
Thermodynamic analysis on sodium carbonate decomposition of
calcium molybdenum
XIA Wen-tang(夏文堂), ZHAO Zhong-wei(赵中伟), LI Hong-gui(李洪桂)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 30 August 2006; accepted 7 December 2006
Abstract:
According to the principle of mass balances and thermodynamic data, lg [Me]—pH diagrams (Me=Ca, Mo) for Ca-Mo-CO3-H2O system at 25 ℃ were presented with total dissolved carbon-containing ions concentrations of 0.01 mol/L and 1 mol/L, and lg [Me]—pH diagram for Ca-Mo-H2O system at 25 ℃ was also depicted. The effects of system pH value and total dissolved carbon-containing ions concentrations on the concentrations of the species in Ca-Mo-CO3-H2O system were studied. The results show that the stability region of CaMoO4 reduces significantly in the presence of sodium carbonate. In order to achieve effective leaching of molybdenum from CaMoO4, a certain concentration of sodium carbonate is necessary. High total dissolved carbon-containing ions concentrations and high pH values facilitate to the leaching of CaMoO4 and dissolved sodium carbonate is an efficient leaching agent for decomposing CaMoO4.
Key words:
calcium molybdenum(CaMoO4); sodium carbonate; decomposition; thermodynamic analysis; concentration logarithm diagram;
1 Introduction
Calcium molybdenum(CaMoO4) mainly exists in seyrigite, molybdenum residue of ammonia-leaching, low-grade concomitancy ore with complexity composition and multi-metal after roasted and spent catalyst with molybdenum, etc. Because of lacking deep study on thermodynamic characteristics in this system, it is difficult to locate the species that play a role in the leaching process with dissolved sodium carbonate. The optimum leaching conditions for decomposing CaMoO4 are usually determined by experiments[1-6].
Since the principle of potential—pH (E—pH) diagrams was created by POURBAIX[7], the diagrams have played a very important role in many fields and are widely used[8-15]. OSSEO-ASARE[16] presented E—pH diagram of Ca-Mo-CO3-H2O system at 25℃([Mo]=[Ca]=10-3 mol/L) for molybdenum leaching. The diagram shows that E—pH predominance area for equilibrium involves Ca2+, CaMoO4, Ca(OH)2, Ca(OH)+ and a large CaMoO4 stability field in high pH region. As a matter of fact, the overall reactions for the decomposition of CaMoO4 by dissolved sodium carbonate may be represented as[4]
CaMoO4(s)+Na2CO3(aq)=Na2MoO4(aq)+CaCO3(s)
It can be seen that the reaction is a double decomposition reaction rather than an oxidation- reduction reaction. In the multi-species system, the practical application of E—pH diagram has its limitation in analyzing the Ca-Mo-CO3-H2O system. In order to judge the conditions under that better results can be obtained, it is often necessary to know how the solution components influence the solubility of poorly soluble mineral by means of concentration logarithm diagrams[17], which is a predictive and analytical tool in hydrometallurgical process. So the concentration logarithm diagram can be used to analyze the carbonate decomposition process. In this paper, a simple and convenient calculation method was introduced according to mass balance principle. Calculations were made to express equilibrium relations among associated aqueous species in terms of total concentrations rather than the individual dissolved species to establish lg [Me]—pH diagrams in Ca-Mo-CO3-H2O system at 25℃. The effects of pH value and total dissolved carbon-containing ions concentration on the equilibrium in Ca-Mo-CO3-H2O systems were also analyzed.
2 Calculation of concentration logarithm diagrams
2.1 Reactions in aqueous solution and thermo- dynamic data
Table 1 lists reactions in aqueous solution and a summary of thermodynamic data in Ca-Mo-CO3-H2O and Ca-Mo-H2O systems at 25 ℃.
Table 1 Reactions in aqueous solutions and selected equilibrium constants for systems of Ca-Mo-CO3-H2O and Ca-Mo-H2O at 25 ℃ [16, 18]

The equilibria of total dissolved molybdenum- containing ions concentration, total dissolved calcium-containing ions concentration and total dissolved carbon-containing ions concentrations were respectively established according to the equilibrium equations in Ca-Mo-CO3-H2O system and the principle of mass balance.
2.2 Calculation method
1) Based on reactions in aqueous solution and selected equilibrium constants of equations No.10 to 14 in Table 1, the species of![]()
![]() MoO2(OH)+,
MoO2(OH)+, ![]() and H2MoO4(aq) exist in Ca-Mo-CO3-H2O system. The mass balance for total soluble Mo is given by
and H2MoO4(aq) exist in Ca-Mo-CO3-H2O system. The mass balance for total soluble Mo is given by
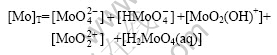
There exist
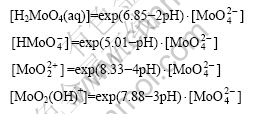
2) From equations No.1 to 4 and equations No.7 to 9 in Table 1, the species of Ca2+, Ca(OH)+, Ca(OH)2(aq), Ca(HCO3)+ and CaCO3(aq) exist in the system. The mass balance for total soluble Ca is given by
[Ca]T=[Ca2+]+[Ca(OH)+]+[Ca(OH)2(aq)]+[Ca(HCO3)+]+
[CaCO3(aq)]
There exist
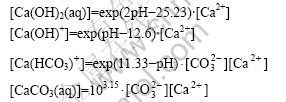
3) From equations No.2 to 6 in Table 1, there are species of H2CO3(aq), ![]() ,Ca(HCO3)+,
,Ca(HCO3)+, ![]() and CaCO3(aq) in the system. The mass balance for total soluble C is given by
and CaCO3(aq) in the system. The mass balance for total soluble C is given by
![]()
There exist

4) When pH value is low, the H2MoO4 precipitation will be formed. According to equations No.10 and 11, there exists
[H2MoO4(aq)]=10-11.99 mol/L
5) In CaMoO4 stability region, there exists
[Ca]T=[Mo]T
6) When pH value increases, CaCO3 precipitation will be formed. According to equations No.2 and 3, there exists
[CaCO3(aq)]=1011.43 mol/L
7) When pH value is high, the Ca(OH)2 precipitation will be formed. According to equations No.8 and 9, there exists
[Ca(OH)2(aq)]=10-2.45 mol/L
Other variables can be obtained from equations in Table 1 by the established simultaneous equations system under given condition.
The determination of stability depends on the availability of relevant equilibrium constants and a variety of soluble ions concentrations that are known to exist[16]. However, there are insufficient data on ion activity coefficient, and so are the ones on temperature effects. The approach taken in this paper is limited at 25℃ and ion activity is replaced by ion concentration in calculation.
3 Results and discussion
3.1 Ca-Mo-H2O system
Fig.1 shows the lg [Me]—pH diagram for Ca-Mo- H2O system at 25 ℃, which indicates that the Ca-Mo- H2O system is dominated by large CaMoO4 stability field between pH 7.3 and pH 14.5. The location of stability field matches the E—pH diagram presented by OSSEO- ASARE[14]. Ca2+ concentration and ![]() concentration are almost constant in this stability field.
concentration are almost constant in this stability field.
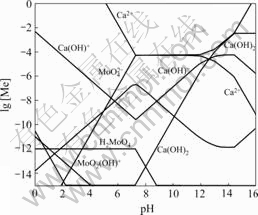
Fig.1 lg [Me]—pH diagram for Ca-Mo-H2O system at 25 ℃
When pH value is less than 7.3, ![]() exists in the form of
exists in the form of ![]() and H2MoO4 precipitations. Meanwhile, the free
and H2MoO4 precipitations. Meanwhile, the free ![]() concentration decreases. Because the solubility product constant of CaMoO4 is unchangeable, the free Ca2+ concentration increases, and the concentration of other species that relate with the concentration of
concentration decreases. Because the solubility product constant of CaMoO4 is unchangeable, the free Ca2+ concentration increases, and the concentration of other species that relate with the concentration of ![]() and Ca2+ change. As a matter of fact, CaMoO4 would be decomposed by acid under this condition.
and Ca2+ change. As a matter of fact, CaMoO4 would be decomposed by acid under this condition.
With the increase of pH value, Ca2+ exists in the form of Ca(OH)+ complex, and the free Ca2+ concentra- tion decreases; while the concentration of ![]() increases because of unchanged solubility product constant.
increases because of unchanged solubility product constant.
When pH value is higher than 14.5, the Ca2+ turns into Ca(OH)2 precipitation with the increase of alkalinity in Ca-Mo-H2O system and the free Ca2+ concentration decreases. Under this condition, it is facilitating to the increasing concentration of the free![]() .
.
3.2 Ca-Mo-CO3-H2O system
Fig.2 shows the lg [Me]—pH diagram for Ca-Mo- CO3-H2O system at 25℃ and [C]T=0.01 mol/L. Compared with Fig.1, Fig.2 shows that the stability region of calcium molybdenum is narrower than that in Fig.1 under the given condition. The CaMoO4 stability region is substantially replaced by CaCO3 region when sodium carbonate is used as a leaching agent. In this region (7.3<pH<8.2), the ![]() concentration is almost constant.
concentration is almost constant.
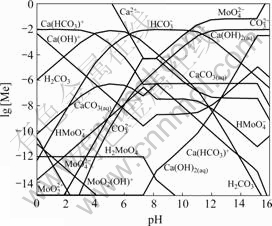
Fig.2 lg [Me]—pH diagram for Ca-Mo-CO3-H2O system at 25℃ and [C]T=0.01mol/L
If pH value is lower than 7.3, most of the free carbon exists in the form ![]() that reacts with Ca2+ to form Ca(HCO3)+.
that reacts with Ca2+ to form Ca(HCO3)+. ![]() also turns into H2MoO4 precipitation. The concentrations of
also turns into H2MoO4 precipitation. The concentrations of![]() and
and ![]() decrease, while the Ca2+ concentration increases. In fact, there is still the decomposition of calcium molybdenum by acidic medium, the same as the analysis mentioned above.
decrease, while the Ca2+ concentration increases. In fact, there is still the decomposition of calcium molybdenum by acidic medium, the same as the analysis mentioned above.
When pH value is more than 8.2, ![]() transmits into
transmits into![]() gradually.
gradually. ![]() and
and ![]() concentrations increase greatly and Ca2+ concentration decreases at this stage due to the solubility product constant. However, when pH value is greater than 10.3, the concentrations of
concentrations increase greatly and Ca2+ concentration decreases at this stage due to the solubility product constant. However, when pH value is greater than 10.3, the concentrations of ![]() , Ca2+ and
, Ca2+ and ![]() have little change. According to thermodynamic analysis, it is unnecessary to over increase pH value for decomposing molybdenum in sodium carbonate solution.
have little change. According to thermodynamic analysis, it is unnecessary to over increase pH value for decomposing molybdenum in sodium carbonate solution.
When pH value is higher than 15.1, the ![]() concentration increases sharply under condition of high alkalinity, and a new reaction takes place at this stage
concentration increases sharply under condition of high alkalinity, and a new reaction takes place at this stage
CaMoO4(s)+2OH-=Ca(OH)2(s)+ ![]() Actually, the process of sodium carbonate decomposition turns into the sodium hydroxide decomposition at high alkalinity.
Actually, the process of sodium carbonate decomposition turns into the sodium hydroxide decomposition at high alkalinity.
In order to investigate the effects of the variation of total dissolved carbon-containing ions concentrations on species in leaching solution, Fig.3 shows a lg [Me]—pH diagram for Ca-Mo-CO3-H2O system at 25℃ under the condition of [C]T=1 mol/L.
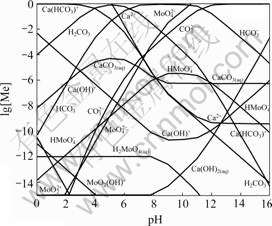
Fig.3 lg [Me]—pH diagram for Ca-Mo-CO3-H2O system at 25℃ and [C]T=1 mol/L
From the above thermodynamic analysis and practical work, sodium carbonate is an effective leaching agent, which can promote the decomposing CaMoO4 in Ca-Mo-CO3-H2O system.
Compared with Fig.2, Fig.3 shows that the stability region of CaMoO4 is constricted significantly with the increase of [C]T and the ![]() concentration also increases efficiently in leaching solution. For the sake of getting best leaching yield of molybdenum in Ca-Mo-CO3-H2O system, certain concentration of sodium carbonate is necessary. For leaching CaMoO4 with sodium carbonate, the higher the
concentration also increases efficiently in leaching solution. For the sake of getting best leaching yield of molybdenum in Ca-Mo-CO3-H2O system, certain concentration of sodium carbonate is necessary. For leaching CaMoO4 with sodium carbonate, the higher the ![]() concentration is, the greater the solubility of molybdenum is in theory, which has been conformed by GU et al[3] and PAN et al[6].
concentration is, the greater the solubility of molybdenum is in theory, which has been conformed by GU et al[3] and PAN et al[6].
4 Conclusions
1) Based on principle of mass balances and thermodynamic data, lg [Me]—pH diagrams for Ca-Mo- CO3-H2O system at 25℃ are presented, which are generated with computer under the conditions of 0.01 mol/L and 1 mol/L of total dissolved carbon- containing ions concentrations, and the diagram for Ca-Mo-H2O system at 25℃ is depicted as well.
2) Compared with the diagram of E—pH, more quantitative information on practical hydrometallurgical processes can be obtained from the given diagrams of lg [Me]—pH for Ca-Mo-CO3-H2O system. The stability region of CaMoO4 reduces significantly in the presence of Na2CO3. For the sake of getting best leaching yield of molybdenum, certain concentration of sodium carbonate is necessary, and dissolved sodium carbonate is an efficient leaching agent. The high total dissolved carbon-containing ions concentrations and high pH values are facilitating to decomposition of CaMoO4.
References
[1] ZELIKEMAN A H, KELIEYIN O E, SAMUNUOFU Г E. SONG Chen-guang Tr. Metallurgy of Rare Metals [M]. Beijing: Metallurgical Industry Press, 1982:120-122.
[2] QIN Wen-feng, PENG Jin-hui, FAN Xi-an, GUO Rong-hui. A new process for preparing ammonium molybdate by using waste calcium molybdate [J]. Multipurpose Utilization of Mineral Resources, 2003(1): 46-47. (in Chinese)
[3] GU Heng, LI Hong-gui, LIU Mao-sheng. Study on the new technology for treatment of the molybdenum residue of ammonia-leaching with sodium carbonate in heat ball-mill reactor [J]. China Molybdenum Industry, 1997, 21(4): 10-18. (in Chinese)
[4] GUTPA C K. Extractive Metallurgy of Molybdenum [M]. Flordia: CRC Press, 1992: 143-145.
[5] LI Hong-gui. Metallurgy of Rare Metals [M]. Beijing: Metallurgical Industry Press, 1990: 82-83. (in Chinese)
[6] PAN Mao-sen, ZHU Yun. Experimental study on leaching molybdenum at high-pressure from calcium molybdenum [J]. China Molybdenum Industry, 2005, 29(6): 19-21. (in Chinese)
[7] POURBAIX M. Atlas of Electrochemical Equilibria in Aqueous Solutions [M]. London: Pergamon Press, 1966.
[8] LUO Ru-tie. Overall equilibrium diagrams for hydrometallurgical systems: Copper-ammonia-water system [J]. Hydrometallurgy, 1987, 17: 177-199.
[9] ANDRZE J A, PATRICK J S. A computational approach to prediction the formation of iron sulfide species using stability diagrams [J]. Computers & Geosciences, 1997, 23(6): 647-658.
[10] JORDAN L R, BETTS A J, DAHM K L, DAHM K L. Corrosion and passivation mechanism of chromium diboride coatings on stainless steel [J]. Corrosion Science, 2005, 47: 1085-1096.
[11] HARUNA T, SHIBATA T, IMOATA T. Potential—pH region for environment-assisted cracking of gamma titanium aluminide [J]. Intermetallics, 2000, 8: 929-935.
[12] BAI X D, ZHU D H, LIU B X. The establishment of a potential-pH diagram for phosphorous implanted iron in aqueous solutions [J]. Nuclear Instruments and Methods in Physics Research, 1995, B103: 440-445.
[13] ZHAO Zhong-wei, LIU Xu-heng. Thermodynamic analysis of Li-Fe-P-H2O system [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(7): 1257-1263. (in Chinese)
[14] MA Yu-tian, GONG Zhu-qing, WU Jun, CHEN Wen-mi, YANG Zheng-hui. Thermodynamic analysis and experimental studies of leaching lead-rich tellurium slag [J]. Journal of Central South University: Science and Technology, 2006, 37(3): 498-503. (in Chinese)
[15] WANG Yun-yan, PENG Wen-jie, CHAI Li-yuan. Thermodynamic equilibrium of bismuth hydrometallurgy in chloride and nitrate solutions [J]. Journal of Central South University of Technology, 2004, 11(4): 410-413.
[16] OSSEO-ASARE K. Solution chemistry of tungsten leaching systems [J]. Metallurgical Transactions, 1982, 13B: 555-563.
[17] WANG Dian-zuo, HU Yue-hua. Solution Chemistry of Flotation [M]. Changsha: Hunan Science and Technology Press, 1988: 8-12. (in Chinese)
[18] SMITH R M, MARTELL A E. Critical Stability Constants (Vol.4) [M]. New York: Plenum Press, 1976: 1-2.
Foundation item: Project(50434010) supported by the National Natural Science Foundation of China
Corresponding author: ZHAO Zhong-wei; Tel: +86-731-8830476; E-mail: zhaozw@mail.csu.edu.cn
Abstract: According to the principle of mass balances and thermodynamic data, lg [Me]—pH diagrams (Me=Ca, Mo) for Ca-Mo-CO3-H2O system at 25 ℃ were presented with total dissolved carbon-containing ions concentrations of 0.01 mol/L and 1 mol/L, and lg [Me]—pH diagram for Ca-Mo-H2O system at 25 ℃ was also depicted. The effects of system pH value and total dissolved carbon-containing ions concentrations on the concentrations of the species in Ca-Mo-CO3-H2O system were studied. The results show that the stability region of CaMoO4 reduces significantly in the presence of sodium carbonate. In order to achieve effective leaching of molybdenum from CaMoO4, a certain concentration of sodium carbonate is necessary. High total dissolved carbon-containing ions concentrations and high pH values facilitate to the leaching of CaMoO4 and dissolved sodium carbonate is an efficient leaching agent for decomposing CaMoO4.


