
Characterization of carbon encapsulated Fe-nanoparticles prepared by confined arc plasma
WEI Zhi-qiang1, 2, LIU Li-gang2, YANG Hua1, 2, ZHANG Cai-rong1, 2, FENG Wang-jun2
1. State Key Laboratory of Gansu Advanced New Nonferrous Metal Materials,
Lanzhou University of Technology, Lanzhou 730050, China;
2. School of Science, Lanzhou University of Technology, Lanzhou 730050, China
Received 21 October 2010; accepted 10 March 2011
Abstract:
Carbon encapsulated Fe nanoparticles were successfully prepared via confined arc plasma method. The composition, morphology, microstructure, specific surface area and particle size of the product were characterized via X-ray diffraction, transmission electron microscopy, high resolution transmission electron microscopy, energy dispersive X-ray spectrometry and Brunauer-Emmett-Teller N2 adsorption. The experiment results show that the carbon encapsulated Fe nanoparticles have clear core-shell structure. The core of the particles is body centered cubic Fe, and the shell is disorder carbons. The particles are in spherical or ellipsoidal shapes. The particle size of the nanocapsules ranges from 15 to 40 nm, with the average value of about 30 nm. The particle diameter of the core is 18 nm, the thickness of the shells is 6-8 nm, and the specific surface area is 24 m2/g.
Key words:
carbon encapsulation; Fe nanoparticles; confined arc plasma;
1 Introduction
During the past years, metal nanoparticles have attracted considerable attention due to their novel physical and chemical properties, and have a broad range of promising applications, such as ultrahigh-density magnetic recording media, environmental protection, biomedicine, catalysis, magnetic toners for xerography, field oriented drug delivery systems, biotoxin scavengers, and magnetic fluid [1-4]. However, the pure metal nanoparticles are activated and unstable due to easy agglomeration, oxidation in air, and dissolution in acid, which limit their potential applications in industry or academic and technological studies. In order to overcome these limitations, depositing a protective shell has been recommended as one prospective method to improve the chemical stability of metal nanoparticles. By far, various coating materials have been proposed, e.g. metal oxides, silica, titanium, boron oxide, and polymers. Carbon seems to be the most desired material for encapsulation because not only can carbon encapsulated metal nanoparticles retain their intrinsic nanocrystalline properties, and offer a good opportunity to investigate the dimensionally confined systems, but also the high steady encapsulation shell can effectively prevent the core metal nanoparticles from agglomeration and oxidation [5-8].
Many methods have been applied to synthesizing carbon encapsulated metal magnetic nanoparticles, such as solution phase chemical reduction, instant pyrolysis, arc discharge, microwave heating, radio frequency thermal plasma, explosion, copyrolysis, and chemical vapor condensation [9-15]. However, to the best of our knowledge, most of the reported experimental techniques for the synthesis of nanopowders were still limited in laboratory scale due to some unresolved problems, such as special reaction conditions, tedious procedures, complex apparatus, low-yield and high-cost. In addition, byproducts such as carbon nanotubes, nanofibers, nanoparticles, and free amorphous carbon were likely to form by using these techniques. Therefore, from a practical viewpoint, it is vital to develop a more efficient and lower cost technique to synthesize carbon encapsulated metal nanoparticles. Arc discharge technique is a conventional and versatile method, which has been widely used for the synthesis of metal nanoparticles, alloy, metal-oxide system, and fullerenes and carbon nanotubes [16-17].
In this work, DC arc plasma equipment was modified: the electrode device was devised to be in vertical position structure; the graphite crucible served as the anode, which was tightly installed in the inner of the water-cooled copper crucible; the cathode was devised to be plasma gun, which was mainly made up of the graphite rod and the water-cooled nozzle. This technique possessed several advantages: 1) as the process was operated in inert atmosphere, no related contaminants were introduced during powder preparation; 2) it possessed some special characteristics, such as high energy density, high temperature, high velocity and rapid cooling, leading to the formation of nanoparticles with high purity, small size, and good surface activity; 3) the physical and chemical properties can be easily improved by varying the technological parameters; 4) the present approach was promising for large scale production of carbon encapsulated metal nanoparticles. In this work, carbon encapsulated Fe nanoparticles were prepared by using modified confined arc plasma technique in inert atmosphere. In addition, the composition, morphology, microstructure, specific surface area, particle size distribution of the product by this process were characterized via X-ray diffraction (XRD), transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM), energy dispersive X-ray spectrometry (EDS) and Brunauer- Emmett-Teller (BET) N2 adsorption.
2 Experimental
The schematic diagram of the experimental installation designed to obtain carbon encapsulated Fe nanoparticles was fully illustrated elsewhere [18]. Before the experiment, the synthesis chamber was evacuated to a base pressure of 10-3 Pa, and diluted twice by Ar, then Ar was filled to a given pressure. The arc in the inert environment was automatically ignited between the graphite electrode and the crucible with the high frequency ignited device, which was maintained by the current source at the pre-established values of the voltage and current. The bulk metal Fe was heated and melted by the high temperature plasma. The metal atoms were detached from the metal surface when their kinetic energy exceeded the metal superficial energy, and evaporated into free atom state. The vaporization of Fe and gasification of carbon occurred synchronously. A plasma plume was produced with super high-density Fe vapor, gasified carbon and argon species, and the free atoms diffused around and collided each other to decrease the nucleus forming energy. When the metal vapor was supersaturated, a new phase nucleated homogeneously out of the aerosol systems. The droplets were rapidly cooled and combined to form primary particles by an aggregation growth mechanism. The carbon encapsulated Fe nanoparticles were formed by free diffusion of internal solid state carbon to the surface and deposition of external gas phase carbon on the outside. The particles were transported from the nucleation and growth region to the inner walls of the cylinder by the free inert gas convection between the hot evaporation source and the cooled collection cylinder. The dispersed carbon encapsulated Fe nanoparticles were collected at the inner walls of the water-cooled collection cylinder after a period of stabilization with working gas.
The crystalline structures of the products were characterized by a rotating-target X-ray diffractometer (Rigaku D/Max-2400, Japan) with graphite monochromatized Cu Ka radiation (1.540 56 ?, 40 kV, 100 mA). The average crystalline grain size of the metal core was estimated from the half maximum width and the peak position of an XRD line broadened according to the Scherrer formula. The particle size and morphology of the samples were examined by JEOL JEM-1200EX transmission electron microscope (TEM) and JEM-2010 high-resolution transmission electron microscope (HRTEM). The chemical composition of the products was analyzed by energy dispersive X-ray spectroscopy (EDS) at an acceleration voltage of 200 keV in TEM. The specific surface area of the samples was calculated from the BET adsorption equation and measured on an accelerated surface area porosimetry instrument (ASAP 2010) produced by Micromerities Corp. at liquid nitrogen temperature (77.8 K).
3 Results and discussion
3.1 Crystalline structures
Figure 1 shows the powder X-ray diffraction (XRD) pattern of carbon encapsulated Fe nanoparticles. It can be seen from Fig. 3 that all the peaks in the curves are significantly broadened, corresponding to the nanocrystalline character of the particles, which is in agreement with the result of TEM characterization. The broad peak at 2θ=26.24° can be attributed to the diffraction of (002) planes of the hexagonal graphite structure, where the broad diffraction signal of 21°-30° is possible from amorphous carbon. Whereas other peaks at 2θ=44.4°, 64.88° and 82.16° can be readily indexed as the crystal planes (110), (200) and (211) of metallic Fe, respectively. All these diffraction peaks can be perfectly indexed to the body centered cubic (BCC) crystalline structure of Fe, not only in peak position, but also in their relative intensity of the characteristic peaks, which is in accordance with the standard spectrum (JCPDS, No. 85—1410). In addition, there are no peaks assigned to Fe carbide compound phase.
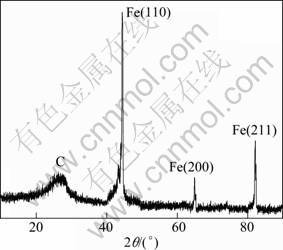
Fig. 1 XRD pattern of carbon encapsulated Fe nanoparticles
The grain size for the metal core can be calculated from the half width of the major diffraction peak (110) according to Scherrer formula, ![]() , where d represents the grain size; K=0.89 is the Scherrer constant related to the shape and index (hkl) of the crystals; λ is the wavelength of the X-ray (Cu Kα, 1.540 56 ?,); θ is the diffraction angle of the peak; B stands for the full width at half-height of the peaks (in radian) given by B2=Bm2-Bs2, where Bm is the full width at half-maximum (FWHM) of the sample and Bs is the half-width of a standard sample with a known crystal size greater than 100 nm. The effect of instrumental broadening on the reflection peaks is calibrated. The crystallite size of the encapsulated Fe nanoparticles is 20 nm which was calculated from the measured values for the spacing of the (110) plane.
, where d represents the grain size; K=0.89 is the Scherrer constant related to the shape and index (hkl) of the crystals; λ is the wavelength of the X-ray (Cu Kα, 1.540 56 ?,); θ is the diffraction angle of the peak; B stands for the full width at half-height of the peaks (in radian) given by B2=Bm2-Bs2, where Bm is the full width at half-maximum (FWHM) of the sample and Bs is the half-width of a standard sample with a known crystal size greater than 100 nm. The effect of instrumental broadening on the reflection peaks is calibrated. The crystallite size of the encapsulated Fe nanoparticles is 20 nm which was calculated from the measured values for the spacing of the (110) plane.
3.2 Morphology and particle size
Figure 2 shows the representative TEM images of carbon encapsulated Fe nanoparticles. It can be seen from Fig. 2 that the carbon encapsulated Fe nanoparticles possess spherical or ellipsoidal shapes, and are well dispersed with smooth surface and uniform size. Most of nanocapsules possess the distinct shell/core structure with isolated cores. The particle size of the nanocapsules ranges from 15 to 40 nm, with an average particle diameter of about 30 nm. The size of the core ranges from several nanometers to 30 nm, and the mean particle size of the core is about 18 nm, while most of shells are 6-8 nm in thickness. The mean particle size of the core determined by TEM is in good agreement with the average crystallite size calculated by Scherrer formula from the typical XRD pattern. It suggests that the core of most of the iron particles of possibly composed of one grain, namely, iron particles are single crystal. According to the TEM image, except for carbon encapsulated Fe nanoparticles, no carbon nanotubes or nanowires, empty carbon cages or other unwanted byproducts are observed. This proves that this technique is an efficient method to obtain carbon encapsulated nanoparticles, which has successfully overcome the problem of agglomeration and oxidation of the core metal nanoparticles.
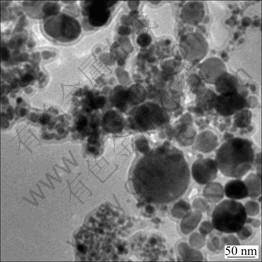
Fig. 2 TEM image of carbon encapsulated Fe nanoparticles
Figure 3 shows the typical HRTEM image of carbon encapsulated Fe nanoparticles. From the HRTEM image, a single core-shell structure with Fe particle as core and carbonaceous material as shell is clearly observed. It is evident that an encapsulation structure of carbon layer is surrounded by a crystalline metal core. The carbon encapsulated metal nanoparticles possess clear core-shell structure, where the core of the particles is iron (dark globules in Fig. 3), and the shell of the particles is carbon (light black shadow). The particle diameter of the core is about 18 nm and the thickness of the cages is about 8 nm. It can be estimated that the interlayer distance of crystalline Fe grain is about 0.20 nm, corresponding to Fe (110) plane.
3.3 Chemical compositions
The chemical compositions of carbon encapsulated Fe nanopowders were examined by using energy dispersive X-ray spectrometry (EDS). The EDS result is shown in Fig. 4. It can be seen from Fig. 4 that the products are mainly composed of elements Fe and C. The characteristic peaks of Fe show that the core of the particles is iron, and the peaks of C show that the shell of the particles is of carbon.
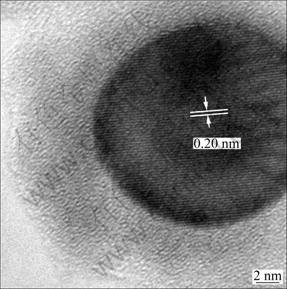
Fig. 3 HRTEM image of carbon encapsulated Fe nanoparticles

Fig. 4 EDS pattern of carbon encapsulated Fe nanoparticles
3.4 Specific surface area
The specific surface area analysis was carried out on carbon encapsulated Fe nanoparticles by BET method. Assuming the particles have solid, spherical shape with smooth surface and same size, the specific surface area can be related to the average equivalent particle size by the equation: DBET=6000/(ρSw), where DBET is the average diameter of a spherical particle, Sw represents the measured surface area of the powder, ρ is the average theoretical density of the Fe core and carbon shell (the particle diameter of the Fe core is 18 nm and the thickness of carbon shell is 6 nm), and the value is 3.75 g/cm3. Figure 5 shows the BET plot of carbon encapsulated Fe nanoparticles. The specific surface area is 24 m2/g, which was calculated with the multi-point BET-equation. The calculated average equivalent particle size is 32 nm. It is noticed that the particle size obtained from the BET methods agrees very well with the result by TEM observations.

Fig. 5 BET plots of carbon encapsulated Fe nanoparticle
4 Conclusions
1) Carbon encapsulated Fe nanoparticles with uniform size, well-dispersed and spherical shape were successfully prepared by confined arc plasma method. These nanoparticles have clear core-shell structure, the core of the particles is of iron, and the shell of the particles is of disorder carbons.
2) The crystalline structure of the encapsulated Fe nanoparticles is BCC. The particle size of the nanocapsules ranges from 15 to 40 nm, with an average value of about 30 nm. The particle diameter of the core is about 18 nm and the thickness of the shells is 6-8 nm, and the specific surface area is 24 m2/g.
References
[1] SUBRAMONEY S. Novel nanocarbons structure, properties, and potential applications [J]. Adv Mater, 1998, 10: 1157-1171.
[2] BYSTRZEJEWSKI M, PYRZYNSKA K, HUCZKO A. Carbon- encapsulated magnetic nanoparticles as separable and mobile sorbents of heavy metal ions from aqueous solutions [J]. Carbon, 2009, 47: 1189-1206.
[3] DOBSON J. Magnetic nanoparticles for drug delivery [J]. Drug Develop Res, 2006, 67: 55-64.
[4] HISATO T, TAKASHI Ni, SHIGEO F. Magnetic iron particles with high magnetization useful for immunoassay [J]. Journal of Magnetism and Magnetic Materials, 2009, 321: 1676-1678.
[5] BAKER C, HASANAIN S K, ISMAT S S. The magnetic behavior of iron oxide passivated iron nanoparticles [J]. Journal of Applied Physics, 2004, 96(1): 6657-6662.
[6] ZHANG X F, DONG X L, HUANG H. Synthesis, growth mechanism and magnetic properties of SiO2-coated Co nanocapsules [J]. Acta Materialia, 2007, 55: 3727-3733.
[7] ZHU L Z, MA J W, JIA N Q. Chitosancoated magnetic nanoparticles as carriers of fluorouracil: Preparation, characterization and cytotoxicity studies [J]. Colloids and Surfaces B, 2009, 68: 1-6.
[8] URSZYLA N, MARCIN P, WALEERIAN A. Carbon-coated cobalt nanoparticles [J]. Materials Science and Engineering C, 2007, 27: 1273-1276.
[9] ZHU G X, WEI X W, JIANG S. A facile route to carbon-coated nickel-based metal nanoparticles [J]. J Mater Chem, 2007, 17: 2301-2306.
[10] JACOB D S, GENISH I, KLEIN L. Carbon-coated core shell structured copper and nickel nanoparticles synthesized in an ionic liquid [J]. J Phys Chem B, 2006, 110: 17711-17714.
[11] BYSTRZEJEWSKI M, HUCZKO A, LANGE H. Large scale continuous synthesis of carbon-encapsulated magnetic nanoparticles [J]. Nanotechnology, 2007, 18: 145608-145617.
[12] WU W, ZHU Z, LIU Z. Preparation of carbon-encapsulated iron carbide nanoparticles by an explosion method [J]. Carbon, 2003, 41: 317-321.
[13] WANG C F, WANG J N, SHEG Z M. Solid-phase synthesis of carbon-encapsulated magnetic nanoparticles [J].J Phys Chem C, 2007, 17: 6303-6307.
[14] GANDHI A S, JAYARAM V, CHOKSHI A H. Low temperature densification behaviour of metastable phases in ZrO2-AlO2 powders produced by spray pyrolysis [J]. Mater Sci Eng A, 2001, 304: 785-789.
[15] HUO J P, SONG H H, CHEN X H. Preparation of carbon-encapsulated iron nanoparticles by co-carbonization of aromatic heavy oil and ferrocene [J]. Carbon, 2004, 42: 3177-3182.
[16] RUOFF R S, LORENTS D C, CHAN B. Single crystal metals encapsulated in carbon nanoparticles [J]. Science, 1993, 259: 346-348.
[17] SAITO Y, YOSHIKAWA T, OKUDA M. Cobalt particles wrapped in graphitic carbon prepared by an arc discharge [J]. Journal of Applied Physics, 1994, 75: 134-137.
[18] WEI Z Q, XIA T D, BAI L F. Efficient preparation for Ni nanopowders by anodic arc plasma [J]. Materials Letters, 2006, 60(3): 766-770.
约束弧等离子体制备碳包覆铁纳米颗粒的性能
魏智强1, 2, 刘立刚2, 杨 华1, 2, 张材荣1, 2, 冯旺军2
1. 兰州理工大学 甘肃省有色金属新材料省部共建国家重点实验室,兰州 730050;
2. 兰州理工大学 理学院,兰州 730050
摘 要:采用约束弧等离子体技术制备碳包覆铁纳米颗粒,利用X射线衍射、透射电子显微镜、高分辨透射电子显微镜、X射线能量色散分析谱仪和N2 吸附等测试手段对样品的化学成分、形貌、微观结构、比表面积和粒度等特征进行表征分析。结果表明:采用约束弧等离子体技术制备的碳包覆纳米金属颗粒具有明显的核-壳结构,内核金属为体心立方结构的铁,外壳为无定形碳。颗粒大多呈球形和椭球形,粒径分布在15~40 nm范围内,平均粒径为30 nm,内核粒径为18 nm,外层碳的厚度为6~8 nm,比表面积为24 m2/g。
关键词:碳包覆;Fe纳米颗粒;约束弧等离子体
(Edited by YANG Hua)
Foundation item: Project (208151) supported by the Key Project of Ministry of Education, China; Project (1014RJZA035) supported by the Natural Science Foundation of Gansu Province, China
Corresponding author: WEI Zhi-qiang; Tel: + 86-931-2973780; E-mail: zqwei7411@163. com
DOI: 10.1016/S1003-6326(11)60967-9


