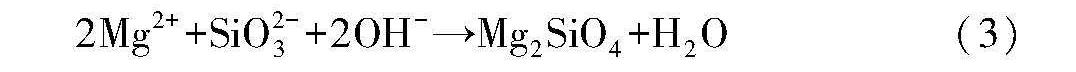网络首发时间: 2018-11-29 17:40
硅酸钠在镁合金表面生成微弧氧化膜时的作用
兰州理工大学省部共建有色金属先进加工与再利用国家重点实验室
摘 要:
分别在不添加和添加硅酸钠的电解液中采用微弧氧化(MAO)技术在AZ91D镁合金表面上制备膜层,研究微弧氧化过程中,电解液中的硅酸钠在镁合金表面形成微弧氧化膜层时的作用,分析电解液中硅酸钠的有无对微弧氧化膜层的影响。膜层的微观结构及物相组成分别通过扫描电镜(SEM)和X射线衍射仪(XRD)进行分析,膜层的耐蚀性通过电化学工作站来表征。结果表明:镁合金微弧氧化过程中,电解液中硅酸钠的加入,将会促使在基材表面生成具有平面连续性的完整的微弧氧化膜层,同时改善膜层的致密性,并获得优质新物相Mg2SiO4。随着电解液中硅酸钠的加入,溶液的电导率增大,基材表面的起弧电压降低,击穿变得容易发生。SiO32-离子与电解液中的其他阴离子协同作用,使得阳极表面微观电位强弱区的对比态势加剧,从而促使放电火花的燃、熄两种状态在基材表面此起彼伏、交替进行,并由此加速了放电火花在基材表面的辗转游移,继而避免了局部热量累积有可能造成的宏观小凹坑的出现,以及微观裂纹的产生。电解液中加入硅酸钠后,所得膜层的腐蚀电流密度减小了1个数量级,线性极化电阻增大了约16倍,膜层的耐蚀性明显得到提高。
关键词:
中图分类号: TG174.4
作者简介:孙乐(1986-),男,江西吉安人,博士研究生,研究方向:轻金属的表面改性与防护;E-mail:sl7398821@163.com;;*马颖,教授;电话:0931-2973563;E-mail:maying@lut.cn;
收稿日期:2018-09-26
基金:甘肃省创新研究群体计划项目(1111RJDA011)资助;
Role of Sodium Silicate for Coating Forming on Magnesium Alloys with Micro Arc Oxidation
Sun Le Ma Ying Dong Hairong An Lingyun Wang Sheng
State Key Laboratory of Advanced Processing and Recycling of Non-ferrous Metals,Lanzhou University of Technology
Abstract:
AZ91 D magnesium alloys were processed by micro are oxidation(MAO) in electrolytes without and with sodium silicate,respectively.The role of sodium silicate during MAO treatment was investigated and its effect on MAO coatings was analyzed.Scanning electron microscope(SEM) and X-ray diffraction(XRD) were employed to characterize the microstructure and phase composition of MAO coatings,respectively.And the corrosion resistance of MAO coatings was evaluated by electrochemical workstation.The results showed that a complete,uniform coating was obtained during MAO treatment once sodium silicate was added into the electrolyte,and in turn,the coating's compactness was improved and a new phase Mg2 Si04 with excellent properties was generated in the coatings.With addition of sodium silicate into the electrolyte,the solution conductivity increased and the ignition voltage on specimen surfaces decreased during MAO process,which facilitated a breakdown easily developing on specimen surfaces.Further on this foundation,the competing state in terms of the potential levels in different areas would be enhanced on the anode surface under the coactions between the silicate ions and other anions in the electrolyte,which,in turn,accelerated the sparks' moving phenomena around the coatings' surfaces due to the igniting and extinguishing of the sparks appearing alternately on specimen surfaces.Consequently,the macro-scale pits and the micro-cracks caused by the local thermal accumulation had eliminated on the coatings' surfaces.As a result,the corrosion resistance of MAO coatings formed in the electrolyte with sodium silicate was improved obviously by demonstration of the decreasing of the corrosion current density of the coatings with one order of magnitude and increasing of their linear polarization resistance with sixteen times.
Keyword:
magnesium alloy; micro arc oxidation; sodium silicate; coating forming; corrosion resistance;
Received: 2018-09-26
镁合金因具有比重小、强度高、优良的切削加工性能和减震性能、以及良好的电磁屏蔽性能和阻尼性能等优点,使其广泛应用在航空航天、电子、汽车、3C产品等领域
研究表明,镁合金微弧氧化膜的微观结构及性能受到多个工艺参数的影响,如电流密度
1 实验
1.1 材料与试剂
本实验选择的基材为商用铸态AZ91D镁合金,其中各元素的质量分数分别为Al 8.3%~9.7%,Zn 0.35%~1.0%,Mn 0.17%~0.27%,Si≤0.05%,Cu≤0.025%,Ni≤0.001%,Fe≤0.004%,其余的为Mg。通过线切割将AZ91D镁合金铸锭加工成大小为:30 mm×20 mm×11 mm的长方体试样,然后在试样的一侧用Φ2.5 mm的钻头钻孔,接下来用Φ3 mm的丝锥攻内螺纹。实验之前,先对试样表面进行预处理,即用120号的粗金相砂纸对试样进行初级打磨,再依次用600,1000,1600,2200号的金相砂纸对试样进行精细打磨,然后抛光处理,再用清水冲洗,最后吹干备用。微弧氧化时镁合金试样与Φ3 mm的铝丝相连,铝丝的另一端用螺纹紧固件固定在电源支架上。实验过程中阳极为镁合金试样,阴极为不锈钢片。
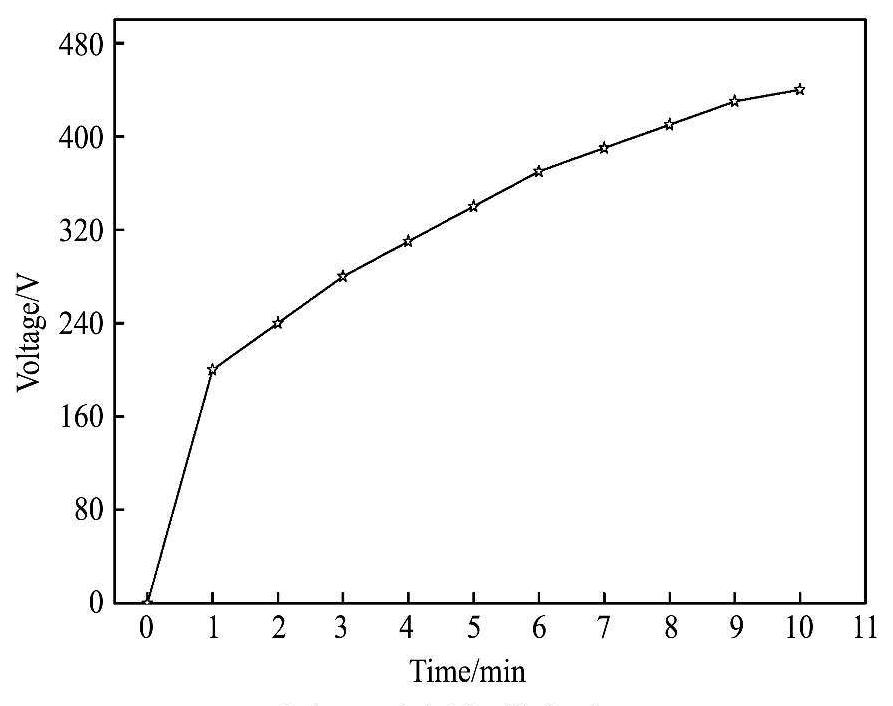
本实验中基础电解液的成分为氟化钾和氢氧化钠,分别往基础电解液中不添加及添加硅酸钠,其中不添加硅酸钠的电解液命名为G1,添加硅酸钠的电解液命名为G2,两种电解液的成分如表1所示。实验过程中采用水冷的方式对电解液进行冷却,使电解液的温度基本控制在20~40℃的范围内。设备采用兰州理工大学自主研发的微弧氧化双极性脉冲电源,输出额定功率为220 kW,额定正负电压分别为500 V和200 V,正负电流分别为400 A和100 A,脉冲频率范围为100~1000 Hz,脉冲占空比范围为10%~90%。实验过程中频率为700 Hz,占空比为20%,并将镁合金试样分别放入G1,G2两种电解液中进行微弧氧化处理,采用相同的电压加载方式(图1),即微弧氧化过程中逐步提高电压。
1.2 表征方法
使用DDS-307W型微处理器电导仪来测量电解液的电导率。使用索尼(SONY) ILCE-7K型照相机来拍摄实验过程中试样表面放电火花形态的变化和试样的宏观形貌。微弧氧化膜层的表面与截面形貌通过HITACHI S-4700型和QUANTA-450FEG型扫描电子显微镜(SEM)来观察。微弧氧化膜层的厚度、平均微孔尺寸和表面孔隙率都采用Image J通用图像分析软件分别对膜层截面形貌和表面形貌进行测量而获得。使用日本理光的D9-ADVANCE型X射线衍射仪(XRD)来检测微弧氧化膜层的物相,其中衍射条件:阳极选用铜靶,电子加速电压:40 kV,电流:60 mA,入射角:3°,扫描的范围、步长分别为:20°~80°和0.02°。通过CHI660C型电化学工作站来表征微弧氧化膜层的耐蚀性,使用三电极体系进行评估,其中工作电极为镁合金试样,参比电极为饱和甘汞电极(SCE),辅助电极为铂电极。在测动电位极化曲线之前,将镁合金试样表面裸露出面积为1cm2大小的区域并在质量分数为3.5%的氯化钠溶液中浸泡30min,电位扫描范围为-1.9~-1.0 V,扫描速率为0.5 mV·s-1。
表1 G1和G2两电解液中的成分 下载原图
Table 1 Compositions of different electrolytes
图1 电压加载方式
Fig.1 Loading mode of voltages
2 结果与讨论
2.1 微弧氧化过程中放电火花状态的变化
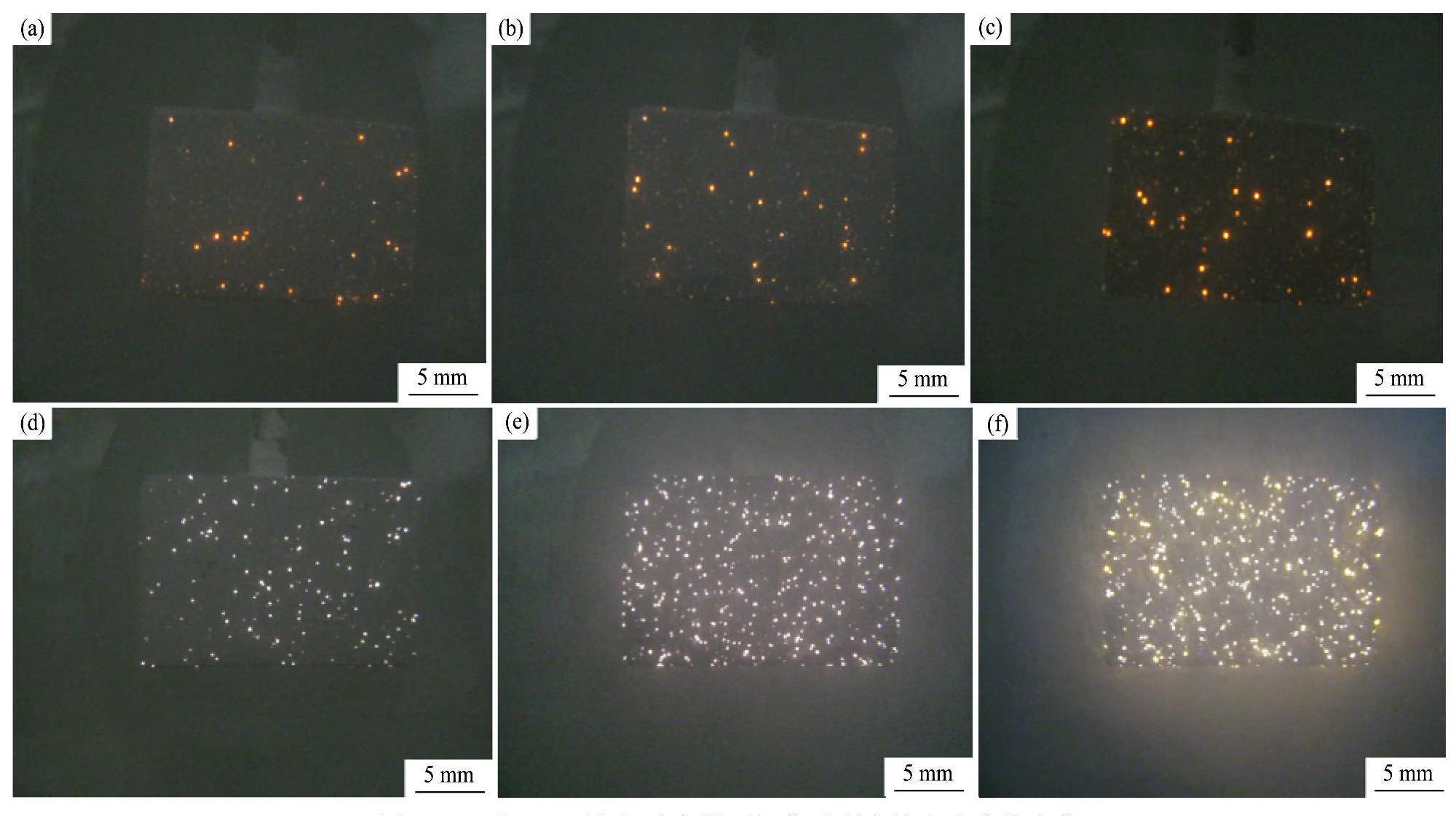
G1和G2两电解液中溶液的电导率、基材表面的起弧电压及放电火花的状态如表2所示,图2则为微弧氧化不同处理时间段基材表面放电火花的变化情形。从表2中明显可以看出,电解液中不添加硅酸钠时,溶液的电导率和起弧电..压分别为22.5 ms·cm-1和285 V,但是,添加硅酸钠后溶液的电导率曾大了10.4 ms·cm-1,起弧电压减小了40 V。在试验过程中可以观察到,电解液中不存在硅酸钠时,起弧后基材表面上大部分放电火花的状态是长时间停留在基材表面的某个固定位置,出现了类烧蚀的现象,并且放电火花的数量不多(图2(a~c))。而电解液中加入硅酸钠后,观察到放电火花在基材表面的位置不再固定,而是不断地辗转游移,并且类烧蚀现象也消失,放电火花变得密集并且数量增多(图2(d~f))。
表2 G1和G2两电解液中溶液电导率、起弧电压及基材表面放电火花状态 下载原图
Table 2 Solution conductivity,ignition voltage and spark state on coated substrate surface in different electrolytes
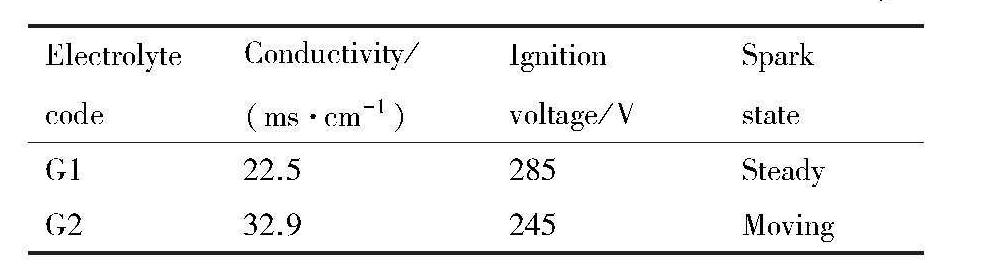
图2 G1和G2两电解液中微弧氧化过程中放电火花的变化
Fig.2 Sparks on substrate surface observed at different time during MAO treatment in different electrolytes
(a) G1 electrolyte-4 min;(b) G1 electrolyte-7 min;(c) G1 electrolyte-10 min;(d) G2 electrolyte-4 min;(e) G2 electrolyte-7 min;(f) G2 electrolyte-10 min
在用微弧氧化技术处理AZ91D镁合金的试验过程中,大量的微区放电击穿在基材表面不断地发生、结束、再发生、再结束

2.2 微弧氧化膜层的宏观形貌及其厚度
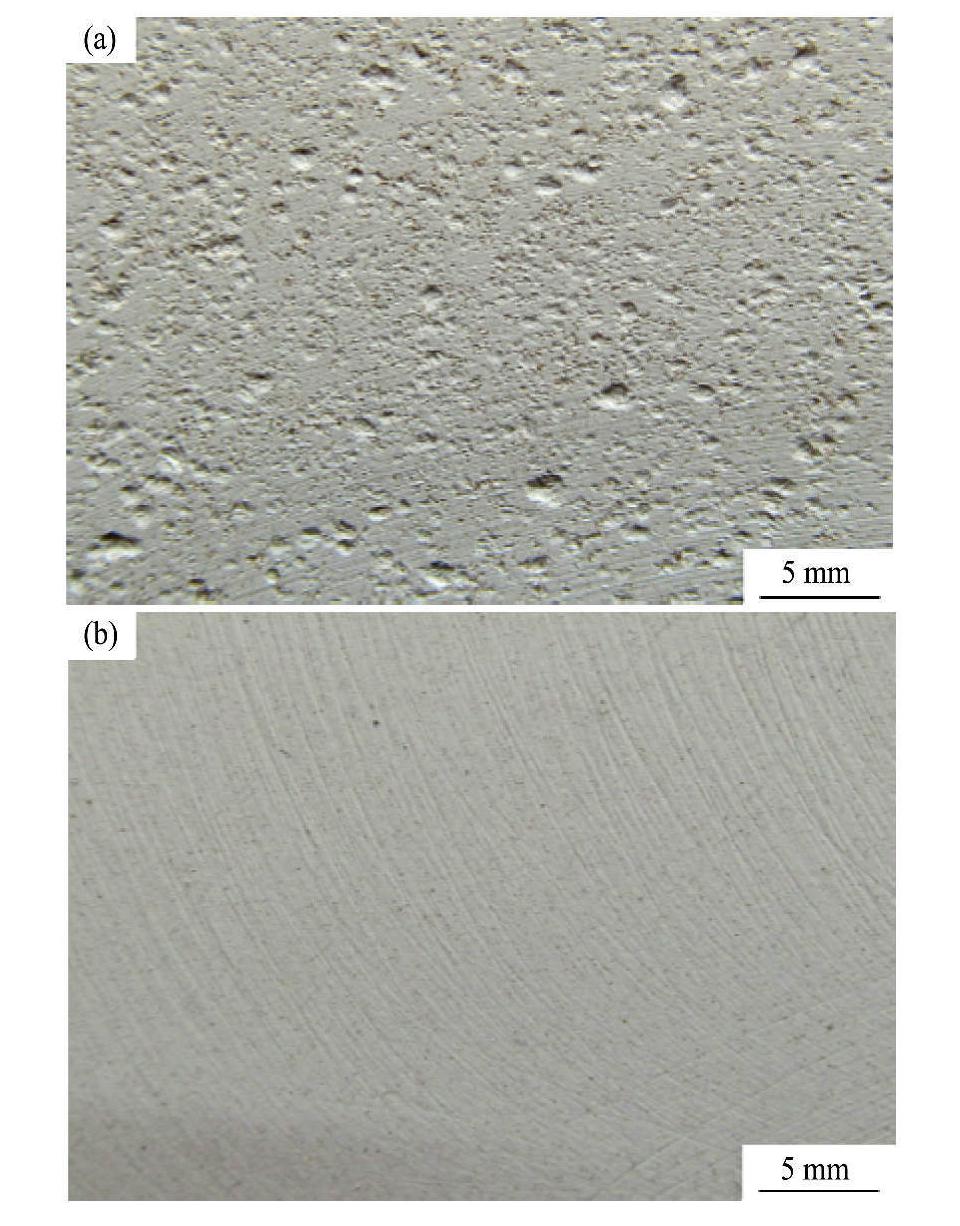
图3为G1和G2两电解液中微弧氧化处理后膜层的宏观形貌。从图3中明显可以观察到,电解液中不存在硅酸钠时,膜层表面的粗糙度较大,而且各个位置均出现了许多大小不一的小凹坑。经实验前后比照发现,在基材表面放电火花滞留时间较长的位置处,小凹坑的尺寸也相对较大(图3(a))。但是,电解液中加入硅酸钠后,膜层表面比较光滑,平整性较好,不再出现小凹坑(图3(b)),膜层表面凹凸不平的状态明显得到了改变。
图3 G1和G2两电解液中微弧氧化膜的宏观形貌
Fig.3 Macroscopic morphologies of MAO coatings obtained in different electrolytes
(a) G1 electrolyte;(b)G2 electrolyte
表3为G1和G2两电解液中制备得到的膜层厚度。从表3中明显可以看出,电解液中不添加硅酸钠时,膜层的厚度为16.5μm,但是,加入硅酸钠后,膜层的厚度增大了3.2μm。
2.3 微弧氧化膜层的微观形貌及物相组成
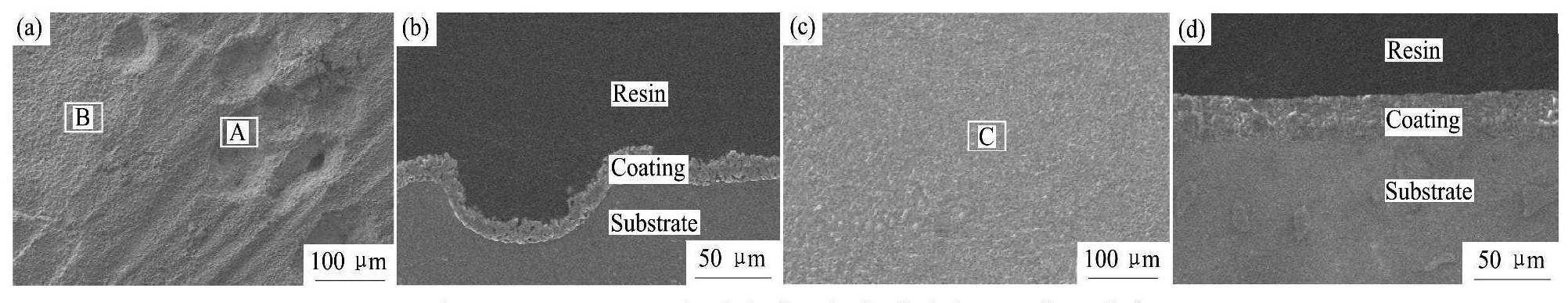
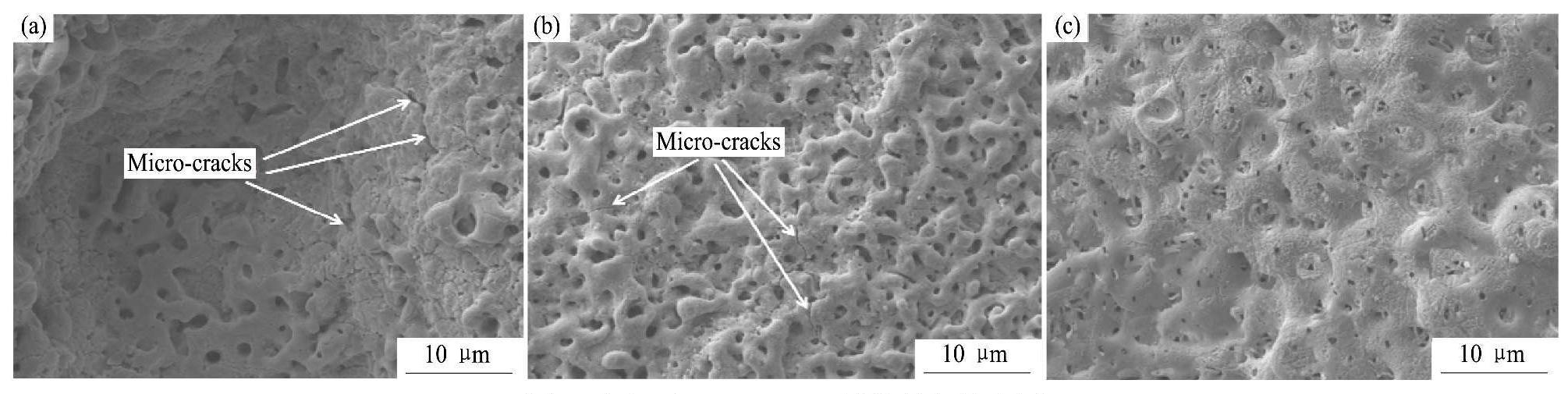
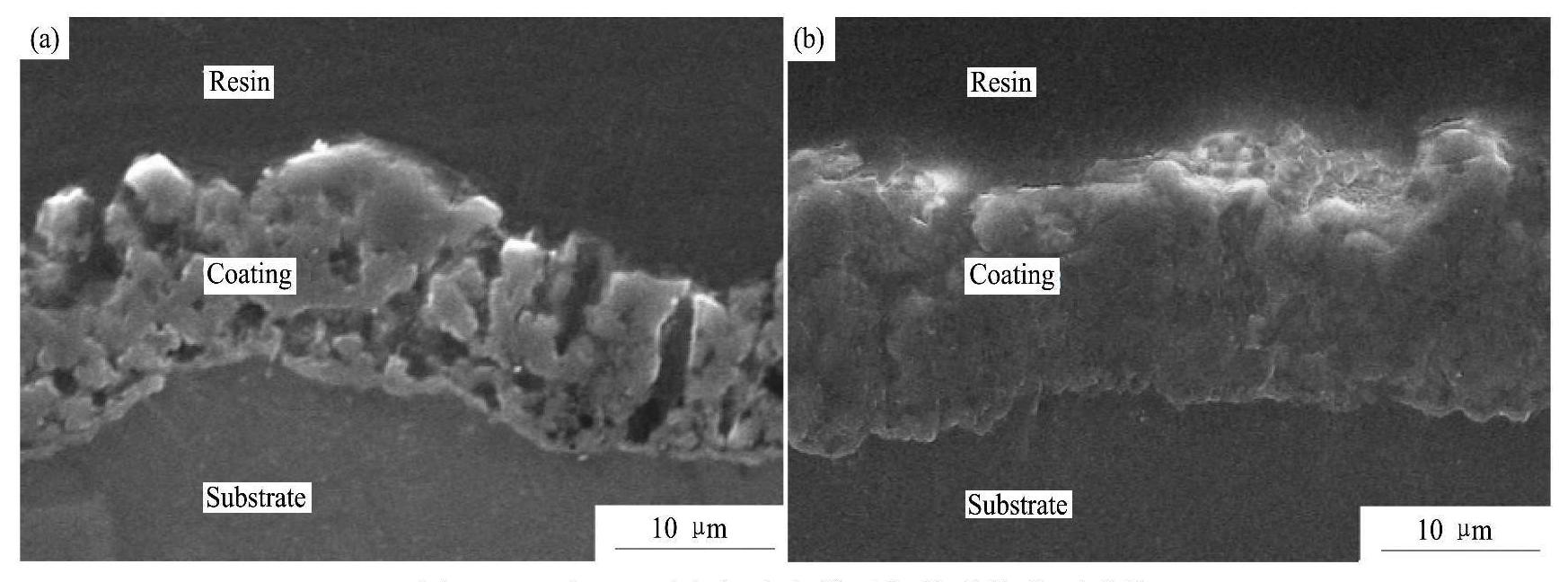
图4为G1和G2两电解液中所获得的微弧氧化膜层的表面和截面形貌,图5为图4(a)和图4(e)中微弧氧化膜层表面不同微观区域的局部放大图,图6是对微弧氧化膜层截面的进一步观察。表4为G1和G2两电解液中所获得的微弧氧化膜层(图5(b,c))表面的平均微孔尺寸及孔隙率。
表3 G1和G2两电解液中所得膜层的厚度 下载原图
Table 3 Thickness of MAO coatings obtained in different electrolytes
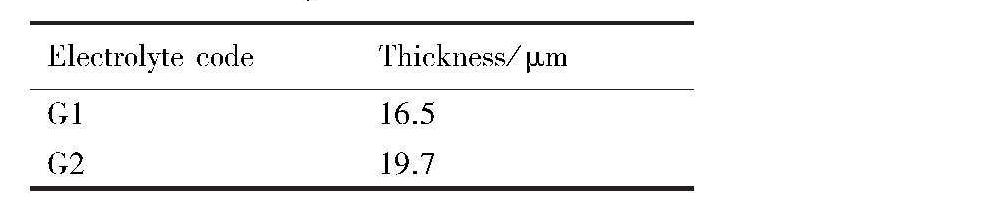
图4 G1和G2两电解液中微弧氧化膜的表面及截面形貌
Fig.4 Surface and cross-section morphologies of MAO coatings obtained in different electrolytes
(a),(b)G1 electrolyte;(c),(d) G2 electrolyte
图5 图4中A,B,C区域的局部放大图
Fig.5 Local magnification images of A,B,C regions shown in Fig.4
v
从图4中可以看出,电解液中不添加硅酸钠时,所得微弧氧化膜层表面凹凸不平,比较粗糙,出现了很多小凹坑(图4(a))。从图4(b)中可以看出,凹坑位置微弧氧化膜层的厚度与其他部位相差不大,并且可以观察到,小凹坑处对应的微弧氧化膜层表面上也同样布满了“火山口”状的微孔(图5(a));而电解液中添加硅酸钠后,所得微弧氧化膜层表面光滑平整,不再出现小凹坑,膜层的平面连续性明显增强(图4(c,d))。
从图5(a,b),图6(a)以及表4中可以看到,电解液中不存在硅酸钠时,所得微弧氧化膜层表面平均微孔尺寸较大,表面孔隙率也较大,膜层表面上微观裂纹较多(图5(a,b)),膜层截面中出现了较多的孔隙,并且有些地方还出现了连通孔(图6 (a))。而电解液中添加硅酸钠后,所得微弧氧化膜层表面平均微孔尺寸减小了1.3μm(表4),表面孔隙率减小了4.7%(表4),膜层表面上基本不存在微裂纹(图5(c)),并且膜层截面中的孔隙数量明显减少,同时连通孔消失(图6(b)),整个膜层的致密度得到了明显提高。
表4 G1和G2两电解液中所得膜层表面的平均微孔尺寸及孔隙率 下载原图
Table 4 Average size of micro pores and surface porosity of MAO coatings obtained in different electrolytes
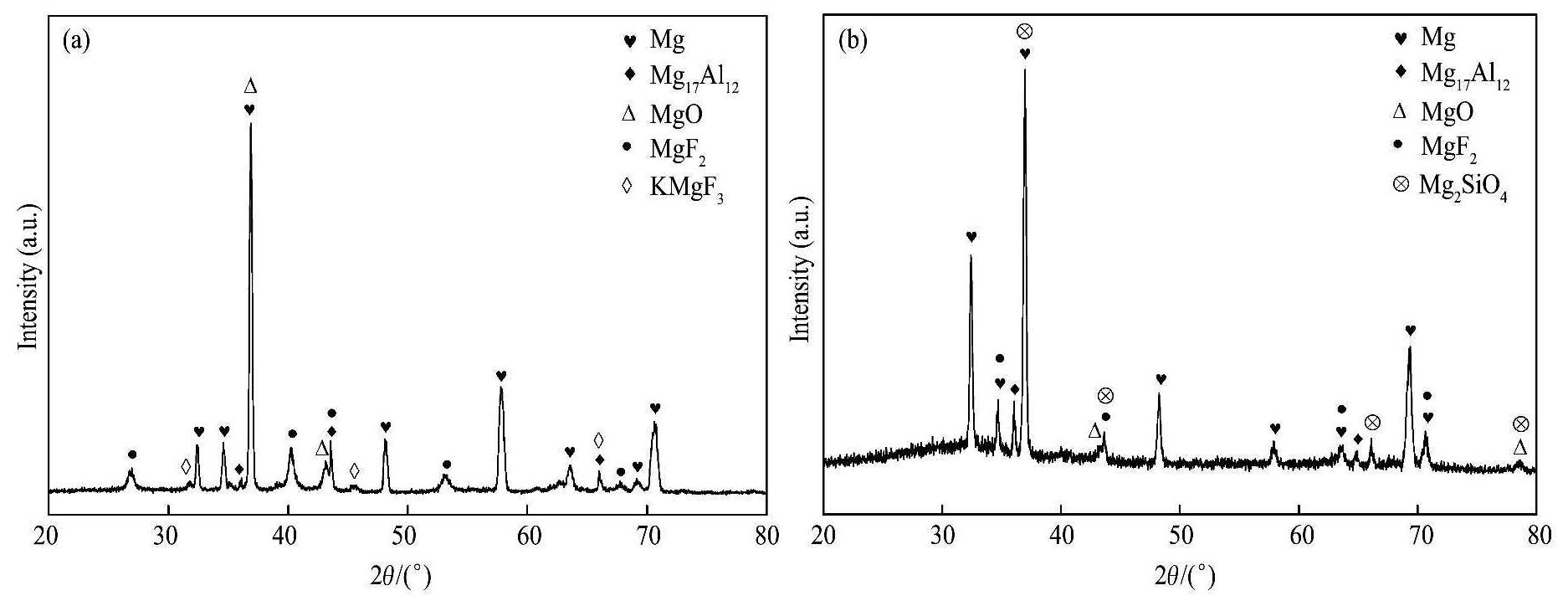
图7为G1和G2两电解液中所获得的微弧氧化膜层的XRD图谱。从图7 (a)中可以看出,电解液中不添加硅酸钠时,微弧氧化膜层中的主要物相为MgO,MgF2等,其可能的形成反应
图6 G1和G2两电解液中微弧氧化膜的截面形貌
Fig.6 Cross-section morphologies of MAO coatings obtained in different electrolytes
(a) G1 electrolyte;(b)G2 electrolyte
图7 G1和G2两电解液中微弧氧化膜的XRD图谱
Fig.7 XRD patterns of MAO coatings obtained in different electrolytes
(a) G1 electrolyte;(b)G2 electrolyte
在用微弧氧化技术处理AZ91D镁合金的试验过程中观察到,当电解液中不存在硅酸钠时,放电火花在基材表面的某些位置持续逗留,这表明这些位置上连续发生了击穿反应。由于击穿过程中会产生大量的热量,并且热量会在这些位置不断地积累,进而使得较多的基体材料被烧蚀熔融继而崩落,同时,由于热量的集中,会使微弧氧化膜层中的热应力形成局部累积,接着导致已形成膜层的局部膨胀甚至崩落,这些因素都可能是膜层表面出现宏观小凹坑以及产生微观裂纹的原因。前已述及,电解液中添加硅酸钠以后,
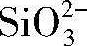
2.4 微弧氧化膜层的耐蚀性
研究表明
式中,V为腐蚀速率(mm·s-1);F为法拉第常数;M为金属的相对原子质量(g·mol-1);n为金属的化合价;ρ为金属的密度;Jcorr为腐蚀电流密度(A·cm-2)。
从式(4)中可以看出:腐蚀电流密度Jcorr表示电化学腐蚀的快慢,即腐蚀速率V,Jcorr越小,电化学腐蚀越慢,腐蚀速率就越小,所得膜层的耐蚀性就越好。腐蚀电位Ecorr则表示电化学腐蚀的倾向,Ecorr越正,腐蚀倾向就越小,所得膜层的耐蚀性也就越好
图8为C1和C2两电解液中所获微弧氧化膜层的动电位极化曲线,表5为动电位极化曲线的拟合数据。结合图8和表5可知,与不添加硅酸钠相比,电解液中添加硅酸钠后,所得微弧氧化膜层的腐蚀电位Ecorr发生了明显正移,正移了约110 mV,腐蚀电流密度Jcorr减小了1个数量级,线性极化电阻Rp、增大了约16倍,膜层的耐蚀性明显得到了提高。
研究表明
图8 G1和G2两电解液中微弧氧化膜层的动电位极化曲线
Fig.8 Potentiodynamic polarization curves of MAO coatings obtained in different electrolytes
表5 G1和G2两电解液中微弧氧化膜层的动电位极化曲线的拟合结果 下载原图
Table 5 Fitted results of potentiodynamic polarization curves of MAO coatings obtained in different e-lectrolytes
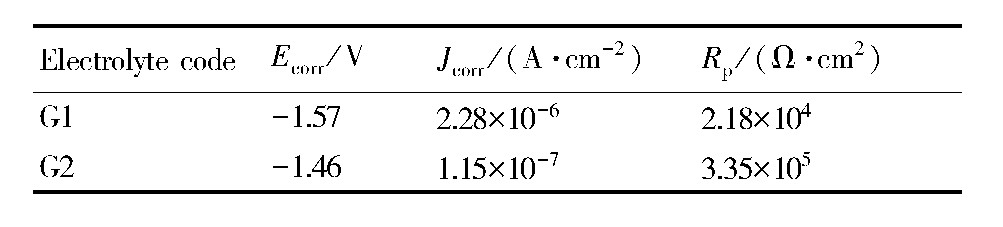
与不含硅酸钠相比,电解液中添加硅酸钠后,微弧氧化膜层的厚度增大了3.2μm(表3),这对提高微弧氧化膜层的耐蚀性有利。并且微弧氧化膜层表面的平均微孔尺寸减小了1.3μm(表4),表面孔隙率减小了4.7%(表4),膜层表面上基本不存在小凹坑(图4(c))和微裂纹(图5(c)),膜层截面中的孔隙数量明显减少,同时连通孔消失(图6(b))),整个膜层的致密性得到了明显改善,因此,外界环境中的腐蚀介质就不容易穿过膜层去腐蚀基体,故微弧氧化膜层的耐蚀性能明显得到提高。另外从物相的角度看,含硅酸钠的电解液制备得到的微弧氧化膜层中出现了新物相Mg2SiO4,这种物相属于氟镁石,结构坚硬、性能稳定,它的存在对微弧氧化膜层耐蚀性能的提高也非常有利。
3 结论
1.电解液中添加硅酸钠后,将会促使在镁合金基材表面生成具有平面连续性的完整的微弧氧化膜层,同时膜层的致密性明显得到改善,并获得优质新物相Mg2SiO4。
2.电解液中添加硅酸钠后,溶液的电导率增大,基材表面的起弧电压降低,击穿变得容易发生。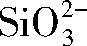
3. 耐蚀性检测结果表明,与不添加硅酸钠相比,电解液中添加硅酸钠后,所得膜层的腐蚀电位正移了110 mV,腐蚀电流密度减小了1个数量级,线性极化电阻增大了约16倍,膜层的耐蚀性明显增强。
参考文献