Effects of low melting point metals (Ga, In, Sn) on hydrolysis properties of aluminum alloys
Fan-qiang WANG1, Hui-hu WANG1,2, Jian WANG1, Jia LU1,Ping LUO1, Ying CHANG3, Xin-guo MA4, Shi-jie DONG1,2
1. School of Mechanical Engineering, Hubei University of Technology, Wuhan 430068, China;
2. Hubei Provincial Key Laboratory of Green Materials for Light Industry,Hubei University of Technology, Wuhan 430068, China;
3. School of Materials Science and Engineering, Hubei University of Technology, Wuhan 430068, China;
4. School of Science, Hubei University of Technology, Wuhan 430068, China
Received 12 January 2015; accepted 2 April 2015
Abstract:
Low melting point metals (Ga, In, Sn) as alloy elements were used to prepare Al-In-Sn and Al-Ga-In-Sn alloys through mechanical ball milling method. The effects of mass ratio of In to Sn and Ga content on the hydrolysis properties of aluminum alloys were investigated. X-ray diffraction (XRD) and scanning electron microscopy (SEM) with energy disperse spectroscopy (EDS) were used to analyze the compositions and morphologies of the obtained Al alloys. The results show that the phase compositions of Al-In-Sn ternary alloys are Al and two intermetallic compounds, In3Sn and InSn4. All Al-In-Sn ternary alloys exhibit poor hydrolysis activity at room temperature. Al-In-Sn alloy with the mass ratio of In to Sn equaling 1:4 has the highest hydrogen yield. After Ga is introduced to the ternary alloys, the hydrolysis activity of aluminum alloys at room temperature is greatly improved. It is speculated that the addition of Ga element promotes the formation of defects inside the Al alloys and Ga-In3Sn-InSn4 eutectic alloys on the alloys surface. Al atoms can be dissolved in this eutectic phase and become the active spots during the hydrolysis process. The small size and uniform distribution of this eutectic phase may be responsible for the enhancement of hydrolysis activity.
Key words:
aluminum alloy; low melting point metal; hydrolysis; hydrogen generation; mechanical ball milling method;
1 Introduction
As a kind of green and renewable energy, hydrogen has been considered as one of the ideal alternatives for fossil fuels [1]. However, the storage and generation of hydrogen are still main issues needed to be solved urgently regarding the use of hydrogen as fuel [2,3]. Recently, the use of aluminum-water reaction to generate hydrogen has attracted more and more attention [4-15]. The main reason is that aluminum is very rich in resources and it also has high hydrogen storage value, thus aluminum can be used as the portable hydrogen source. Furthermore, aluminum-water reaction system is very simple and the hydrolysis reactants can be easily recycled [16]. It is known that pure aluminum is hard to react with water for hydrogen production due to a dense oxide layer on its surface [17,18]. In order to improve the aluminum reactivity under mild conditions, this dense oxide layer should be destroyed or removed, thus water can come into contacting with the fresh aluminum surface for reaction.
Till now, two ways have been developed to improve the aluminum reactivity for hydrogen production. One is to use salts or oxides as additives to ruin the oxide layer on the aluminum surface by mechanical ball milling method [6,8]. The other is to use low melting point metals, such as Bi, Ga, In and Sn, as alloy elements to moisten the aluminum surface and destroy the surface oxide layer by mechanical ball milling method or melting method. For comparison, the aluminum alloys prepared by the second way exhibited higher hydrolysis activity under mild conditions than those obtained from the first way. Therefore, the preparation and hydrogen generation of aluminum alloys have been reported in Refs. [9-15]. FAN et al [9,10] found that the addition of Bi could produce a lot of defects on the aluminum surface during the ball milling process and greatly promote the aluminum-water reaction at room temperature. The highest hydrogen generation volume and hydrogen conversion efficiency can reach 1050 mL/g and 93.4%, respectively. ILYUKHINA et al [11] prepared a series of aluminum alloys with low melting metals, such as Ga, In, Sn and Zn, by mechanical milling method. These alloys could react well with water at 20 and 25 °C. The hydrogen conversion efficiency of the aluminum alloy with 6% Ga (mass fraction) nearly reached 90%. Our group [12] also fabricated aluminum alloys containing Ga, In and Sn through mechanical ball milling method which had a high hydrogenation rate (1080 mL/(g·min)) at room temperature. For the aluminum alloys prepared by melting method, the hydrogen conversion efficiency of aluminum alloy 50%Al-34%Ga-11%In-5%Sn (mass fraction) could reach 83% at room temperature [13]. It was also observed that the hydrolysis activity of aluminum alloys gradually declined at room temperature with the decrease of Ga addition content [14]. However, the current researches about the causes of the differences on the hydrogen conversion efficiency are very few. Especially, the relationship between the hydrolysis reactivity of aluminum alloys and its element compositions, including the element component and their relative mass ratio, has not been well studied and explained reasonably.
In this study, a series of aluminum alloys were prepared using mechanical ball milling method. By carefully controlling the kinds of added alloy elements and their content, the effects of added alloy elements, including Ga, In and Sn, on the microstructure and the hydrolysis properties of aluminum alloys were analyzed and measured. The activation mechanism of added alloy elements was also discussed.
2 Experimental
Industrial pure Al powder (~75 μm, 99.99%), Ga (99.8%), Sn (~75 μm, 99.9%), In (~75 μm, 99%) were used as starting materials. The typical preparation process was expressed as follows. Firstly, 20 g mixture with different alloy elements and mass ratios was weighed and put into the milling tanks. For each mixture, the mass ratio of Al powder to low melting point metals was set as 9:1. The detailed chemical compositions of mixtures are shown in Table 1. Secondly, 200 g grinding ball (Ball-to-powder ratio equals 10:1) was added to the mixtures. After that, the milling tanks were sealed in an argon-filled glove box. The mechanical alloying process was performed in a planetary ball miller. The milling speed and milling time were set as 360 r/min and 12 h, respectively. After the ball milling process, the as-prepared aluminum alloys were collected in an argon-filled glove box and stored in the argon-filled sample bag for further use. Although the preparation process may cause the mass ratio to deviate from the original experimental design, the deviation degree may be negligible for ball milling which is a uniform mixing process. Therefore, it was suggested that the element mass ratio of each sample was similar to the experimental design.
Table 1 Element compositions of aluminum alloys (mass fraction, %)
The aluminum-water hydrolysis reaction test was carried out in a 500 mL four-necked bottle. For each test, 1 g aluminum alloy was added to 300 mL water in a four-necked bottle. The hydrogen volume was tested through drainage gas-collecting method. The hydrogen generation rate was calculated by the hydrogen volume and reaction time.
The phase compositions were recorded by an XD-2 type diffractometer (Beijing Purkinje General Co., Ltd.) with Cu Kα radiation. The step size was set as 0.02 (°)/step and the time per step was 1.2 s. The morphology and element analysis of aluminum alloys were characterized by field emission scanning electron microscopy (FE-SEM) (Quanta FEG 450) equipped with an energy-dispersive spectrometer (EDS).
3 Results and discussion
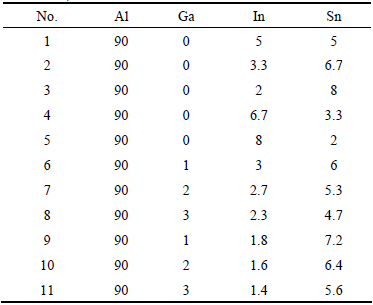
Figure 1 shows the hydrogen yields of as-prepared Al-In-Sn ternary alloys reacting with water at room temperature. The as-prepared Al-In-Sn ternary alloys were designed and produced with different mass ratios of In to Sn in order to study the effect of the mass ratio of In to Sn on the hydrolysis properties of aluminum alloys. It can be seen from Fig. 1(a) that the mass ratio of In to Sn has an obvious effect on the final hydrogen yields of ternary aluminum alloys. When the mass ratio of In to Sn equals 1, the hydrogen yield is only 330 mL, which is the lowest among all the samples. With the increase of either In or Sn mass, the hydrolysis activity of Al-In-Sn ternary alloys is enhanced. Especially, Al-In-Sn ternary alloy with the mass ratio of In to Sn equaling 1:4 exhibits the highest hydrogen yield, reaching 430 mL. The hydrogen conversion efficiency of Al-In-Sn ternary alloys with different mass ratios of In to Sn is revealed in Fig. 1(b). It indicates that there is an optimum mass ratio of In to Sn for activating Al-In-Sn ternary alloys. Furthermore, the Al-In-Sn ternary alloys with higher Sn content demonstrates enhanced hydrolysis activity.
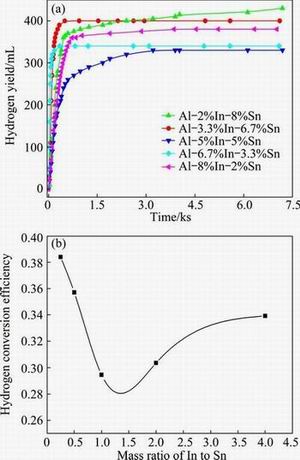
Fig. 1 Hydrogen yields (a) and conversion efficiency (b) of Al-In-Sn alloys with different mass ratios of In to Sn at room temperature
From Fig. 1, it can be observed that the hydrogen yields and conversion efficiency of Al-In-Sn ternary alloys are very low at room temperature. For further studying the hydrolysis properties of Al-In-Sn alloys, the ternary alloys with higher Sn content were selected and hydrolyzed in water at 60 °C. As this temperature is much lower than the boiling point of water, little water steam will be produced. Therefore, the water steam may have little influence on the final hydrogen yields. The hydrogen yields of these three Al-In-Sn ternary alloys with different mass ratios of In to Sn are shown in Fig. 2(a). The variation trend on hydrogen yields at 60 °C is similar to that obtained at room temperature. The Al-In-Sn ternary alloy with the mass ratio of In to Sn equaling 1:4 shows the highest hydrogen yield, reaching 690 mL. The hydrogen generation rates of Al-In-Sn ternary alloys at 60 °C were calculated and are shown in Fig. 2(b). For each aluminum alloy, higher hydrogen generation rate is obtained at 0-400 s. After 400 s, the hydrogen generation rate declines obviously although the reaction still continues. The total hydrolysis reaction ends in 4000 s. Figure 2(b) also shows that the hydrogen generation rate increases rapidly when the reaction is induced. It gets the maximum value at the reaction time of about 20 s. The highest hydrogen generation rate is 14.6 mL/(g·s) for Al-In-Sn ternary alloy with the mass ratio of In to Sn equaling 1:4 at 60 °C. Compared with Fig. 1, Fig. 2 also demonstrates that higher reaction temperature may promote the hydrolysis activity of aluminum alloys and can obtain higher hydrogen yields as well as higher hydrogen generation rates.
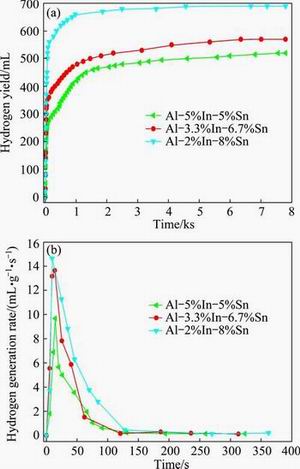
Fig. 2 Hydrogen yields (a) and generation rates (b) of Al-In-Sn alloys reacting with water at 60 °C
In order to improve the hydrolysis activity of Al-In-Sn ternary alloys at room temperature, low melting point metal Ga was added to prepare Al-Ga-In-Sn quaternary alloys. The total alloy elements amount accounts for 10% of mass of quaternary alloys, while the mass ratio of In to Sn is fixed as 1:2 or 1:4 and the Ga content varies from 1% to 3%. Figure 3 shows the hydrogen yields of Al-Ga-In-Sn alloys reacting with water at room temperature. For comparison, the hydrolysis curves of Al-In-Sn ternary alloys with the mass ratios of In to Sn equaling 1:2 and 1:4 were also added in Fig. 3. For both kinds of Al-Ga-In-Sn alloys with the mass ratios of In to Sn equaling 1:2 and 1:4, the lower the Ga content, the higher the hydrogen yield. Interestingly, Al-Ga-In-Sn alloys with the mass ratio of In to Sn equaling 1:2 exhibit higher hydrolysis activity at room temperature in contrast to those with the mass ratio of In to Sn equaling 1:4, which is different from the Al-In-Sn ternary alloys. Al-1%Ga-3%In-6%Sn quaternary alloy shows the highest hydrogen yield, reaching 840 mL.
Fig. 3 Hydrogen yields of Al-Ga-In-Sn alloys with various Ga contents and mass ratios of In to Sn at room temperature
From Fig. 3, it can be found that the addition of metal Ga greatly improves the hydrolysis activity of aluminum alloys at room temperature. The total hydrogen yield and hydrogen conversion efficiency of Al-Ga-In-Sn quaternary alloys (m(In):m(Sn)=1:2) with 1% Ga are 2.1 times those of Al-In-Sn ternary alloys (m(In):m(Sn)=1:2), as shown in Fig. 4.
As the hydrolysis properties of aluminum alloys are determined by their chemical compositions and microstructures, the crystal structures and morphologies of different alloys were analyzed using XRD and SEM techniques. Figure 5(a) shows the XRD patterns of different Al-In-Sn ternary alloys. All samples contain the diffraction peaks of crystalline Al phase, while single In and Sn phases are not found. Two intermetallic compounds, In3Sn and InSn4, are found in the patterns which may be ascribed to the low solid solubility of In and Sn in the aluminum matrix [19]. Both kinds of compounds exist in Al-In-Sn ternary alloys with the mass ratios of In to Sn equaling 1:1 and 1:2. When the mass ratio of In to Sn decreases to 1:4, only the diffraction peaks of InSn4 are observed in the pattern. In the meanwhile, In3Sn and Al phases are detected in Al-In-Sn ternary alloy when the mass ratio of In to Sn increases to 4. The diffraction peaks of InSn4 compound phase are not found in the XRD pattern. Actually, the relative atomic mass of In element (114.818) nearly equals that of Sn (118.710), thus the mass ratio of In to Sn can be regarded as the mole ratio. Therefore, it is easy to understand the change of intermetallic compounds with the increase of mass ratio of In to Sn.

Fig. 4 Hydrogen yields and conversion efficiencies of Al-Ga-In-Sn alloys reacting with water at room temperature
The crystal structures of Al-Ga-In-Sn quaternary alloys were also detected, as shown in Fig. 5(b). However, the XRD patterns are nearly the same as those of Al-In-Sn alloys. No diffraction peaks of Ga are found in the patterns. The same result was also reported by FAN et al [10] who prepared aluminum alloys using Ga as alloy element by mechanical ball-milling method. This may be attributed to the high solid solubility of Ga in the aluminum matrix (10%, mole fraction) and the low content of metal Ga (less than 3%, mass fraction).
The SEM images of Al-Ga-In-Sn quaternary alloys with the same mass ratio of In to Sn (1:2) and different Ga contents (1% and 3%) are shown in Fig. 6. A lot of defects, including cracks and grooves, form on the surface of alloys. It has been studied that low melting point metals (Ga, In, Sn) can be deposited on the surface of aluminum as single or multiple atoms and produce a lot of defects, resulting in the alumina membrane separating and abating [10-12]. The newly formed aluminum surface during the process of mechanical ball grinding could react well with water at room temperature. From Fig. 6, it can also be found that there are alloy phases which are distributed on the surface of alloys. Furthermore, these alloy phases are smaller and more uniform in Al-1%Ga-3%In-6%Sn alloy than those in Al-3%Ga-2.3%In-4.7%Sn alloy.
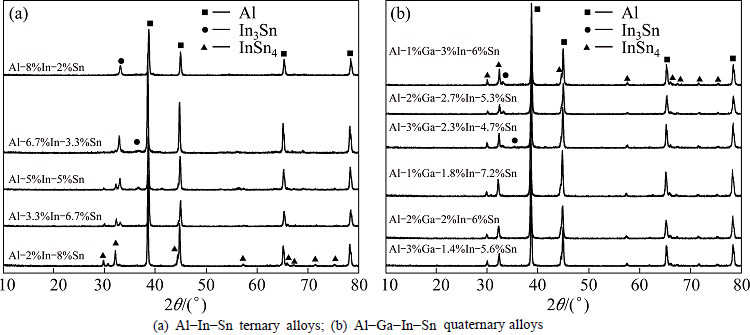
Fig. 5 XRD patterns of all alloys with different mass ratios of Ga, In and Sn: (a) Al-In-Sn ternary alloys; (b) Al-Ga-In-Sn quaternary alloys
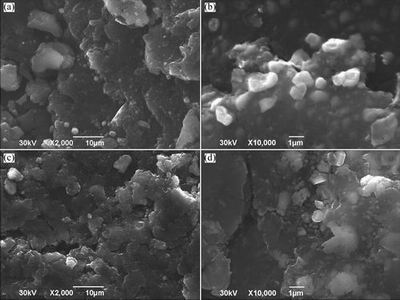
Fig. 6 FE-SEM images of Al-1%Ga-3%In-6%Sn (a, b) and Al-3%Ga-2.3%In-4.7%Sn (c, d)
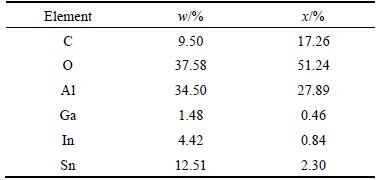
In order to investigate the chemical compositions of these alloy phases, EDS map and spot scannings have been used to analyze Al-3%Ga-2.3%In-4.7%Sn alloy which are shown in Figs. 7 and 8, respectively. From Fig. 7, it can be observed that Ga element is homogeneously dispersed in the whole alloy, while In and Sn alloy elements gather together. The spot scanning results (Fig. 8) of the alloy phases demonstrates that they are mainly composed of Al, Ga, In and Sn. The detailed mass fraction and mole fraction of different elements at spot A are shown in Table 2. According to the XRD results (Fig. 5) and Refs. [15,19], it can be speculated that these alloy phases may be Ga-In3Sn-InSn4 (Ga-In-Sn) eutectic alloy where a lot of Al atoms are solved in it. They are similar to the zinc amalgam formed on the surface of aluminum when zinc powder and mercury were added to the aluminum matrix [20].
Thus, the effects of different alloy elements on the hydrolysis properties of aluminum alloys as well as its activation mechanism can be discussed. Firstly, the low melting point metals (Ga, In, Sn) may promote the formation of defects and second phase in the aluminum matrix during the mechanical ball milling process, as shown in Fig. 6. It is known that the embrittlement of metal can be caused by Rebinder’s effect through simultaneously adding liquid metal and mechanical loading. Therefore, the addition of low melting point metals during the mechanical ball milling process can lead to the embrittlement of aluminum powder. The aluminum cracks form and the alumina membrane is abated. Apparently, more cracks are produced, and more seriously the alumina membrane are destroyed. The liquid low melting point metals will cover and protect the fresh aluminum surface without the oxide layer [11]. Meanwhile, these second phases may enlarge the contact area with water during the aluminum-water reaction. Therefore, the aluminum can react well with water at room temperature. Secondly, Ga can form the eutectic alloy with In3Sn and InSn4 phases, which may have a lower melting point than itself. Al atoms may be easily solved in the liquid eutectic alloy Ga-In3Sn-InSn4 considering the solubility of Al in Ga, In and Sn [19,21]. As the chemical activities of Ga, In and Sn are very low, which may protect Al atoms from oxidation. Thus, this part of Al atoms may become the active spots during the hydrolysis. As the aluminum-water reaction is an exothermal reaction, the Ga-In3Sn-InSn4 eutectic alloy may become semisolid state and Al atoms are more easily to be solved in this kind of eutectic alloy with the development of reaction. More Ga-In3Sn-InSn4 eutectic alloy with small size and uniform distribution will obtain higher hydrolysis activity of aluminum alloys. Furthermore, a part of Ga dissolved in aluminum matrix may distribute along the grain boundary and increase the activated area of aluminum alloys [22]. Therefore, it can be well explained that the hydrogen yield of Al-1%Ga- 3%In-6%Sn is higher than that of other aluminum alloys./P>
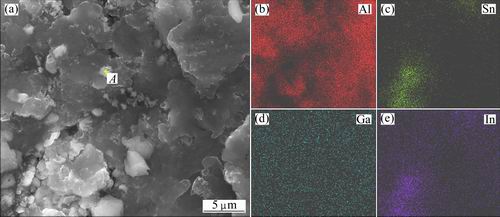
Fig. 7 SEM image (a) and EDS mappings (b-e) of Al-3%Ga-2.3%In-4.7%Sn alloy
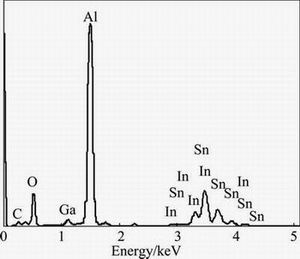
Fig. 8 EDS pattern of spot A labeled in Fig. 7(a)
Table 2 Mass fraction and mole fraction of different elements at spot A in Fig. 7(a)
4 Conclusions
1) Two intermetallic compounds InSn4 and In3Sn form in the aluminum alloys. The highest hydrogen yield was obtained at room temperature for ternary alloys with the mass ratio of In to Sn equaling 1:4.
2) After Ga was introduced to the ternary alloys, the hydrolysis activity of alloys was greatly improved. It is interesting that the quaternary alloy Al-1%Ga-3%In- 6%Sn with the mass ratio of In to Sn equaling 1:2 exhibits the highest hydrogen yield, reaching 840 mL at room temperature. However, the higher the content of Ga is, the lower the hydrolysis activity of Al-Ga-In-Sn quaternary alloys is.
3) The experiment results indicate that the alumina membrane on the Al surface may be separated and abated during the mechanical process for the addition of low melting point metals. The newly formed Al surface can react well with water, thus improving the hydrolysis activity of Al alloys.
4) The formation of Ga-In3Sn-InSn4 eutectic alloy on the quaternary alloy surface can dissolve Al atoms and protect it from oxidation, which may become the active spots during the hydrolysis. The Ga-In3Sn-InSn4 eutectic alloy with smaller size and more uniform distribution will obtain higher hydrolysis activity of aluminum alloys.
References
[1] LATTIN W C, UTGIKAR V P. Transition to hydrogen economy in the United States: A 2006 status report [J]. International Journal of Hydrogen Energy, 2007, 32(15): 3230-3237.
[2] LIU Shu, WANG Liang-liang, YAO Jun, SUN Wen-qiang, FAN Mei-qiang. Hydrogen generation from coupling reactions of AlLi/NaBH4 mixture in water activated by Ni powder [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1140-1145.
[3] CHEN Li-xin, FAN Xiu-lin, XIAO Xue-zhang, XUE Jing-wen, LI Shou-quan, GE Hong-wei, CHEN Chang-pin. Influence of TiC catalyst on absorption/desorption behaviors and microstructures of sodium aluminum hydride [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(6): 1297-1302.
[4] DEYAB M A. Effect of halides ions on H2 production during aluminum corrosion in formic acid and using some inorganic inhibitors to control hydrogen evolution [J]. Journal of Power Sources, 2013, 242: 86-90.
[5] GAI Wei-zhou, DENG Zhen-yan. Effect of trace species in water on the reaction of Al with water [J]. Journal of Power Sources, 2014, 245: 721-729.
[6] WANG Hui-hu, LU Jia, DONG Shi-jie, CHANG Ying, FU Yi-gen, LUO Ping. Preparation and hydrolysis of aluminum based composites for hydrogen production in pure water [J]. Materials Transactions, 2014, 55(6): 892-898.
[7] ALINEJAD B, MAHMOODI K. A novel method for generating hydrogen by hydrolysis of highly activated aluminum nanoparticles in pure water [J]. International Journal of Hydrogen Energy, 2009, 34(19): 7934-7938.
[8] WANG H W, CHUNG H W, TENG H T, CAO G Z. Generation of hydrogen from aluminum and water—Effect of metal oxide nanocrystals and water quality [J]. International Journal of Hydrogen Energy, 2011, 36(23): 15136-15144.
[9] FAN Mei-qiang, XU Fen, SUN Li-xian. Studies on hydrogen generation characteristics of hydrolysis of the ball milling Al-based materials in pure water [J]. International Journal of Hydrogen Energy, 2007, 32(14): 2809-2815.
[10] FAN M Q, XU F, SUN L X, ZHAO J N, JIANG T, LI W X. Hydrolysis of ball milling Al-Bi–hydride and Al-Bi–salt mixture for hydrogen generation [J]. Journal of Alloys and Compounds, 2008, 460(1): 125-129.
[11] ILYUKHINA A V, ILYUKHIN A S, SHKOLNIKOV E I. Hydrogen generation from water by means of activated aluminum [J]. International Journal of Hydrogen Energy, 2012, 37(21): 16382-16387.
[12] WANG Hui-hu, CHANG Yin, DONG Shi-jie, LEI Zhi-feng, ZHU Qing-biao, LUO Ping, XIE Zhi-xiong. Investigation on hydrogen production using multicomponent aluminum alloys at mild conditions and its mechanism [J]. International Journal of Hydrogen Energy, 2013, 38(3): 1236-1243.
[13] ZIEBARTHA J T, WOODALLA J M, KRAMERB R A, CHOI G. Liquid phase-enabled reaction of Al-Ga and Al-Ga-In-Sn alloys with water [J]. International Journal of Hydrogen Energy, 2011, 36(9): 5271-5279.
[14] KRAVCHENKO O V, SEMENENKO K N, BULYCHEV B M, KALMYKOV K B. Activation of aluminum metal and its reaction with water [J]. Journal of Alloys and Compounds, 2005, 397(1): 58-62.
[15] PARMUZINA A V, KRAVCHENKO O V. Activation of aluminium metal to evolve hydrogen from water [J]. International Journal of Hydrogen Energy, 2008, 33(12): 3073-3076.
[16] MURRAY J P. Aluminum production using high-temperature solar process heat [J]. Journal of Solar Energy, 1999, 66(2): 133-142.
[17] MA Guang-lu, ZHUANG Da-wei, DAI Hong-bin, WANG Ping. Controlled hydrogen generation by reaction of aluminum with water [J]. Progress in Chemistry, 2012, 24(4): 650-658.
[18] ZHANG Yi-jie, MA Nai-heng, WANG Hao-wei. Materials to generate hydrogen continuously by aluminum and water reaction at room temperature and normal pressure [J]. Journal of Shanghai Jiao Tong University, 2014, 48(3): 427-429. (in Chinese)
[19] BHATTACHARYA V, CHATTOPADHYAY K. Morphology and phase transformation of nanoscaled indium–tin alloys in aluminum [J]. Materials Science and Engineering A, 2004, 375-377: 932-935.
[20] HUANG Xia-ni,  Chun-ju, HUANG Yue-xiang, LIU Shu, WANG Chao, CHEN Da. Effects of amalgam on hydrogen generation by hydrolysis of aluminum with water [J]. International Journal of Hydrogen Energy, 2011, 36(23): 15119-15124.
Chun-ju, HUANG Yue-xiang, LIU Shu, WANG Chao, CHEN Da. Effects of amalgam on hydrogen generation by hydrolysis of aluminum with water [J]. International Journal of Hydrogen Energy, 2011, 36(23): 15119-15124.
[21] 
 Calorimetric study of Al-Ga system using Oelsen method [J]. Thermochimica Acta, 2012, 544: 6-9.
Calorimetric study of Al-Ga system using Oelsen method [J]. Thermochimica Acta, 2012, 544: 6-9.
[22] CHENG Kai, FU Rui-dong, SANG De-li, JING Lei, LI Yi-jun. A novel method to prepare Al-Ga alloy with intergranular penetrating [J]. Materials Letters, 2014, 129: 84-87.
低熔点金属(Ga,In,Sn)对铝合金水解性能的影响
王凡强1,王辉虎1,2,王 建1,芦 佳1,罗 平1,常 鹰3,马新国4,董仕节1,2
1. 湖北工业大学 机械工程学院,武汉 430068;
2. 湖北工业大学 绿色轻工材料湖北省重点实验室,武汉 430068;
3. 湖北工业大学 材料科学与工程学院,武汉 430068;
4. 湖北工业大学 理学院,武汉 430068
摘 要:以低熔点金属(Ga,In,Sn)作为合金元素,采用机械球磨法制备Al-In-Sn和Al-Ga-In-Sn合金。研究In和Sn的质量比以及Ga的含量对铝合金水解性能的影响。采用X射线衍射、扫面电镜和能谱等手段分析铝合金的成分和形貌。结果表明:Al-In-Sn三元合金主要由Al和两种金属间化合物In3Sn和InSn4组成。所有Al-In-Sn三元合金在室温下的水解活性都很低。当In和Sn的质量比为1:4时,三元合金具有最高的产氢性能。当Ga加入三元合金后,铝合金在室温下的水解活性得到极大的改善,这可能是由于Ga的加入一方面促使铝合金缺陷的产生,另一方面促进Ga-In3Sn-InSn4(Ga-In-Sn)共晶合金在铝合金表面的形成。Al原子能溶入该共晶合金并成为水解过程中的活性点。共晶合金的尺寸小及其在Al合金表面分布均匀是Al-Ga-In-Sn四元合金较强水解活性的主要原因。
关键词:铝合金;低熔点金属;水解;产氢;机械球磨法
(Edited by Mu-lan QIN)
Foundation item: Project (2010CB635107) supported by the Major State Basic Research Development Program of China; Projects (51202064, 51472081) supported by the National Natural Science Foundation of China; Project (2013CFA085) supported by the Natural Science Foundation of Hubei Province, China; Project (2013070104010016) supported by Wuhan Youth Chenguang Program of Science and Technology, China; Project ([2013]2-22) supported by the Open Fund of Key Laboratory of Green Materials for Light Industry of Hubei Province, China
Corresponding author: Hui-hu WANG; Tel: +86-27-59750418; E-mail: wanghuihu@mail.hbut.edu.cn
DOI: 10.1016/S1003-6326(16)64100-6
Abstract: Low melting point metals (Ga, In, Sn) as alloy elements were used to prepare Al-In-Sn and Al-Ga-In-Sn alloys through mechanical ball milling method. The effects of mass ratio of In to Sn and Ga content on the hydrolysis properties of aluminum alloys were investigated. X-ray diffraction (XRD) and scanning electron microscopy (SEM) with energy disperse spectroscopy (EDS) were used to analyze the compositions and morphologies of the obtained Al alloys. The results show that the phase compositions of Al-In-Sn ternary alloys are Al and two intermetallic compounds, In3Sn and InSn4. All Al-In-Sn ternary alloys exhibit poor hydrolysis activity at room temperature. Al-In-Sn alloy with the mass ratio of In to Sn equaling 1:4 has the highest hydrogen yield. After Ga is introduced to the ternary alloys, the hydrolysis activity of aluminum alloys at room temperature is greatly improved. It is speculated that the addition of Ga element promotes the formation of defects inside the Al alloys and Ga-In3Sn-InSn4 eutectic alloys on the alloys surface. Al atoms can be dissolved in this eutectic phase and become the active spots during the hydrolysis process. The small size and uniform distribution of this eutectic phase may be responsible for the enhancement of hydrolysis activity.


