] 胡传跃, 李新海, 王志兴, 等. 材料对锂离子电池热稳定性的影响[J]. 中南大学学报(自然科学版), 2005, 36(4): 587-593.
HU Chuanyue, LI Xinhai, WANG Zhixing, et al. Influence of materials on thermal stability of lithium-ion batteries[J]. Journal of Central South University(Science and Technology), 2005, 36(4): 587-593.
[33] 徐彩娣, 祝成炎, 王荣根. 锂离子电池PVDF纳米隔膜材料的制备工艺研究[J]. 轻纺工业与技术, 2019, 48(3): 5-7.
XU Caidi, ZHU Chengyan, WANG Ronggen. Study on the preparation technology of PVDF nano-diaphragm material for lithium ion battery[J]. Light Industry and Technology, 2019, 48(3): 5-7.
[34] BAUER W, NOTZEL D. Rheological properties and stability of NMP based cathode slurries for lithium ion batteries[J]. Ceramics International, 2014, 40(3): 4591-4598.
[35] LI Li, CHEN Renjie, SUN Feng, et al. Preparation of LiCoO2 films from spent lithium-ion batteries by a combined recycling process[J]. Hydrometallurgy, 2011, 108(3/4): 220-225.
[36] LI LI, DUNN J B, ZHANG Xiaoxiao, et al. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment[J]. Journal of Power Sources, 2013, 233: 180-189.
[37] XU Yanan, SONG Dawei, LI Li, et al. A simple solvent method for the recovery of LixCoO2 and its applications in alkaline rechargeable batteries[J]. Journal of Power Sources, 2014, 252: 286-291.
[38] BOYDEN A, SOO V K, DOOLAN M. The environmental impacts of recycling portable lithium-ion batteries[J]. Procedia CIRP, 2016, 48: 188-193.
[39] HE Lipo, SUN Shuying, SONG Xingfu, et al. Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning[J]. Waste Management, 2015, 46: 523-528.
[40] GAO Shiyan, SU Yuefeng, BAO Liying, et al. High-performance LiFePO4/C electrode with polytetrafluoroethylene as an aqueous-based binder[J]. Journal of Power Sources, 2015, 298: 292-298.
[41] ZENG Xianlai, LI Jinhui. Innovative application of ionic liquid to separate Al and cathode materials from spent high-power lithium-ion batteries[J]. Journal of Hazardous Materials, 2014, 271: 50-56.
[42] WANG Mengmeng, TAN Quanyin, LIU Lili, et al. A low-toxicity and high-efficiency deep eutectic solvent for the separation of aluminum foil and cathode materials from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2019, 380: 120846.
[43] CHEN Yongming, LIU Nannan, HU Fang, et al. Thermal treatment and ammoniacal leaching for the recovery of valuable metals from spent lithium-ion batteries[J]. Waste Management, 2018, 75: 469-476.
[44] WANG Mengmeng, TAN Quanyin, LIU Lili, et al. A facile, environmentally friendly, and low-temperature approach for decomposition of polyvinylidene fluoride from the cathode electrode of spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(15): 12799-12806.
[45] WANG Shubin, WANG Chao, LAI Fengjiao, et al. Reduction-ammoniacal leaching to recycle lithium, cobalt, and nickel from spent lithium-ion batteries with a hydrothermal method: Effect of reductants and ammonium salts[J]. Waste Management, 2020, 102: 122-130.
[46] JOULIE M, LAUCOURNET R, BILLY E.Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries[J]. Journal of Power Sources, 2014, 247: 551-555.
[47] BARIK S P, PRABAHARAN G, KUMAR L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study[J]. Journal of Cleaner Production, 2017, 147: 37-43.
[48] YANG Yue, LEI Shuya, SONG Shaole, et al. Stepwise recycling of valuable metals from Ni-rich cathode material of spent lithium-ion batteries[J]. Waste Management, 2020, 102: 131-138.
[49] CHENG Qian, CHIRDON W M, LIN Meiduan, et al. Characterization, modeling, and optimization of a single-step process for leaching metallic ions from LiNi1/3Co1/3Mn1/3O2 cathodes for the recycling of spent lithium-ion batteries[J]. Hydrometallurgy, 2019, 185: 1-11.
[50] ZHU Shuguang, HE Wenzhi, LI Guangming, et al. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(9): 2274-2281.
[51] 陆修远, 张贵清, 曹佐英, 等. 采用硫酸-还原剂浸出工艺从废旧锂离子电池中回收LiNi0.6Mn0.2Co0.2O2[J]. 稀有金属与硬质合金, 2017, 45(6): 14-23.
LU Xiuyuan, ZHANG Guiqing, CAO Zuoying, et al. Recovery of LiNi0.6Mn0.2Co0.2O2 from spent lithium ion batteries by leaching with H2SO4 and reductants[J]. Rare Metals and Cemented Carbides, 2017, 45(6): 14-23.
[52] MESHRAM P, PANDEY B D, MANKHAND T R.Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching[J]. Chemical Engineering Journal, 2015, 281: 418-427.
[53] VIECELI N, NOGUEIRA C A, GUIMARAES C, et al. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite[J]. Waste Management, 2018, 71: 350-361.
[54] MENG Qi, ZHANG Yingjie, DONG Peng. Use of glucose as reductant to recover Co from spent lithium ions batteries[J]. Waste Management, 2017, 64: 214-218.
[55] 赖延清, 杨健, 张刚, 等. 废旧三元锂离子电池正极材料的淀粉还原浸出工艺及其动力学[J]. 中国有色金属学报, 2019, 29(1): 159-166.
LAI Yanqing, YANG Jian, ZHANG Gang, et al. Optimization and kinetics of leaching valuable metals from cathode materials of spent ternary lithium ion batteries with starch as reducing[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(1): 159-166.
[56] NAYAKA G P, PAI K V, SANTHOSH G, et al. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co[J]. Hydrometallurgy, 2016, 161: 54-57.
[57] GAO Wenfang, ZHANG Xihua, ZHENG Xiaohong, et al. Lithium carbonate recovery from cathode scrap of spent lithium-ion battery:a closed-loop process[J]. Environmental Science & Technology, 2017, 51(3): 1662-1669.
[58] PAGNANELLI F, MOSCARDINI E, GRANATA G, et al. Acid reducing leaching of cathodic powder from spent lithium ion batteries:glucose oxidative pathways and particle area evolution[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 3201-3207.
[59] SUN Liang, QIU Keqiang. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries[J]. Waste Management, 2012, 32(8): 1575-1582.
[60] 苏继桃, 苏玉长, 赖智广. 制备镍、钴、锰复合氢氧化物的热力学分析[J]. 电池工业, 2008, 13(1): 18-22.
SU Jitao, SU Yuchang, LAI Zhiguang. Thermodynamic analysis of preparation of multiple hydroxid of Ni, Co and Mn[J]. Chinese Battery Industry, 2008, 13(1): 18-22.
[61] CHEN Xiangping, LUO Chuanbao, ZHANG Jinxia, et al. Sustainable recovery of metals from spent lithium-ion batteries: a green process[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(12): 3104-3113.
[62] ZENG Xianlai, LI Jinhui, SHEN Bingyu. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid[J]. Journal of Hazardous Materials, 2015, 295: 112-118.
[63] XIN Yayun, GUO Xingming, CHEN Shi, et al. Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery[J]. Journal of Cleaner Production, 2016, 116: 249-258.
[64] MISHRA D, KIM D J, RALPH D E, et al. Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans[J]. Waste Management, 2008, 28(2): 333-338.
[65] ZENG Guisheng, DENG Xiaorong, LUO Shenglian, et al. A copper-catalyzed bioleaching process for enhancement of cobalt dissolution from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2012, 199/200: 164-169.
[66] HOREH N B, MOUSAVI S M, SHOJAOSADATI S A.Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus Niger[J]. Journal of Power Sources, 2016, 320: 257-266.
[67] BANIASADI M, VAKILCHAP F, BAHALOO-HOREH N, et al. Advances in bioleaching as a sustainable method for metal recovery from e-waste:a review[J]. Journal of Industrial and Engineering Chemistry, 2019, 76: 75-90.
[68] WU Caibin, LI Bensheng, YUAN Chengfang, et al. Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching[J]. Waste Management, 2019, 93: 153-161.
[69] ZHENG Xiaohong, GAO Wenfang, ZHANG Xihua, et al. Spent lithium-ion battery recycling-reductive ammonia leaching of metals from cathode scrap by sodium sulphite[J]. Waste Management, 2017, 60: 680-688.
[70] WANG Shubin, WANG Chao, LAI Fengjiao, et al. Reduction-ammoniacal leaching to recycle lithium, cobalt, and nickel from spent lithium-ion batteries with a hydrothermal method: Effect of reductants and ammonium salts[J]. Waste Management, 2020, 102: 122-130.
[71] JIANG Feng, CHEN Yuqian, JU Shaohua, et al. Ultrasound-assisted leaching of cobalt and lithium from spent lithium-ion batteries[J]. Ultrasonics Sonochemistry, 2018, 48: 88-95.
[72] 陈宇乾. 微波焙烧预处理-超声波辅助浸出锗精矿的基础研究[D]. 昆明: 昆明理工大学冶金能源工程学院, 2018: 1-2.
CHEN Yuqian. Basic Research on microwave roasting pretreatment-ultrasonic assisted leaching of germanium concentrate[D]. Kunming:Kunming University of Science and Technology, School of Metallurgy and Energy Engineering, 2018: 1-2.
[73] 杨玮娇, 马保中, 蒋兴明, 等. 褐铁型红土镍矿活化预处理后选择性浸出镍钴[J]. 有色金属(冶炼部分), 2018(1): 16-19.
YANG Weijiao, MA Baozhong, JIANG Xingming, et al. Selective leaching of nickel and cobalt from limonitic laterite after activation pretreatment[J]. Nonferrous Metals(Extractive Metallurgy), 2018(1): 16-19.
[74] ZHANG Qiwu, SAEKI S, TANAKA Y, et al. A soft-solution process for recovering rare metals from metal/alloy-wastes by grinding and washing with water[J]. Journal of Hazardous Materials, 2007, 139(3): 438-442.
[75] WANG Mengmeng, ZHANG Congcong, ZHANG Fushen. An environmental benign process for cobalt and lithium recovery from spent lithium-ion batteries by mechanochemical approach[J]. Waste Management, 2016, 51: 239-244.
[76] BERTUOL D A, MACHADO C M, SILVA M L, et al. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction[J]. Waste Management, 2016, 51: 245-251.
[77] LIU Kang, ZHANG Fushen. Innovative leaching of cobalt and lithium from spent lithium-ion batteries and simultaneous dechlorination of polyvinyl chloride in subcritical water[J]. Journal of Hazardous Materials, 2016, 316: 19-25.
[78] 罗希韬, 王志奇, 武景丽, 等. 基于热重红外联用分析的PE、PS、PVC热解机理研究[J]. 燃料化学学报, 2012, 40(9): 1147-1152.
LUO Xitao, WANG Zhiqi, WU Jingli, et al. Study on the pyrolysis mechanism of polyethylene, polystyrene, and polyvinyl chloride by TGA-FTIR[J]. Journal of Fuel Chemistry and Technology, 2012, 40(9): 1147-1152.
[79] MENG Qi, ZHANG Yingjie, DONG Peng. Use of electrochemical cathode-reduction method for leaching of cobalt from spent lithium-ion batteries[J]. Journal of Cleaner Production, 2018, 180: 64-70.
[80] CHEN Xiangping, CAO Ling, KANG Duozhi, et al. Recovery of valuable metals from mixed types of spent lithium ion batteries.Part II:Selective extraction of lithium[J]. Waste Management, 2018, 80: 198-210.
[81] PENG Chao, HAMUYUNI J, WILSON B P, et al. Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system[J]. Waste Management, 2018, 76: 582-590.
[82] 胡国荣, 李国, 邓新荣, 等. 针铁矿法从铬铁合金硫酸浸出液中除铁[J]. 湿法冶金, 2006, 25(4): 198-201.
HU Guorong, LI Guo, DENG Xinrong, et al. Removal of iron from sulfuric acid leaching solution of ferrochromium alloy by goethite[J]. Hydrometallurgy of China, 2006, 25(4): 198-201.
[83] 邓永贵, 陈启元, 尹周澜, 等. 锌浸出液针铁矿法除铁[J]. 有色金属, 2010, 62(3): 84-88.
DENG Yonggui, CHEN Qiyuan, YI Zhoulan, et al. Removal of ferrous/ferric ions from zinc leaching solution by goethite process[J]. Nonferrous Metals, 2010, 62(3): 84-88.
[84] 阳征会, 龚竹青, 李宏煦, 等. 用黄钠铁矾渣制备复合镍锌铁氧体[J].中南大学学报(自然科学版), 2006, 37(4): 685-691.
YANG Zhenghui, GONG Zhuqing, LI Hongxu, et al. Preparation of Ni-Zn ferrite from sodium jarosite residue[J]. Journal of Central South University(Science and Technology), 2006, 37(4): 685-691.
[85] 杨玮娇, 马保中, 杨卜, 等. 硝酸浸出液中的铝高效脱除[J]. 有色金属工程, 2018, 8(4): 61-65.
YANG Weijiao, MA Baozhong, YANG Pu, et al. High-efficiency removal of aluminum from nitric acid leaching liquor[J]. Nonferrous Metals Engineering, 2018, 8(4): 61-65.
[86] 罗炎, 皮露, 何克杰, 等. 软锰矿烟气脱硫浸出液同步去除铁、铝试验研究[J]. 湿法冶金, 2015, 34(3): 218-221, 224.
LUO Yan, PI Lu, HE Kejie, et al. Simultaneously removing of iron and aluminum from leaching liquor of flue gas desulfurization with pyrolusite[J]. Hydrometallurgy of China, 2015, 34(3): 218-221, 224.
[87] 李学鹏, 杨斌, 刘大春, 等. 硫酸镍电解液净化除杂工艺研究[J]. 稀有金属, 2010, 34(2): 271-275.
LI Xuepeng, YANG Bin, LIU Dachun, et al. Purification of nickel sulphate electrolyte[J]. Chinese Journal of Rare Metals, 2010, 34(2): 271-275.
[88] LI Jinhui, LI Xinhai, HU Qiyang, et al. Study of extraction and purification of Ni, Co and Mn from spent battery material[J]. Hydrometallurgy, 2009, 99(1/2): 7-12.
[89] 胡宝兰. 含镍废料的综合利用研究[D]. 合肥: 合肥工业大学化学与化工学院, 2002: 1-2.
HU Baolan. Research on comprehensive utilization of nickel-containing waste [D]. Hefei:Hefei University of Technology. School of Chemical Engineering, 2002: 1-2.
[90] 刘争伟, 于枭影, 钟晓聪, 等. 含Al、Ca复合除氟剂在含氟硫酸锌溶液中的除氟性能[J]. 中国有色金属学报, 2016. 26(5): 1151-1157.
LIU Zhengwei, YU Xiaoying, ZHONG Xiaocong, et al. Performance of Ca, Al-containningcomposite defluoridation agent in zinc sulfate solution[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(5): 1151-1157.
[91] 张萌, 邱琳, 于晓晴, 等. 亚铁盐与高铁盐除磷工艺的对比研究[J]. 高校化学工程学报, 2013, 27(3): 159-165.
ZHANG Meng, QIU Lin, YU Xiaoqing, et al. Comparison of phosphorus removal processes by ferrous salts and ferric salts[J]. Journal of Chemical Engineering of Chinese Universities, 2013, 27(3): 159-165.
[92] PANT D, DOLKER T. Green and facile method for the recovery of spent lithium nickel manganese cobalt oxide(NMC) based Lithium ion batteries[J]. Waste Management, 2017, 60: 689-695.
[93] CONTESTABILE M, PANERO S, SCROSATI B. A laboratory-scale lithium-ion battery recycling process[J]. Journal of Power Sources, 2001, 92(1/2): 65-69.
[94] HUANG Yanfang, HAN Guihong, LIU Jiongtian, et al. A stepwise recovery of metals from hybrid cathodes of spent Li-ion batteries with leaching-flotation-precipitation process[J]. Journal of Power Sources, 2016, 325: 555-564.
[95] SA Qina, GRATZ E, HE Meinan, et al. Synthesis of high performance LiNi1/3Mn1/3Co1/3O2 from lithium ion battery recovery stream[J]. Journal of Power Sources, 2015, 282: 140-145.
[96] ZOU Haiyang, GRATZ E, APELIAN D, et al. A novel method to recycle mixed cathode materials for lithium ion batteries[J]. Green Chemistry, 2013, 15(5): 1183-1191.
[97] HE Lipo, SUN Shuying, YU Jianguo. Performance of LiNi1/3Co1/3Mn1/3O2 prepared from spent lithium-ion batteries by a carbonate co-precipitation method[J]. Ceramics International, 2018, 44(1): 351-357.
[98] LI Li, ZHANG Xiaoxiao, CHEN Renjie, et al. Synthesis and electrochemical performance of cathode material Li1.2Co0.13Ni0.13Mn0.54O2 from spent lithium-ion batteries[J]. Journal of Power Sources, 2014, 249: 28-34.
[99] ZHAO J M, SHEN X Y, DENG F L, et al. Synergistic extraction and separation of valuable metals from waste cathodic material of lithium ion batteries using Cyanex272 and PC-88A[J]. Separation and Purification Technology, 2011, 78(3): 345-351.
[100] CHEN Xiangping, ZHOU Tao, KONG Jiangrong, et al. Separation and recovery of metal values from leach liquor of waste lithium nickel cobalt manganese oxide based cathodes[J]. Separation and Purification Technology, 2015, 141: 76-83.
[101] RABAHARAN G, BARIK S P, KUMAR N, et al. Electrochemical process for electrode material of spent lithium ion batteries[J]. Waste Management, 2017, 68: 527-533.
[102] LUPI C, PASQUALI M. Electrolytic nickel recovery from lithium-ion batteries[J]. Minerals Engineering, 2003, 16(6): 537-542.
[103] YAO Lu, FENG Yong, XI Guoxi. A new method for the synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries[J]. RSC Advances, 2015, 5: 44107-44114.
[104] LI Li, CHEN Renjie, ZHANG Xiaoxiao, et al. Preparation and electrochemical properties of re-synthesized LiCoO2 from spent lithium-ion batteries[J]. Chinese Science Bulletin, 2012, 57(32): 4188-4194.
(编辑 陈灿华)
收稿日期: 2020 -06 -16; 修回日期: 2020 -08 -22
基金项目(Foundation item):国家自然科学基金资助项目(51674298);博士后科学基金资助项目(2018M630910);湖南省自然科学基金资助项目(2017JJ3384) (Project(51674298) supported by the National Natural Science Foundation of China;Project(2018M630910) supported by the Postdoctoral Science Foundation of China; Project(2017JJ3384) supported by the Natural Science Foundation of Hunan Province)
通信作者:蒋良兴,教授,从事湿法冶金及光电催化研究;E-mail:lxjiang@csu.edu.cn
摘要:随着电子产品的快速更新换代和动力汽车的飞速发展,产生了越来越多的废旧锂离子电池。废旧锂离子电池中含有的大量有毒有害物质,会对环境和人类健康产生严重危害,此外,废旧锂离子电池中含有丰富有价金属,可作为重要的二次资源,因此,废旧锂离子电池的回收已成为全球关注的热点。湿法冶金过程被认为是低能耗、低成本、低污染以及更适合规模化应用的废旧离子电池回收技术。湿法冶金回收过程包括电池预处理、有价金属浸出以及高附加值产品回收。本文对采用湿法冶金技术回收废旧锂离子电池中有价金属的研究现状进行综述,对比分析各个步骤中不同处理技术之间的优劣,提出当前湿法冶金回收过程中存在的问题,并对湿法冶金技术回收废旧锂离子电池发展方向进行展望。
[1] 吴越, 裴锋, 贾蕗路,等 . 废旧锂离子电池中有价金属的回收技术进展[J]. 稀有金属, 2013, 37(2): 320-329.
[5] 李建波, 徐政, 纪仲光, 等. 废旧锂离子动力电池回收的研究现状[J]. 稀有金属, 2019, 43(2): 201-212.
[7] 尹文艳, 魏致慧. 废旧锂离子电池中有价金属回收利用技术研究进展[J]. 金属材料与冶金工程, 2018(2): 56-60.
[10] 吴越, 裴锋, 贾蕗路, 等. 从废旧磷酸铁锂电池中回收铝、铁和锂[J]. 电源技术, 2014, 38(4): 629-631.
[15] 汤依伟, 黄家奇, 彭灿. 一种废旧锂离子电池的回收方法[P]. CN: 201810894917.9. [2018-08-08].
[16] 张湘红. 一种废旧锂离子电池的回收方法[P]. CN: 201910571441.X. [2019-06-28].
[17] 史红彩. 废旧锂离子动力电池中镍钴锰酸锂正极材料的回收及再利用[D]. 郑州: 郑州大学化学学院, 2017: 1-2.
[22] 宋秀玲, 戴书琪, 徐永胜, 等. 废旧锂离子电池放电的实验研究[J]. 应用化工, 2015(4): 594-597.
[25] 范汇吉, 孙虎元, 孙立娟, 等. 电解液的浓度和温度对铝空气电池负极性能的影响[J]. 腐蚀科学与防护技术, 2012, 24(2): 149-152.
[30] 王洪彩. 含钴废旧锂离子电池回收技术及中试工艺研究[D]. 哈尔滨: 哈尔滨工业大学化学与化工学院, 2013: 1-2.
[32] 胡传跃, 李新海, 王志兴, 等. 材料对锂离子电池热稳定性的影响[J]. 中南大学学报(自然科学版), 2005, 36(4): 587-593.
[33] 徐彩娣, 祝成炎, 王荣根. 锂离子电池PVDF纳米隔膜材料的制备工艺研究[J]. 轻纺工业与技术, 2019, 48(3): 5-7.
[51] 陆修远, 张贵清, 曹佐英, 等. 采用硫酸-还原剂浸出工艺从废旧锂离子电池中回收LiNi0.6Mn0.2Co0.2O2[J]. 稀有金属与硬质合金, 2017, 45(6): 14-23.
[55] 赖延清, 杨健, 张刚, 等. 废旧三元锂离子电池正极材料的淀粉还原浸出工艺及其动力学[J]. 中国有色金属学报, 2019, 29(1): 159-166.
[60] 苏继桃, 苏玉长, 赖智广. 制备镍、钴、锰复合氢氧化物的热力学分析[J]. 电池工业, 2008, 13(1): 18-22.
[72] 陈宇乾. 微波焙烧预处理-超声波辅助浸出锗精矿的基础研究[D]. 昆明: 昆明理工大学冶金能源工程学院, 2018: 1-2.
[73] 杨玮娇, 马保中, 蒋兴明, 等. 褐铁型红土镍矿活化预处理后选择性浸出镍钴[J]. 有色金属(冶炼部分), 2018(1): 16-19.
[78] 罗希韬, 王志奇, 武景丽, 等. 基于热重红外联用分析的PE、PS、PVC热解机理研究[J]. 燃料化学学报, 2012, 40(9): 1147-1152.
[82] 胡国荣, 李国, 邓新荣, 等. 针铁矿法从铬铁合金硫酸浸出液中除铁[J]. 湿法冶金, 2006, 25(4): 198-201.
[83] 邓永贵, 陈启元, 尹周澜, 等. 锌浸出液针铁矿法除铁[J]. 有色金属, 2010, 62(3): 84-88.
[84] 阳征会, 龚竹青, 李宏煦, 等. 用黄钠铁矾渣制备复合镍锌铁氧体[J].中南大学学报(自然科学版), 2006, 37(4): 685-691.
[85] 杨玮娇, 马保中, 杨卜, 等. 硝酸浸出液中的铝高效脱除[J]. 有色金属工程, 2018, 8(4): 61-65.
[86] 罗炎, 皮露, 何克杰, 等. 软锰矿烟气脱硫浸出液同步去除铁、铝试验研究[J]. 湿法冶金, 2015, 34(3): 218-221, 224.
[87] 李学鹏, 杨斌, 刘大春, 等. 硫酸镍电解液净化除杂工艺研究[J]. 稀有金属, 2010, 34(2): 271-275.
[89] 胡宝兰. 含镍废料的综合利用研究[D]. 合肥: 合肥工业大学化学与化工学院, 2002: 1-2.
[90] 刘争伟, 于枭影, 钟晓聪, 等. 含Al、Ca复合除氟剂在含氟硫酸锌溶液中的除氟性能[J]. 中国有色金属学报, 2016. 26(5): 1151-1157.
[91] 张萌, 邱琳, 于晓晴, 等. 亚铁盐与高铁盐除磷工艺的对比研究[J]. 高校化学工程学报, 2013, 27(3): 159-165.


