
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1209-1216
Determination of arsenic speciation in secondary zinc oxide and arsenic leachability
LI Yu-hu, LIU Zhi-hong, ZHAO Zhong-wei, LI Qi-hou, LIU Zhi-yong, ZENG Li
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 23 May 2011; accepted 30 August 2011
Abstract:
The species of arsenic in secondary zinc oxide generated from fuming furnace were investigated. The results revealed that there are mainly three types of secondary zinc oxide based on three arsenic species. The main phase of As is As2O3 in type I, zinc arsenite (Zn(AsO2)2) in type II and lead arsenate (Pb(As2O6), Pb4As2O9) in type III, respectively. Selective leaching of zinc oxide of type II was carried out. The leaching rate of As kept at 65%-70% with 30 g/L NaOH and L/S ratio of 3 at 20 ℃ for 1 h, while the losses of Pb and Zn were both below 1%.
Key words:
secondary zinc oxide; arsenic species; alkali leaching; arsenic removal;
1 Introduction
Arsenic contamination is a worldwide problem, and the safe treatment of arsenic is becoming a focus. The current method to treat arsenic is to transform arsenic into insoluble compounds by solidification, rather than to recover as a product [1].
Arsenic exists in the forms of realgar (As4S4) and orpiment (As2S3) in nature, however, it normally co-exists with other metalliferous deposits, such as iron pyrite, galena and chalcopyrite [2,3]. Abundant arsenic is enriched in flue dust in the process of roasting and smelting. The arsenic-bearing dusts are fine particles, containing a mass of metal values, such as Zn, Cu, Pb and Sb, which are very attractive for their economic values [4,5]. The treatment of dusts can be classified into two ways. One is hydrometallurgical process, in which As and metal values are leached out by acid, alkaline or bacteria [6-9]. The solidification of arsenic and the recovery of metal values are achieved by precipitation, solvent extraction and ion-exchange process [10-14]. The other is the combined hydrometallurgical and pyrometallurgical process [15].
Although there are many reports on the treatment of arsenic, little concern was given to arsenic phases. It normally takes As2O3 as the only arsenic species in dust [16,17]. CONTRERAS et al [18] reported that the interactions of arsenic with other elements in the vaporization might result in the formation of new arsenic species, such as Cd3(AsO4)2, As3SbO6 and As2Sb2O6. The removal of arsenic strongly depends on the species of arsenic. Secondary zinc oxide was a representative dust, which was generated in zinc and lead production by reduction-roasting method from fuming furnace or rotary kiln. Several methods were reported on the treatment of secondary zinc oxide, such as caustic soda roasting-leaching [19], oxidation hydrolysis [20], sulphating roasting-leaching [21] and ammonia leaching [22]. However, the industrial application of these processes was limited due to low removal of arsenic or poor economic values, as well as formation of secondary pollutant. Therefore, a sampling plan of secondary zinc oxide from the production site was established for characterizing arsenic species and exploring the interaction between metal values and arsenic in vaporization process. The removal of As from secondary zinc oxide was also investigated.
2 Experimental
2.1 Materials
15 samples with each secondary zinc oxide of 15 kg were collected from Shaoguan Smelter during research periods (May, 2004—November, 2007), which were
named Z1 to Z15. All samples were analyzed to determine their physical and chemical properties. It was found that the compositions of secondary zinc oxide and the arsenic phase can be classified into three types. Sample Z1 to Z8, sample Z9 to Z12, and sample Z13 to Z15 belonged to type I, type II and type III, respectively. Thus, three representative samples, named Z1, Z9 and Z13, were chosen as experimental materials.
Reagent of NaOH was analytical pure and deionized water was used in the experiment.
2.2 Experimental procedures
2.2.1 Occurrence forms of arsenic
The composition, phase and microscopic characteristics of the three samples were characterized by chemical composition analysis, chemical phase analysis, X-ray diffraction (XRD) and scanning electron microscopy (SEM/EDS) analysis.
2.2.2 Leaching experiment of secondary zinc oxide
All leaching experiments were conducted in a 1 L beaker fully enclosed with a thermometer fixed for monitoring temperature and gas exhaust pipe as cooling system to prevent solution from evaporation. The beaker was immersed in a water bath. In each experiment, 100 g secondary zinc oxide was mixed with leaching reagent to form proper slurry and then stirred at 500 r/min and certain temperature. After leaching and vacuum filtration, the solid residue was washed by hot water three times, then dried; the filtrate and wash water were collected together for further treatment. Finally, the solid residue was recycled to sintering burden process to recover zinc and lead, and the leach solution was oxidized by hydrogen peroxide and precipitated with lime.
2.3 Analysis and characterization
X-ray diffractometer (Siemens D5000, Cu Kα, λ= 1.542×10-10 m) was employed to qualitatively analyze the phase of samples and leach residue. The total arsenic content in leach residue was determined by the sodium hypophosphite reduction-iodometric method. The contents of As(III), As(V) in the leach solution were determined by extracting-iodometric method and the contents of Zn and Pb were determined by EDTA complex titration or ICP-MS (IRIS InterpidII XSP, Thermo Electron Corporation) method when they are present in trace amount. The morphologies and particle size of secondary zinc oxide were observed using a JSM-6360LV apparatus. The micro area component was measured by energy dispersive X-ray spectrometric microanalyzer (EDX- GENESIS 60S).
3 Results and discussion
3.1 Characteristic of secondary zinc oxide
The chemical composition of samples is listed in Table 1. It is shown that the content of As varies slightly around 6%; however, samples Z1 and Z9 contain more than 50% Zn and only 5%-10% Pb, and sample Z13 contains 30% Zn and 20% Pb.
Table 1 Chemical composition of secondary zinc oxide samples

Figure 1 shows the XRD patterns of secondary zinc oxide samples. As can be seen, arsenic in sample Z1 mainly exists as As2O3 in comparison to ZnAs2O4 and Pb4As2O9 or Pb(As2O6) in samples Z9 and Z13, respectively.
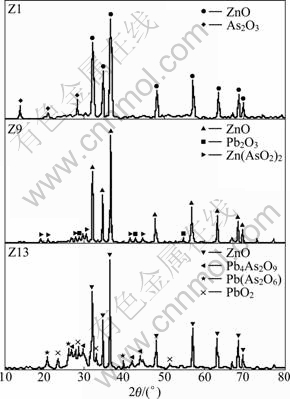
Fig. 1 XRD patterns of secondary zinc oxide samples
The chemical phase analysis results are shown in Table 2. The arsenic species are consistent with XRD analysis results. For samples Z9 and Z13, the total As amount detected by chemical phase analysis is a little lower than that detected by chemical composition analysis.
SEM micrographs of the samples are shown in Fig. 2. There is great difference in morphology and particle size among the samples. For sample Z1, the coarse polyhedral particles in micro-size are massive and well crystallized, which are supposed to be ZnO particles. The minimal spherical nano-particles adhered to the coarse ZnO particles are assumed to be As2O3. Both samples Z9 and Z13 possess spherical and polyhedral particles, but the average particle size of sample Z9 is finer than that of sample Z13. It can be inferred that the formation mechanism of spherical particles is melting and solidification of Pb-bearing compounds with low melting point, while the polyhedral particles are formed by the crystallized growth of ZnO.
Table 2 Chemical phase analysis results of secondary zinc oxide samples (mass fraction, %)

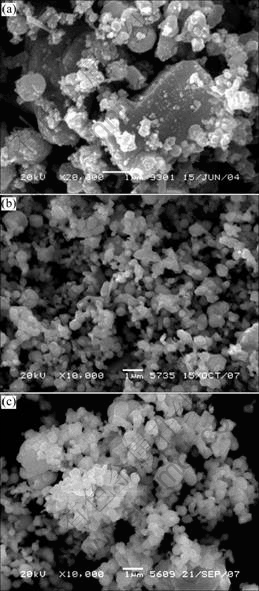
Fig. 2 SEM images of secondary zinc oxide: (a) Sample Z1; (b) Sample Z9; (c) Sample Z13
The EDS analysis spectra of secondary zinc oxide are shown in Fig. 3. As can be seen from Fig. 3, only Pb and Zn peaks are present in sample Z1. Therefore, As is dispersed with Pb and Zn phases, or exist as ultrafine As2O3 particles.
The EDS analysis results of sample Z9 show that there exist two kinds of particles: one is grey powder, and the other is white one. According to EDS point scanning analysis, Zn is the main composition of grey powders, which is assumed to be ZnO particles, while the white one is rich in Pb and As.
The EDS analysis results of sample Z13 show that the white spherical particles contain more Pb and As, which is consistent with the XRD analysis result that the main As phase in sample Z13 is lead arsenate. Those particles with Zn and O as major elements are assigned to ZnO.
In conclusion, arsenic in secondary zinc oxide does not always exist as As2O3. The interaction with Pb and Zn would result in the formation of lead and zinc arsenite, or arsenate, which is greatly different from generally acknowledged recognition. According to arsenic species, secondary zinc oxide can be classified into three types: Type I with the main phase of As2O3, and type II and type III with zinc arsenite (Zn(AsO2)2) and lead arsenate (Pb(As2O6), Pb4As2O9), respectively.
The reasons for producing different types of secondary zinc oxide are as follows. Firstly, acidic oxides As2O3 and As2O5 tend to react with ZnO and PbO in thermodynamics according to reactions (1) and (2). Secondly, production atmosphere has significant influence on the formation of Zn and Pb complex oxides. Generally, it is favorable to the combination of complex oxide in oxidative atmosphere and dissociation in reductive atmosphere.
ZnO+As2O3=Zn(AsO2)2, ΔG298=-49.68 kJ/mol (1)
PbO+As2O5=Pb(As2O6), ΔG298=-124.28 kJ/mol (2)
Although secondary zinc oxide is generated by the reductive volatilization under the weak reductive atmosphere, the composition fluctuation of raw material can change the atmosphere because of the variation of oxygen consumption for different elements. Zn would consume almost three times as many oxygen as Pb, thus, even controlling the same production conditions, the actual atmosphere may be totally different. As seen from Table 1, there is a distinctive difference between Pb and Zn contents in the three samples. Therefore, the composition fluctuation of raw materials results in the diversification of secondary zinc oxide.
Undoubtedly, the diversification of secondary zinc oxide affects the leaching behavior of As. There are little reports about the removal of As from arsenite, or arsenate, and only the similar research of the treatment of chromated copper arsenate was reported [23,24]. Since the study of type I and type III has been well developed [25,26], it is necessary to investigate the removal of As in type II secondary zinc oxide. The leach behavior of As from type II secondary zinc oxide was thus studied in this study.
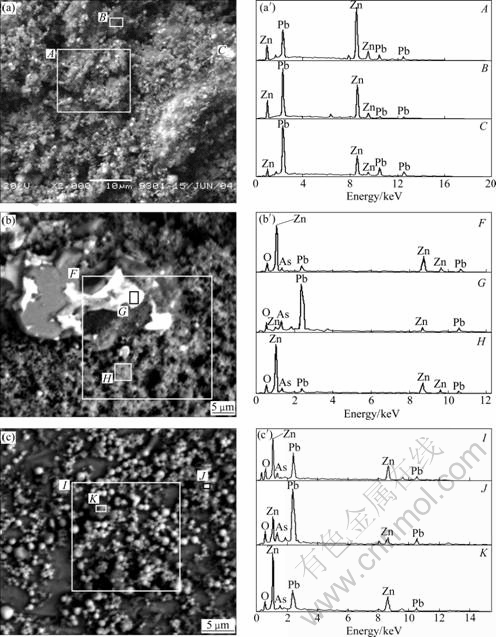
Fig. 3 EDS analysis spectra of secondary zinc oxide: (a), (a′) Sample Z1; (b), (b′) Sample Z9; (c), (c′) Sample Z13
3.2 Foundation of leaching arsenic from type II secondary zinc oxide
To investigate the leachability of type II secondary zinc oxide, the equilibrium concentration diagram of type II secondary zinc oxide was plotted. The main phases in type II secondary zinc oxide are Zn(AsO2)2, ZnO and Pb2O3. The Gibbs free energy of Zn(AsO2)2 is equal to the sum of Gibbs free energy of formation of corresponding cation, anion and complex [27]. Thus the Gibbs free energy of Zn(AsO2)2 was estimated to be -847.1 kJ/mol, and the Ksp value of this insoluble product was 10-9.59.
Dissociated As(III), Zn(II) and Pb(II) might exist in aqueous solution in the following forms: AsO+, ![]() HAsO2(aq),
HAsO2(aq), ![]()
![]()
![]() Zn2+, ZnOH+, Zn(OH)2(aq),
Zn2+, ZnOH+, Zn(OH)2(aq), ![]()
![]() Pb2+, PbOH+, Pb(OH)2(aq),
Pb2+, PbOH+, Pb(OH)2(aq), ![]() and
and ![]() [28].
[28].
Based on the thermodynamic calculations, the equilibrium concentration logarithm diagram of type II secondary zinc oxide was plotted and is shown in Fig. 4. The total concentration of As(III), Zn(II) and Pb(II) at different pH values are described by curves. The regions enclosed by curves are stable regions of Zn(AsO2)2, ZnO and Pb2O3, respectively.
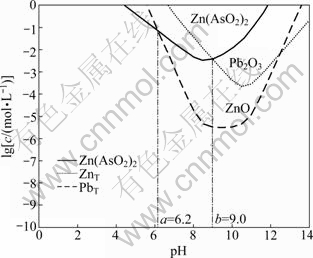
Fig. 4 lg c–pH plot for type II secondary zinc oxide
When system pH<6.2, the stability order is Zn(AsO2)2>ZnO>Pb2O3; when 6.2
Zn(AsO2)2>Pb2O3; and when pH>9.0, the order is ZnO>Pb2O3>Zn(AsO2)2. Therefore, the separation of As and metal values could be achieved by selective alkali leaching, especially when system pH is above 9. For example, when pH value is 10.5, the theoretical concentrations of As, Zn and Pb in solution are 1.92, 0.000029 and 0.045 g/L, respectively. Zn(AsO2)2 can dissolve effectively, leaving ZnO and Pb2O3 in residue, meanwhile, high recovery of metal values is ensured. After liquid and solid separation, the leach residue is returned to pyrometallurgical process for recycling Zn and Pb. The leach solution is returned to leaching step after arsenic precipitation.
3.3 Selective leaching of type II secondary zinc oxide
Alkali leaching experiment was carried out to remove arsenic and recover metal values. The aim of this process is to leach more As and less Pb and Zn as much as possible. Zinc and lead in leach solution were counted as loss of metal values.
Both water leaching and alkali leaching systems were studied. Alkali leaching was conducted with 10 g/L NaOH solution at 70 ℃ for 2 h. Water leaching was carried out under the same conditions with only water as the leaching reagent. The effect of leaching system on the leaching rate of As is shown in Fig. 5.
As can be seen from Fig. 5, the leaching rate of As both increases with the increase of leaching reagent amount. However, the leaching rate of As improves dramatically in alkali leaching. At 70 ℃, the leaching rate of As with 300 mL water is 1.24%, while that is 11.42% with 300 mL of 10 g/L NaOH solution. The difference essentially derives from the effect of pH value on the leaching rate of As. According to Fig. 4, Zn(AsO2)2 is relatively stable in the pH range of 6-11, and it is favorable to leach Zn(AsO2)2 beyond this range. Given the efficiency and selectivity for leaching system, alkali leaching is chosen for the following experiment.

Fig. 5 Effect of leaching reagents on leaching rate of As
The effect of leaching time on the leaching rate of As and metal values was studied with 50 g/L NaOH solution and liquid/solid ratio (L/S) of 4 at 80 ℃. The results are shown in Fig. 6.
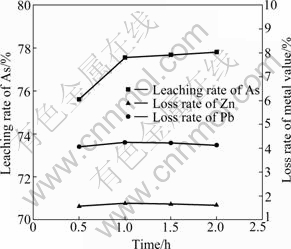
Fig. 6 Effect of leaching time on leaching rate of As and metal values
It can be seen from Fig. 6 that 77% of As is leached out in 1 h and the leaching rate is improved slightly with the increase of leaching time. Meanwhile, the leaching time has little effect on the loss of metal values, thus the dissolution balance of Zn and Pb in aqueous solution is easily reached. Since As partially exists as encapsulation or inclusion phase, the leaching time of As is longer than that of metal values. Therefore, the leaching time is determined to be 1 h.
The effect of temperature on the leaching rate of arsenic and metal values was studied with 50g/L NaOH solution and L/S of 4 for 1 h. The results are shown in Fig. 7.
As seen from Fig. 7, the leaching rate of As increases slightly with the increase of temperature, while the loss of Pb and Zn increases rapidly. The leaching rates of As, Pb and Zn at 20 ℃ are 62.18%, 1.8% and 0.6% respectively, but are 75.03%, 4.2% and 1.6% at 80 ℃, correspondingly. In consideration of the cost and energy comsuption, 20 ℃ was determined to be the optimum leaching temperature with alkali leaching.

Fig. 7 Effect of leaching temperature on leaching rate of As and metal values
The effect of NaOH concentration on the leaching rate of As and metal values was studied with L/S ratio of 4 at 20 ℃ for 1 h. The results are shown in Fig. 8.
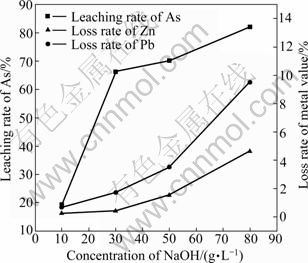
Fig. 8 Effect of NaOH concentration on leaching rate of As and metal values
As seen from Fig. 8, the leaching rate of As improves rapidly with the increase of NaOH concentration from 10 g/L to 30 g/L. Further increase of NaOH concentration has little effect on the leaching rate of As, but the leaching rates of Pb and Zn increase dramatically. As seen from Fig. 4, Zn(AsO2)2 existing in type II secondary zinc oxide dissolves in priority, thus the leaching rate of As increases with the increase of NaOH concentration. However, the rest of arsenic encapsulated by ZnO or Pb2O3 is difficult to dissolve, which can be only leached out after the dissolution of ZnO and Pb2O3, resulting in the loss of metal values increasing rapidly and the leaching rate of As increasing slightly. The leaching rates of As, Pb and Zn with 30 g/L NaOH are 66.33%, 1.71% and 0.43%, respectively, in comparison to 82.04%, 9.49% and 4.66% with 80 g/L NaOH. Given the leaching rate of As and the loss of metal values, 30 g/L NaOH was determined.
The effect of liquid/solid ratio (L/S) on the leaching rate of As and metal values was studied with 30 g/L NaOH at 20 ℃ for 1 h. The results are shown in Fig. 9.

Fig. 9 Effect of L/S ratio on leaching rate of As and metal values
The leaching rates of As, Zn and Pb are improved with the increase of L/S ratio. The leaching rates of As, Pb and Zn at L/S ratio of 10 are 81.24%, 10.39% and 4.76%, respectively, in comparison to 62.29%, 0.92% and 0.45% at L/S ratio of 3, correspondingly. The leaching rate of As increases less than 1.5 times when L/S ratio increases more than three times (from 3 to 10), which demonstrates that the arsenic occurance in type II secondary zinc oxide is partially inclusion. Further decrease in L/S ratio is unfavorable for the dispersion of leaching system, therefore, L/S ratio of 3 is chosen.
Based on the results above, the optimum leaching conditions for removing arsenic from type II secondary zinc oxide were determined as follows: 30 g/L NaOH solution, L/S ratio of 3, temperature of 20 ℃, leaching time of 1 h. The scale-up test was carried out at the optimum conditions using 2.5 kg type II secondary zinc oxide. The results are shown in Table 3, and the XRD pattern of leach residue is presented in Fig. 10. The results of scale-up test indicate that the leaching rate of As kept at 65%-70% and only less than 1% Pb or Zn is lost. As seen from Fig. 10, only ZnO diffraction peaks are detected and ZnAs2O4 peaks disappear, indicating that the removal of As is satisfied. The rest of arsenic exists as either amorphous state or multiple species but low content, which results in the absence of arsenic species in XRD patterns.
Table 3 Results of scale-up test

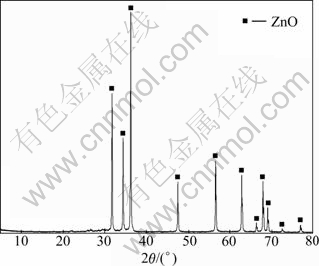
Fig. 10 XRD pattern of scale-up leaching residue
4 Conclusions
1) To better understand the leachability of arsenic in secondary zinc oxide from fuming furnace, the speciation of arsenic was invenstigated. The results showed that arsenic in secondary zinc oxide does not always exist as As2O3. The interaction of As with Pb and Zn would form new species of zinc arsenite (Zn(AsO2)2) and lead arsenate (Pb(As2O6), Pb4As2O9), which is greatly different from general recognition.
2) Further leaching experiment of type II secondary zinc oxide in alkaline media was also investigated. The results of scale-up test indicated that the leaching rate of As kept at 65%-70% with 30 g/L NaOH and L/S ratio of 3 at 20 ℃ for 1 h, while the losses of Pb and Zn were both below 1%.
References
[1] LEIST M, CASEY R J, CARIDI D. The management of arsenic wastes: Problems and prospects [J]. Journal of Hazardous Materials, 2000, B76(1): 125-138.
[2] JAIN C K, ALI I. Arsenic: Occurrence, toxicity and speciation techniques [J]. Water Research, 2000, 34(17): 4304-4312.
[3] MANDAL B K, SUZUKI K T. Arsenic round the world: A review [J]. Talanta, 2002, 58(1): 201-235.
[4] AGRAWAL A, SAHU K K, PANDEY B D. Solid waste management in non-ferrous industries in India [J]. Resources Conservation and Recycling, 2004, 42(2): 99-120.
[5] MONTENEGRO V, SANO H, FUJISAWA T. Recirculation of high arsenic content copper smelting dust to smelting and converting processes [J]. Minerals Engineering, 2010, doi: 10.1016/j.mineng. 2010.03.020.
[6] XU Zhi-feng, NIE Hua-ping, LI Qiang, LU Qiu-hu, WANG Wei, YUE Ri-hui. Pressure leaching technique of smelter dust with high-copper and high arsenic [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(s1): s59-s63. (in Chinese)
[7] XU Zhi-feng, LI Qiang, NIE Hua-ping. Pressure leaching technique of smelter dust with high-copper and high arsenic [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(s1): s176-s181.
[8] MORALES A, CRUELLS M, ROCA A, BERGO R. Treatment of copper flash smelter flue dusts for copper and zinc extraction and arsenic stabilization [J]. Hydrometallurgy, 2010, 105(1-2): 148-154.
[9] CUI Ri-cheng, YANG Hong-ying, CHEN Sen, ZHANG Shuo, LI Ke-feng. Valence variation of arsenic in bioleaching process of arsenic-bearing gold ore [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(6): 1171-1176.
[10] IBERHAN L,WISNIEWSKI M. Extraction of arsenic (III) and arsenic (V) with Cyanex 925, Cyanex 301 and their mixtures [J]. Hydrometallurgy, 2002, 63(1): 23-30.
[11] ORHAN G. Leaching and cementation of heavy metals from electric arc furnace dust in alkaline medium [J]. Hydrometallurgy, 2005, 78(3-4): 236-245.
[12] IMPELLITTERI C A. Effect of pH and phosphate on metal distribution with emphasis on As speciation and mobilization in soils from a lead smelting site [J]. Science of the Total Environment, 2005, 345(1-3): 175-190.
[13] DUTRA A J B, PAIVA P R P, TAVARES L M. Alkaline leaching of zinc from electric arc furnace steel dust [J]. Minerals Engineering, 2006, 19(5): 478-485.
[14] YU Xiao-hua, XIE Gang, LI Rong-xing, LI Yong-gang, LU Ying. Behavior of arsenic in the zinc electrowining [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(s1): s50-s54.
[15] SHIBAYAMA A, TAKASAKI Y, WILLIAM T, YAMATODANI A, HIGUCHI Y, SUNAGAWA S, ONO E. Treatment of smelting residue for arsenic removal and recovery of copper using pyro-hydrometallurgical process [J]. Journal of Hazardous Materials, 2010, 181(1-3): 1016-1023.
[16] SHIH C J, LIN C F. Arsenic contaminated site at an abandoned copper smelter plant: Waste characterization and solidification/ stabilization treatment [J]. Chemosphere, 2003, 53(7): 691-703.
[17] STERLING R O, HELBLE J J. Reaction of arsenic vapor species with fly ash compounds: Kinetics and speciation of the reaction with calcium silicates [J]. Chemosphere, 2003, 51(10): 1111-1119.
[18] CONTRERAS M L, AROSTEGUI J M, ARMESTO L. Arsenic interactions during co-combustion processes based on thermodynamic equilibrium calculations [J]. Fuel, 2009, 88(3): 539-546.
[19] TAN Xiang-ting. Recycling and reclamation research of acid leaching residue from secondary zinc oxide [J]. Nonferrous Metals: Extractive Metallurgy, 1998(5): 18-21. (in Chinese)
[20] ZHANG Cai-ming. Research and application of the process for removing As from secondary zinc oxide by oxidation-hydrolysis [J]. Nonferrous Metals: Extractive Metallurgy, 1997(2): 9-11. (in Chinese)
[21] CHEN Shi-ming, CHENG Dong-kai, LI Yu-hou. Study of comprehensive recovery of high-contents As secondary zinc oxide [J]. Non-ferrous Mining and Metallurgy, 2001, 17(5): 29-32. (in Chinese)
[22] YI Qiu-shi. Preparation of activated zinc oxide from arsenic- containing crude zinc oxide [J]. Environmental Protection of Chemical Industry, 2001, 21(4): 217-220. (in Chinese)
[23] JANIN A, ZAVISKA F, DROGUI P, BLAIS J F, MERCIER G. Selective recovery of metals in leachate from chromated copper arsenate treated wastes using electrochemical technology and chemical precipitation [J]. Hydrometallurgy, 2009, 96(4): 318-326.
[24] JEAN A, BLAIS J F, MERCIER G, DROGUI P. Optimization of a chemical leaching process for decontamination of CCA-treated wood [J]. Journal of Hazardous Materials, 2009, 169(1-3): 136-145.
[25] LI Y H, LIU Z H, LI Q H, ZHAO Z W, LIU Z Y, ZENG L. Removal of arsenic from Waelz zinc oxide using a mixed NaOH-Na2S leach [J]. Hydrometallurgy, 2011, 108(3-4): 165-170.
[26] LI Yu-hu, LIU Zhi-hong, LI Qi-hou, ZHAO Zhong-wei, LIU Zhi-yong, ZENG Li, LI Li. Removal of arsenic from arsenate complex contained in secondary zinc oxide [J]. Hydrometallurgy, 2011, 109(3-4): 237-244.
[27] GOLAM MOSTAFA A T M, EAKMAN J M. Prediction of standard heats and Gibbs free energies of formation of solid inorganic salts from group contributions [J]. Industrial & Engineering Chemistry Research, 1995, 34(12): 4577-4582.
[28] DEAN J A. Lange’s Handbook of Chemistry [M]. WEI J F, transl. Beijing: Science Press, 2003. (in Chinese)
次氧化锌中砷的物相及砷的浸出
李玉虎,刘志宏,赵忠伟,李启厚,刘智勇,曾 理
中南大学 冶金科学与工程学院,长沙 410083
摘 要:研究烟化炉次氧化锌中砷的物相类型。结果表明:按砷的物相可将次氧化锌分为3种类型。在一型次氧化锌中砷以As2O3形态存在,而在二型和三型次氧化锌中砷分别以亚砷酸锌(Zn(AsO2)2)和砷酸铅(Pb(As2O6), Pb4As2O9)形态存在。在热力学分析基础上,对二型次氧化锌进行浸出脱砷。结果表明:采用30 g/L NaOH溶液,在液固比3、温度20 ℃的条件下,砷的浸出率在1 h内可达到65%~70%,而铅、锌的损失均小于1%。
关键词:次氧化锌;砷物相;碱性浸出;脱砷
(Edited by YUAN Sai-qian)
Foundation item: Project (50874121) supported by the National Natural Science Foundation of China
Corresponding author: LI Yu-hu; Tel: +86-731-88879850; E-mail:lyh_csu@163.com
DOI: 10.1016/S1003-6326(11)61307-1
Abstract: The species of arsenic in secondary zinc oxide generated from fuming furnace were investigated. The results revealed that there are mainly three types of secondary zinc oxide based on three arsenic species. The main phase of As is As2O3 in type I, zinc arsenite (Zn(AsO2)2) in type II and lead arsenate (Pb(As2O6), Pb4As2O9) in type III, respectively. Selective leaching of zinc oxide of type II was carried out. The leaching rate of As kept at 65%-70% with 30 g/L NaOH and L/S ratio of 3 at 20 ℃ for 1 h, while the losses of Pb and Zn were both below 1%.


