Trans. Nonferrous Met. Soc. China 27(2017) 2656-2662
Dielectric properties and point defect behavior of antimony oxide doped Ti deficient barium strontium titanate ceramics
Chen ZHANG, Zhi-xin LING, Gang JIAN, Fang-xu CHEN
Provincial Key Lab of Advanced Welding Technology, Jiangsu University of Science and Technology, Zhenjiang 212003, China
Received 27 September 2016; accepted 17 March 2017
Abstract:
The microstructures and dielectric properties of Sb2O3-doped Ti deficient barium strontium titanate ceramics prepared by solid state method were investigated with non-stoichiometric level and Sb2O3 content by SEM, XRD and LCR measure system. It is found that with the increase of δ, (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics transform from single phase solid solutions with typical cubic perovskite structure to multiphase compounds while (Ba0.75Sr0.25)Ti0.998O2.996 ceramics remain to be single-phase with the increasing Sb2O3 content. The distortion of the ABO3 perovskite lattice caused by VTi″″ and 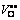 induces the drop of Curie temperature and the rise of relative dielectric constant in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics with increasing δ value. The orientation of
induces the drop of Curie temperature and the rise of relative dielectric constant in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics with increasing δ value. The orientation of 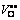 elastic dipoles results in the domain-wall pinning and thus the reduction of the dielectric loss. With increasing Sb2O3 content, the relative dielectric constant, dielectric constant maximum and Curie temperature of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics decrease dramatically while the dielectric loss increases.
elastic dipoles results in the domain-wall pinning and thus the reduction of the dielectric loss. With increasing Sb2O3 content, the relative dielectric constant, dielectric constant maximum and Curie temperature of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics decrease dramatically while the dielectric loss increases.
Key words:
barium strontium titanate; defects; dielectric properties; non-stoichiometric ceramics;
1 Introduction
Barium strontium titanate (Ba1-xSrxTiO3, BST) as a ferroelectric material with perovskite structure has drawn considerable attention in the microelectronic industries because of its outstanding properties such as adjustable phase transition temperature, high dielectric constant and low dielectric loss [1], which allows it to have a great potentiality for phase shifter, phased array antenna, resonator and capacitor applications. However, the conflict between the fine dielectric properties at room temperature and good dielectric temperature stability remains in the uniform BST ceramics [2]. In order to overcome the above mentioned drawback, the traditional doping method applied widely in electronic ceramics has been introduced into BST ceramics [3-5].
Due to the intermediate size and charge of trivalent ions compared with the Ba2+, Sr2+ and Ti4+ ions, the aliovalent doping mechanism and the substitution preference of trivalent ions in BST ceramics as well as in BaTiO3 ceramics have been debated for many years [6]. However, it is no doubt that the dielectric properties of perovskite BST ceramics are related to the doping mechanism and correspondingly the point defect behavior. In many cases, the doping mechanisms are not only dependent on the oxygen partial pressure (pO2) and sintering temperature/time but also the overall nA/nB ratio [7]. Many literatures have been focused on the relationships between the point defect behavior and nA/nB ratio in BaTiO3 ceramics [8,9]. Also, some influences of nA/nB ratio on the microstructures and dielectric properties in BST ceramics are gradually understood. DONG et al [2] concluded that adding excessive TiO2 could remarkably inhibit grain growth, suppress and broaden the Curie peaks in compositionally inhomogeneous BST ceramics. SYAMAPRASAD et al [10] found that excess Ba led to high un-changed dielectric constant with a slight decrease of loss tangent in Ba0.71Sr0.29TiO3 ceramics.
In our previous work, the dielectric properties of Sb2O3-doped stoichiometric BST ceramics such as (Ba0.992-xSrxY0.008)TiO3.004 ceramics (nA/nB=1) have been investigated and the Sb2O3 dopant showed extraordinary effects on the dielectric temperature stability improvement [11,12]. So, in this work, we report a systematic study of the microstructure, point defect behavior and dielectric properties of Ti deficient (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics (nA/nB>1) still taking the trivalent Sb3+ ions as dopant. The influences of δ value (namely the nA/nB ratio) and doping content on point defect behavior and the dielectric properties in barium strontium titanate ceramics are discussed.
2 Experimental
The chemical compositions of the Sb2O3-doped Ti deficient barium strontium titanate specimens were given by the formula (Ba0.75Sr0.25)Ti1-δO3-2δ+0.6%Sb2O3 (δ= 0.002, 0.004, 0.006, 0.008) and (Ba0.75Sr0.25)Ti0.998O2.996 + xSb2O3 (x=0, 0.4%, 0.8%, 1.2%). High purity BaCO3 (>99.0%), SrCO3 (>99.0%) and TiO2 (>98.0%) powders used as starting raw materials were weighed according to the above specimens, ball-milled, dried and calcined at 1080 °C for 2 h. The calcined powders were mixed with Sb2O3 (>99.0%), reground, dried and added with 5% polyvinyl alcohol (PVA) as a binder for granulation. The mixture was sieved through 250 μm screen and then pressed into pellets of 10 mm in diameter and 2 mm in thickness under 250 MPa. Sintering was conducted in air at 1300-1320 °C for 2 h. For dielectric measurement, both the flat surfaces of the specimens were coated with BQ-5311 silver paste after ultrasonic bath cleaning and then fired at 800 °C for 10 min.
The crystal structures of the specimens were confirmed by X-ray diffraction analysis (XRD, Rigaku D/max 2500v/pc) with Cu Kα radiation. The surface morphologies of the specimens were observed using the SEM (JSM-6480 ESEM). The capacitance quantity (C) and dissipation factor (D) were measured with LCR-8101G Automatic LCR Meter at 1 kHz. The relative dielectric constant (εr) and the loss tangent (tan δ) were calculated as follows:
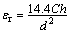 (1)
(1)
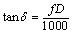 (2)
(2)
where h is the thickness (cm), d is the diameter of the electrode (cm) and f is the test frequency (Hz). An automatic measuring system consisting of Automatic LCR Meter and THP-F-100 temperature control unit was used to record the capacitance quantity and dissipation factor from -20 °C to 50 °C at 1 kHz for measuring the temperature dependence of dielectric parameters.
3 Results and discussion
3.1 XRD and SEM analysis
The X-ray diffraction patterns of 0.6%Sb2O3-doped (Ba0.75Sr0.25)Ti1-δO3-2δ bulk ceramics are shown in Fig. 1. With the increase of δ value, (Ba0.75Sr0.25)Ti1-δO3-2δ+ 0.6%Sb2O3 ceramics transform from single phase solid solutions with typical cubic perovskite structure to multiphase compounds. In other words, to maintain the ABO3 perovskite single phase structure, the δ value should be restricted to a very narrow range which is no more than 0.006 in present samples. Also, a slight shift of diffraction peaks to higher 2θ values with the increasing δ value was observed, especially for the (110), (200) and (211) peaks, which indicated that the unit cell volumes of (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics decreased as the non-stoichiometric level of Ti ions in present ABO3 perovskite structure increased. Similar phenomena have been previously reported in Ca substituted BST ceramics [13] and in our previous work for (La, Sb)- doped (Ba0.74Sr0.26)TiO3 ceramics [14]. Apparently, this shrinkage of unit cell volume is mainly attributed to the appearance of B-site vacancies VTi″″ and oxygen vacancies 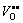 in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics which is revealed by the following point defect reaction equation:
in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics which is revealed by the following point defect reaction equation:
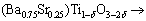
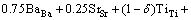
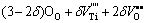 (3)
(3)
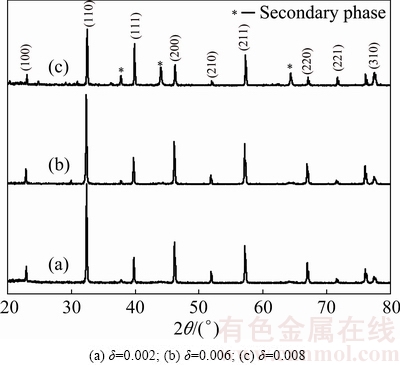
Fig. 1 XRD patterns for (Ba0.75Sr0.25)Ti1-δO3-2δ+0.6%Sb2O3 ceramics
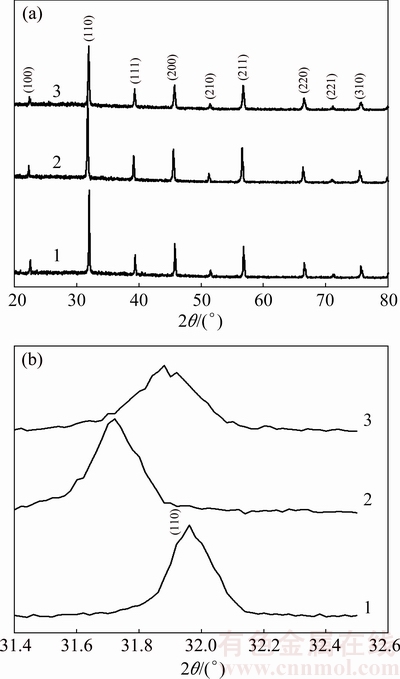
Fig. 2 XRD patterns for Sb2O3-doped (Ba0.75Sr0.25)Ti0.998O2.996 ceramics with different Sb2O3 contents (1—0; 2—0.4%; 3—1.2%)
The X-ray diffraction patterns of sintered Sb2O3- doped (Ba0.75Sr0.25)Ti0.998O2.996 bulk ceramics are shown in Fig. 2. All these polycrystals are single-phase compounds with perovskite structure, which implies that Sb3+ ions have incorporated into the lattice and thus maintain the perovskite structure of non-stoichiometric barium strontium titanate solid solution. The XRD profiles focusing on the (110) diffraction peaks are presented in Fig. 2(b). It shows that the diffraction peaks move towards lower 2θ values and then shift to higher 2θ values as the Sb2O3 doping content increases in (Ba0.75Sr0.25)Ti0.998O2.996 ceramics, which reveals a variation of the unit cell volume for Sb2O3-doped (Ba0.75Sr0.25)Ti0.998O2.996 ceramics. To be specific, with the increase of Sb2O3 content, the unit cell volume increases and then decreases slightly. In terms of size, the ionic radii of Ba2+, Sr2+ in 12 coordination and Ti4+ in 6 coordination are 0.161, 0.144 and 0.061 nm, respectively. The radius of Sb3+ ion in 6 coordination is 0.076 nm which is bigger than that of Ti4+ ion but smaller than that of host A-site ion. Therefore, B-sites in the perovskite lattice are partially occupied by bigger Sb3+ ions for present low Sb2O3 doping content samples, consequently causing the increase of the unit cell volume. While the above shrinkage of unit cell suggests that the substitution of Sb3+ ions for the host A-site ions takes place in high Sb2O3 doping content samples. The substitution preference of Sb3+ ions in present system is opposite to that in (Ba0.992-xSrxY0.008)TiO3.004 ceramics [11]. This difference of Sb3+ substitution preference in perovskite lattice can be explained by the existence of B-site vacancies VTi″″ in non-stoichiometric (Ba0.75Sr0.25)- Ti0.998O2.996 ceramics as shown in Eq. (3). The incorporation of Sb3+ ions into the lattice also brings about some kinds of point defects which turn out to be the main factors changing the dielectric characteristics of non-stoichiometric barium strontium titanate ceramics. In (Ba0.75Sr0.25)Ti0.998O2.996 samples with low Sb2O3 doping concentration, Sb3+ ions enter B-sites in the perovskite structure and serve as an acceptor dopant. The defect reaction is as follows:
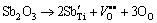 (4)
(4)
For high Sb2O3 doping concentration samples, Sb3+ ions tend to occupy the A-sites and serve as a donor dopant. The defect reaction is as follows:
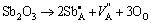 (5)
(5)
Figure 3 shows the surface morphologies of 0.6%Sb2O3-doped Ti deficient (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics. And the surface morphologies of Sb2O3-doped (Ba0.75Sr0.25)Ti0.998O2.996 ceramics are shown in Fig. 4. It appears that all samples exhibit dense microstructure and no abnormal grain growth is observed. There is no obvious change in the average grain size of (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics as the δ value increases, presenting that the non-stoichiometric level makes little contribution to refine the grain size. Distinguishingly, the average grain size of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics decreases dramatically after applying the Sb2O3 dopant while remains almost the same when gradually increasing the Sb2O3 content. And the grain size distribution of sintered ceramics can be refined by Sb2O3 addition.
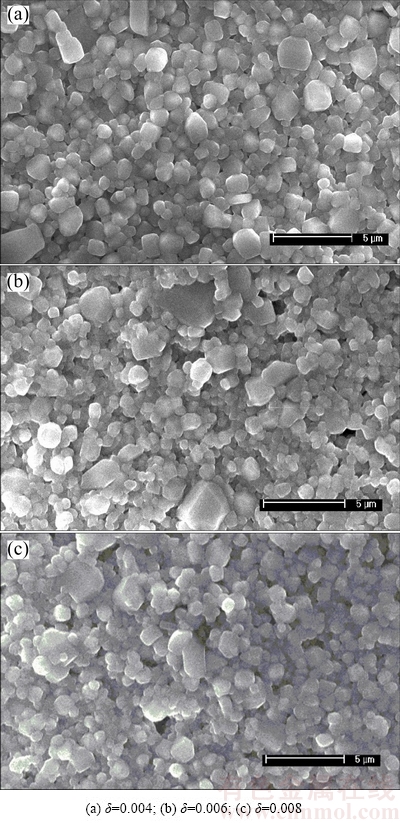
Fig. 3 SEM images of 0.6%Sb2O3-doped (Ba0.75Sr0.25)- Ti1-δO3-2δ ceramics
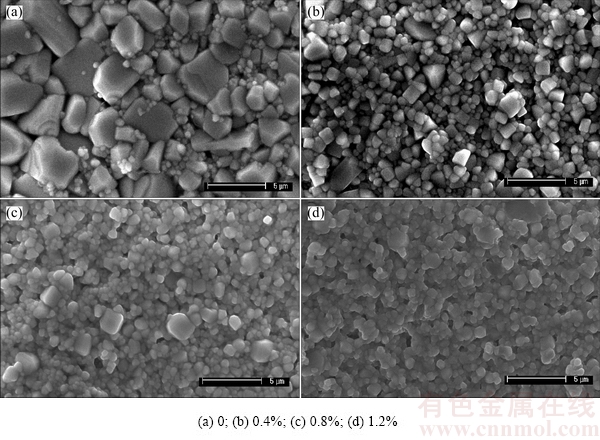
Fig. 4 SEM images of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics with various Sb2O3 contents
3.2 Dielectric characteristics
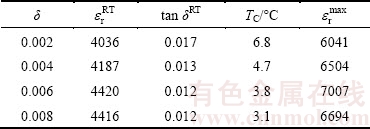
Table 1 shows the relative dielectric constant and dielectric loss of 0.6%Sb2O3-doped (Ba0.75Sr0.25)Ti1-δ- O3-2δ ceramics with different δ values at room temperature. It is obvious that all the non-stoichiometric (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics possess high relative dielectric constant (more than 4000) at room temperature. With the increase of δ value which is in the range of 0.002-0.006, the relative dielectric constant increases notably while the dielectric loss decreases considerably. This demonstrates that a proper non-stoichiometric level in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics helps to improve the dielectric properties at room temperature. nBa2+/nTi4+>1 in BaTiO3 has been demonstrated by HYATT et al [15] to reduce the loss tangent particularly in the paraelectric region above the Curie temperature. In our present (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics satisfying the condition of (nBa2++nSr2+)/nTi4+>1, the dielectric loss is now proved to decrease with the increasing non-stoichiometric level, which is in accord with Hyatt’s results.
Table 1 Dielectric properties of (Ba0.75Sr0.25)Ti1-δO3-2δ+ 0.6% Sb2O3 ceramics
Temperature dependence of relative dielectric constant and dielectric loss for 0.6%Sb2O3-doped (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics is shown in Fig. 5. The relative dielectric constant first increases, achieves a maximum remarked as  and then decreases with increasing temperature. Contrarily, the dielectric loss decreases at the beginning, achieves a minimum and then increases with increasing temperature. The temperature corresponding to the relative dielectric constant maximum is taken as the Curie temperature TC. It is obvious that the Curie temperature (see Table 1) decreases with increasing δ value. The B-site vacancies VTi″″ and oxygen vacancies
and then decreases with increasing temperature. Contrarily, the dielectric loss decreases at the beginning, achieves a minimum and then increases with increasing temperature. The temperature corresponding to the relative dielectric constant maximum is taken as the Curie temperature TC. It is obvious that the Curie temperature (see Table 1) decreases with increasing δ value. The B-site vacancies VTi″″ and oxygen vacancies 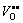 in (Ba0.75Sr0.25)Ti1-δ- O3-2δ ceramics resulting in the shrinkage of crystal lattice as mentioned above lead to a distortion of the ABO3 perovskite structure thus inducing a drop in the Curie temperature. Also, the lattice deformation and inner stress were reported to cause the rise of dielectric constant [16], which exactly explains the increase of relative dielectric constant with the increasing δ value in present (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics. It is noteworthy that in the whole temperature range the higher the δ value is, the lower the dielectric loss of (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics becomes. This phenomenon is related to the existing point defects as well. Oxygen vacancies
in (Ba0.75Sr0.25)Ti1-δ- O3-2δ ceramics resulting in the shrinkage of crystal lattice as mentioned above lead to a distortion of the ABO3 perovskite structure thus inducing a drop in the Curie temperature. Also, the lattice deformation and inner stress were reported to cause the rise of dielectric constant [16], which exactly explains the increase of relative dielectric constant with the increasing δ value in present (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics. It is noteworthy that in the whole temperature range the higher the δ value is, the lower the dielectric loss of (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics becomes. This phenomenon is related to the existing point defects as well. Oxygen vacancies  residing at the corners of octahedra are well interconnected and therefore can be regarded as relatively mobile defects. The mobile defects migrate to domain boundaries. Orientation of the elastic dipoles caused by
residing at the corners of octahedra are well interconnected and therefore can be regarded as relatively mobile defects. The mobile defects migrate to domain boundaries. Orientation of the elastic dipoles caused by  results in the domain-wall pinning and thus the reduction of the dielectric loss.
results in the domain-wall pinning and thus the reduction of the dielectric loss.
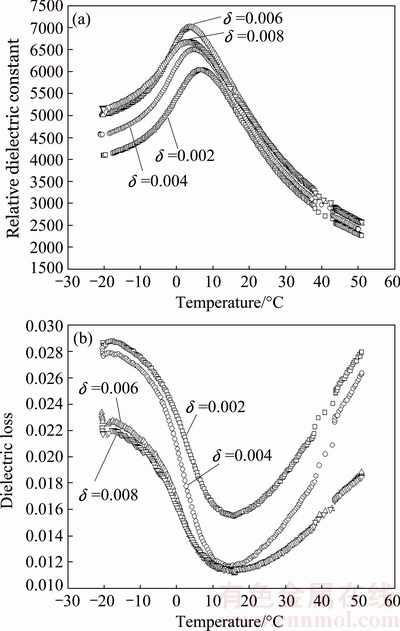
Fig. 5 Temperature dependence of relative dielectric constant (a) and dielectric loss (b) for (Ba0.75Sr0.25)Ti1-δO3-2δ+0.6% Sb2O3 ceramics
The relative dielectric constant and dielectric loss of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics with different Sb2O3 doping contents at room temperature are shown in Table 2. It is clear that the relative dielectric constant of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics decreases dramatically with increasing Sb2O3 addition content. The substitution for A/B-site with any of the Sb3+ ions leads to a shorter distance between the B-site ion and its nearest neighbors of the octahedron, so the movement of B-site ion is restricted, which weakens the spontaneous polarization of grain lattice, and consequently, the dielectric constant decreases with increasing Sb2O3 content macroscopically. The dielectric loss of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics increases with the increase of Sb2O3 content. Orientation of the electric dipoles formed from 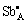 -V″A/Sb′Ti-
-V″A/Sb′Ti-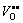 complexes shown in Eqs. (4) and (5) leads to domain-wall pinning and thus a reduction of the dielectric loss. In titanium-containing ceramics the dissipation factor is always related to the reduction of Ti4+ ion [4]. When the Sb3+ ions substitute for host ions in present samples, the electrons will be generated and then trapped by the Ti4+ ions. Hereby, the limited reduction of Ti4+ ion in (Ba0.75Sr0.25)Ti0.998O2.996 ceramics is sufficient to cause a severe deterioration in dielectric loss. Therefore, the electric dipoles related to the point defect and the reduction of Ti4+ ion caused by Sb3+ ion substitution for host ion become two conflicting factors for the dielectric loss. Apparently, the latter is the controlling factor for Sb2O3-doped (Ba0.75Sr0.25)Ti0.998-O2.996 ceramics since the dielectric loss increases with the increasing Sb2O3 content.
complexes shown in Eqs. (4) and (5) leads to domain-wall pinning and thus a reduction of the dielectric loss. In titanium-containing ceramics the dissipation factor is always related to the reduction of Ti4+ ion [4]. When the Sb3+ ions substitute for host ions in present samples, the electrons will be generated and then trapped by the Ti4+ ions. Hereby, the limited reduction of Ti4+ ion in (Ba0.75Sr0.25)Ti0.998O2.996 ceramics is sufficient to cause a severe deterioration in dielectric loss. Therefore, the electric dipoles related to the point defect and the reduction of Ti4+ ion caused by Sb3+ ion substitution for host ion become two conflicting factors for the dielectric loss. Apparently, the latter is the controlling factor for Sb2O3-doped (Ba0.75Sr0.25)Ti0.998-O2.996 ceramics since the dielectric loss increases with the increasing Sb2O3 content.
Table 2 Dielectric properties of Sb2O3-doped (Ba0.75Sr0.25)Ti0.998O2.996 ceramics
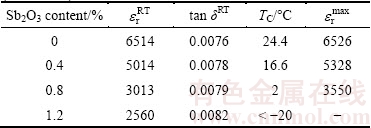
Temperature dependence of relative dielectric constant and dielectric loss for Sb2O3-doped (Ba0.75Sr0.25)Ti0.998O2.996 ceramics is shown in Fig. 6. It is obvious that the Curie temperature of Sb2O3-doped (Ba0.75Sr0.25)Ti0.998O2.996 ceramics (see Table 2) shifts to lower value with increasing Sb2O3 doping content, which is attributed to the weakening of spontaneous polarization of the grain lattice caused by the substitution for host ion with any of the Sb3+ ions as mentioned before. The charged vacancies caused by A-site /B-site substitution give rise to the local deformation of the perovskite unit cells, which also causes the reduction of Curie temperature. In the whole temperature range relative dielectric constants of Sb2O3-doped (Ba0.75Sr0.25)Ti0.998O2.996 ceramics are significantly suppressed as the Sb2O3 content increases. At high Sb2O3 doping contents (>0.8%), the curves become flat, which implies the high thermal stability of Sb2O3-doped non-stoichiometric barium strontium titanate ceramics. Particularly, the peak value remarked as 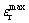 decreases with the increase of Sb2O3 content. The effects of Sb2O3 content on dielectric maximum are caused by the weakening of ferroelectricity, which is attributed to the replacing reaction of Sb3+ ions for the host ions in perovskite lattice. As shown in Fig. 6(b) the dielectric loss decreases dramatically with the increasing temperature in low temperature range and reaches the minimum at around 35 °C. Also, (Ba0.75Sr0.25)Ti0.998O2.996 ceramics with high Sb2O3 content exhibit better thermal stability of dielectric loss than that with low Sb2O3 content.
decreases with the increase of Sb2O3 content. The effects of Sb2O3 content on dielectric maximum are caused by the weakening of ferroelectricity, which is attributed to the replacing reaction of Sb3+ ions for the host ions in perovskite lattice. As shown in Fig. 6(b) the dielectric loss decreases dramatically with the increasing temperature in low temperature range and reaches the minimum at around 35 °C. Also, (Ba0.75Sr0.25)Ti0.998O2.996 ceramics with high Sb2O3 content exhibit better thermal stability of dielectric loss than that with low Sb2O3 content.
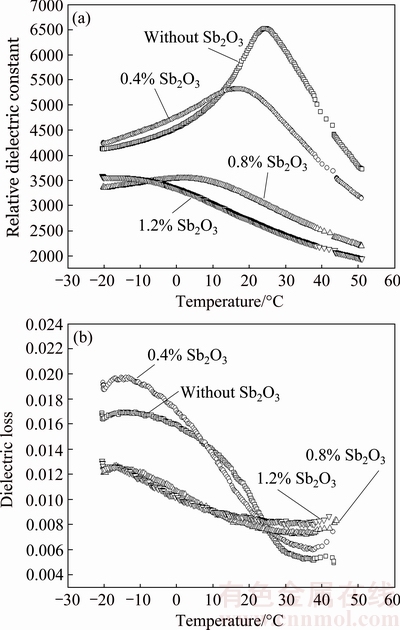
Fig. 6 Temperature dependence of relative dielectric constant (a) and dielectric loss (b) for Sb2O3-doped (Ba0.75Sr0.25)Ti0.998- O2.996 ceramics
4 Conclusions
1) (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics transform from single phase solid solutions with typical cubic perovskite structure to multiphase compounds with the increase of δ value while (Ba0.75Sr0.25)Ti0.998O2.996 ceramics remain to be single-phase solid solutions with the increasing Sb2O3 doping content.
2) The B-site vacancies VTi″″ as well as oxygen vacancies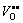 exist in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics and the
exist in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics and the 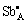 -V″A/Sb′Ti-
-V″A/Sb′Ti-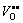 complexes show in Sb2O3- doped (Ba0.75Sr0.25)Ti0.998O2.996 ceramics. Due to the existence of VTi″″ in Ti deficient (Ba0.75Sr0.25)Ti0.998O2.996 ceramics, the substitution preference of Sb3+ ions is opposite to that in stoichiometric barium strontium titanate ceramics.
complexes show in Sb2O3- doped (Ba0.75Sr0.25)Ti0.998O2.996 ceramics. Due to the existence of VTi″″ in Ti deficient (Ba0.75Sr0.25)Ti0.998O2.996 ceramics, the substitution preference of Sb3+ ions is opposite to that in stoichiometric barium strontium titanate ceramics.
3) The distortion of the ABO3 perovskite structure caused by VTi″″ and 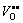 facilitates the drop of Curie temperature and the rise of relative dielectric constant in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics with increasing δ value. The orientation of the elastic dipoles caused by
facilitates the drop of Curie temperature and the rise of relative dielectric constant in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics with increasing δ value. The orientation of the elastic dipoles caused by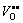 leads to the domain-wall pinning and thus the reduction of the dielectric loss. With increasing Sb2O3 content, the relative dielectric constant, dielectric constant maximum and Curie temperature of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics decrease dramatically. Contrarily, the dielectric loss increases with the increasing Sb2O3 content because of the reduction of Ti4+ ion induced by Sb3+ ion substitution for host ion.
leads to the domain-wall pinning and thus the reduction of the dielectric loss. With increasing Sb2O3 content, the relative dielectric constant, dielectric constant maximum and Curie temperature of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics decrease dramatically. Contrarily, the dielectric loss increases with the increasing Sb2O3 content because of the reduction of Ti4+ ion induced by Sb3+ ion substitution for host ion.
4) The average grain size and grain size distribution of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics can be refined by Sb2O3 addition.
Acknowledgements
This work is supported by the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and also sponsored by Suzhou Pante Electric Ceramics Tech. Co., Ltd., China.
References
[1] SAEED A, RUTHRAMURTHY B, YONG W H, HOONG O B, BAN T K, KWANG Y H. Structural and dielectric properties of iron doped barium strontium titanate for storage applications [J]. Journal of Materials Science: Materials in Electronics, 2015, 26: 9859-9864.
[2] DONG Han-ting, JIN Deng-ren, XIE Chao-jun, CHENG Jin-rong, ZHOU Li-xin, CHEN Jian-guo. Compositionally inhomogeneous Ti-excess barium strontium titanate ceramics with a robust dielectric temperature stability [J]. Materials Letters, 2014, 135: 83-86.
[3] SU B, BUTTON T W. Microstructure and dielectric properties of Mg-doped barium strontium titanate ceramics [J]. Journal of Applied Physics, 2004, 95: 1382-1385.
[4] LI Yuan-liang, QU Yuan-fang. Dielectric properties and substitution mechanism of samarium-doped Ba0.68Sr0.32TiO3 ceramics [J]. Materials Research Bulletin, 2009, 44: 82-85.
[5] LIU S J, ZENOU V Y, SUS I, KOTANI T, SCHILFGAARDE M, NEWMAN N. Structure-dielectric property relationship for vanadium- and scandium-doped barium strontium titanate [J]. Acta Materialia, 2007, 55: 2647-2657.
[6] FREEMAN C L, DAWSON J A, CHEN H R, BEN L B, HARDING J H, MORRISON F D, SINCLAIR D C, WEST A R. Energetics of donor-doping, metal vacancies, and oxygen-loss in A-site rare-earth-doped BaTiO3 [J]. Advanced Functional Materials, 2013, 23: 3925-3928.
[7] KISHI H, MIZUNO Y, CHAZONO H. Base metal-electrode- multilayer ceramic capacitors: Past, present and future perspective [J]. Japanese Journal of Applied Physics, 2003, 42: 1-15.
[8] LU Da-yong. Self-adjustable site occupations between Ba-site Tb3+ and Ti-site Tb4+ ions in terbium-doped barium titanate ceramics [J]. Solid State Ionics, 2015, 276: 98-106.
[9] LU Da-Yong, CUI Shu-Zhen. Defects characterization of Dy-doped BaTiO3 ceramics via electron paramagnetic resonance [J]. Journal of the European Ceramic Society, 2014, 34: 2217-2227.
[10] SYAMAPRASAD U, GALGALI R K, MOHANTY B C. Capacitor ceramics in pure and doped Ba0.71Sr0.29TiO3 [J]. Materials Letters, 1989, 8: 36-40.
[11] ZHANG Chen, QU Yuan-fang, MA Shi-cai. Structural and dielectric properties of Sb2O3-doped (Ba0.992-xSrxY0.008)TiO3.004 ceramics [J]. Materials Science and Engineering B, 2007, 136: 118-122.
[12] ZHANG Chen, QU Yuan-fang, MA Shi-cai. Dielectric properties of Sb2O3-doped Ba0.672Sr0.32Y0.008TiO3 [J]. Materials Letters, 2007, 61: 1007-1010.
[13] YUN Si-ning, WANG Xiao-li, LI Bo, XU De-long. Dielectric properties Ca-substituted barium strontium titanate ferroelectric ceramics [J]. Solid State Communications, 2007, 143: 461-465.
[14] ZHANG Chen, QU Yuan-fang. Dielectric properties and phase transitions of La2O3- and Sb2O3-doped barium strontium titanate ceramics [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2742-2748.
[15] HYATT E P, LONG S A, ROSE R E. Sintering high-purity BaTiO3 [J]. American Ceramic Society Bulletin, 1967, 46: 732-736.
[16] LI Wen, QI Jian-quan, WANG Yong-li, LI Long-tu, GUI Zhi-lun. Doping behaviors of Nb2O5 and Co2O3 in temperature stable BaTiO3-based ceramics [J]. Materials Letters, 2002, 57: 1-5.
氧化锑掺杂贫Ti钛酸锶钡陶瓷的介电性能及缺陷行为
张 晨,凌志新,简 刚,陈方旭
江苏科技大学 江苏省先进焊接技术重点实验室,镇江 212003
摘 要:采用固相法制备Sb2O3掺杂的贫Ti钛酸锶钡陶瓷,通过SEM、XRD和LCR测试系统研究其显微结构及介电性能随非化学计量比及Sb2O3含量的变化。结果表明:随着δ值增大,(Ba0.75Sr0.25)Ti1-δO3-2δ陶瓷由典型立方钙钛矿结构单相固溶体转变为多相化合物,而(Ba0.75Sr0.25)Ti0.998O2.996陶瓷随Sb2O3掺杂量增加始终为单相固溶体。由VTi″″及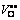 引起的ABO3型钙钛矿晶胞畸变导致(Ba0.75Sr0.25)Ti1-δO3-2δ陶瓷随δ值增大居里温度降低且相对介电常数增高。弹性偶极子
引起的ABO3型钙钛矿晶胞畸变导致(Ba0.75Sr0.25)Ti1-δO3-2δ陶瓷随δ值增大居里温度降低且相对介电常数增高。弹性偶极子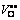 的定向引起畴壁钉扎而导致其介电损耗降低。(Ba0.75Sr0.25)Ti0.998O2.996陶瓷的相对介电常数、介电常数最大值及居里温度均随着Sb2O3掺杂量增加显著降低而其介电损耗却随Sb2O3掺杂量的增加而 增大。
的定向引起畴壁钉扎而导致其介电损耗降低。(Ba0.75Sr0.25)Ti0.998O2.996陶瓷的相对介电常数、介电常数最大值及居里温度均随着Sb2O3掺杂量增加显著降低而其介电损耗却随Sb2O3掺杂量的增加而 增大。
关键词:钛酸锶钡;缺陷;介电性能;非化学计量陶瓷
(Edited by Xiang-qun LI)
Foundation item: Project (BK20140517) supported by the Natural Science Foundation of Jiangsu Province, China; Project (14KJB430011) supported by Jiangsu Provincial Natural Science Foundation for Colleges and Universities, China
Corresponding author: Chen ZHANG; Tel:+86-511-84401184; Fax: +86-511-84407381; E-mail: czhang1981@hotmail.com
DOI: 10.1016/S1003-6326(17)60294-2
Abstract: The microstructures and dielectric properties of Sb2O3-doped Ti deficient barium strontium titanate ceramics prepared by solid state method were investigated with non-stoichiometric level and Sb2O3 content by SEM, XRD and LCR measure system. It is found that with the increase of δ, (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics transform from single phase solid solutions with typical cubic perovskite structure to multiphase compounds while (Ba0.75Sr0.25)Ti0.998O2.996 ceramics remain to be single-phase with the increasing Sb2O3 content. The distortion of the ABO3 perovskite lattice caused by VTi″″ and 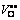 induces the drop of Curie temperature and the rise of relative dielectric constant in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics with increasing δ value. The orientation of
induces the drop of Curie temperature and the rise of relative dielectric constant in (Ba0.75Sr0.25)Ti1-δO3-2δ ceramics with increasing δ value. The orientation of 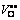 elastic dipoles results in the domain-wall pinning and thus the reduction of the dielectric loss. With increasing Sb2O3 content, the relative dielectric constant, dielectric constant maximum and Curie temperature of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics decrease dramatically while the dielectric loss increases.
elastic dipoles results in the domain-wall pinning and thus the reduction of the dielectric loss. With increasing Sb2O3 content, the relative dielectric constant, dielectric constant maximum and Curie temperature of (Ba0.75Sr0.25)Ti0.998O2.996 ceramics decrease dramatically while the dielectric loss increases.


