DOI: 10.11817/j.ysxb.1004.0609.2021-40009
赤泥活化焙烧-联合浸出制备富钛料及组分溶解行为
朱晓波,巩文辉,李 望
(河南理工大学 化学化工学院,焦作 454000)
摘 要:
赤泥活化焙烧-联合浸出制备富钛料并分离有价组分,考察参数对各金属浸出率的影响,并对活化焙烧-联合浸出过程中组分溶解行为进行了理论分析。结果表明:在盐酸浓度为5 mol/L、液固比为6 mL/g、浸出温度为80 ℃和浸出时间为90 min的条件下,铝、铁、钠、钙和钛的浸出率分别为81.2%、76.3%、99.2%、99.3%和3.2%,同时稀有金属钒、钪和钇的浸出率均超过95%。在焙烧温度为550 ℃、碱渣比为2、焙烧时间为40 min、水浸温度为80 ℃、液固比为7 mL/g、浸出时间为60 min的条件下,硅浸出率可达85.7%,可获得TiO2品位为71.8%的富钛料。钛酸钠和少量未反应的石英存在于富钛料中,TiO2能够稳定存在于Fe3+、Al3+和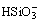 的溶液体系中。钒和硅的浸出过程均受内扩散控制,浸出表观活化能分别为10.76 kJ/mol和10.61 kJ/mol,而铁的酸浸表观活化能达到23.86 kJ/mol。
的溶液体系中。钒和硅的浸出过程均受内扩散控制,浸出表观活化能分别为10.76 kJ/mol和10.61 kJ/mol,而铁的酸浸表观活化能达到23.86 kJ/mol。
关键词:
文章编号:1004-0609(2021)-08-2227-11 中图分类号:TF11 文献标志码:A
引文格式:朱晓波, 巩文辉, 李 望. 赤泥活化焙烧-联合浸出制备富钛料及组分溶解行为[J]. 中国有色金属学报, 2021, 31(8): 2227-2237. DOI: 10.11817/j.ysxb.1004.0609.2021-40009
ZHU Xiao-bo, GONG Wen-hui, LI Wang. Preparation of titanium-rich materials and dissolution behavior of components from red mud by active roasting and combined leaching[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(8): 2227-2237. DOI: 10.11817/j.ysxb.1004.0609.2021-40009
赤泥是生产氧化铝过程中排放的尾渣,全球累计堆存量已超过30亿t,并以1.2亿t/a的排放量堆存筑坝,造成土地资源浪费[1-3]。赤泥中不仅含有钠、钾等金属阳离子,还含有碳酸根、氢氧根等碱性阴离子,学者们考察了淋滤或浸出对这些离子溶出影响,表明赤泥中超过80%的碱性离子可以溶解,会造成严重的水体和土壤污染[4-6]。因此,关于赤泥的处置和综合利用得到国内外越来越多的关注。目前,赤泥综合利用主要包括制备建筑材料、吸附材料、催化材料和回收有价组分[7-11]。
赤泥中富含铝、铁、钛、钙等元素,同时还含有一定量的钪、钒、钇等稀有金属,因此,从赤泥中提取有价组分不仅可以改善环境,还可实现资源的二次回收[12-14]。目前,关于赤泥中铁回收多采用磁化焙烧-磁选工艺,采用碱浸方法回收铝,采用酸浸方法回收钛、钪、钒、钇等稀有金属[15-20]。例如,在焙烧温度为950 ℃、焙烧时间为60 min、氯化钠用量为10%、焦炭用量为15%、焦炭粒度为0.5~0.25 mm、弱磁选磁场强度为0.12 T的条件下,焙烧赤泥可得到铁精矿的铁品位为73.99%,回收率为88.99%[21]。在赤泥中添加适量石灰和纯碱,于1000~1050 ℃下焙烧30~40 min,控制熟料中Na2O·Fe2O3的含量为10%~12%(质量分数),钙铁摩尔比为1.0~1.2,在80 ℃下浸出15 min,铝浸出率可达85%~90%[22]。该方法通过添加活化剂高温焙烧,可获得较高的铁、铝回收率,但难以联合回收其他多种有价组分。许多学者利用高浓度硫酸浸出赤泥回收钪、钒和钛,稀有金属的浸出率可达80%以上[23-24]。由于硫酸的浸出选择性较差,浸出液中除了含有钪、钛、钒之外,还含有铁、铝等多种杂质离子,导致后续的溶剂萃取等分离纯化技术难以操作[25]。
因此,本文依据赤泥的理化特性,提出了活化焙烧-联合浸出制备富钛料的方法,选择性地浸出分离和回收赤泥中的钛、钒和钪,实现了多种有价组分的联合回收。研究盐酸浓度、液固比、酸浸温度、焙烧温度、碱渣比和水浸液固比等参数对赤泥中有价组分回收的影响,利用微观物相分析、溶液热力学和浸出动力学理论,分析阐述该工艺过程有价组分的转移规律,为赤泥制备富钛料和联合回收多种有价组分提供新的思路和研究基础。
1 实验
1.1 实验原料
赤泥试样取至山西某地,通过电感耦合等离子体原子发射光谱仪(ICP-AES)和X射线衍射仪 (XRD)分析试样的化学成分和矿物组成,结果见表1和图1。
由表1可知,该赤泥中主要含有铁、铝、钛、钙、硅、钠、镁等氧化物,此外还含有一定量的钒、钇和钪等稀有金属。
由图1可知,该赤泥的主要矿物组成包括钙霞石、板钛矿、赤铁矿、方解石和石英等。实验过程所用的盐酸、氢氧化钠等药剂均为分析纯,溶剂为蒸馏水。
1.2 实验过程
根据前期研究结果,赤泥经预焙烧后,矿物组成发生改变,晶型易出现缺陷,容易发生溶解反应。
表1 赤泥的化学成分分析
Table 1 Chemical constituent of red mud (mass fraction, %)

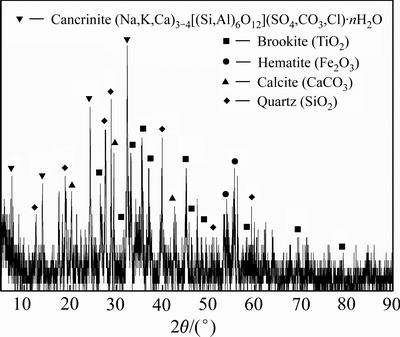
图1 赤泥的XRD物相分析
Fig. 1 XRD pattern of red mud
该赤泥于750 ℃条件下焙烧1 h,冷却后备用[26]。每次取100 g赤泥与一定浓度的盐酸溶液混合均匀,然后在不同浸出温度、液固比、浸出时间和搅拌速率的条件下进行搅拌浸出,搅拌结束后经固液分离得到酸浸液和酸浸渣。酸浸液可通过离子交换、溶剂萃取和聚合反应回收其中的钒、钪、铝和铁[27-28]。酸浸渣经蒸馏水洗涤烘干后,与氢氧化钠按照一定质量的碱渣比混合均匀,在一定的焙烧温度和焙烧时间条件下于马弗炉中进行活化焙烧,冷却后取出试样。将焙烧试样与蒸馏水按照一定的液固比混合均匀,在不同的浸出温度和浸出时间条件下进行水浸实验,水浸结束经固液分离得到水浸液和富钛料。赤泥、酸浸渣和富钛料在进行ICP-AES测试之前,需对其进行溶解预处理。该过程是将样品研磨至粒径小于0.047 mm,然后取0.1 g研磨后样品置于聚四氟乙烯烧杯中,然后向烧杯中加入20 mL氢氟酸、盐酸和硝酸混合溶液进行搅拌,直至样品完全溶解,备用待测。各元素浸出率可由式(1)表示。
 (1)
(1)
式中:R为元素浸出率(%);m1为原料质量(g);r1为原料中相应元素的品位(%);m2为浸出浸出渣质量(g);r2为浸出渣中相应元素的品位(%)。
2 结果与讨论
2.1 盐酸浸出参数的影响
考察盐酸浸出参数对赤泥中主要成分浸出率的影响,其结果如图2所示。
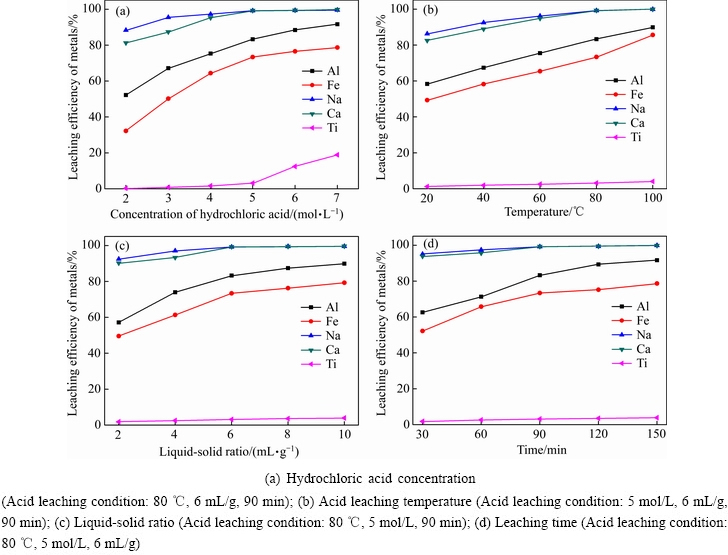
图2 酸浸参数对赤泥中主要成分浸出率的影响
Fig. 2 Effects of acid leaching parameters on leaching efficiency of main elements
由图2(a)可知,随着盐酸浓度的升高,赤泥中各组分的浸出率均呈增长趋势。随着盐酸浓度从2 mol/L提高至5 mol/L,钠和钙的浸出率从80%提高至98%以上;此时,铝和铁的浸出率分别从52.4%和31.4%提高至81.2%和76.3%。当盐酸浓度小于5 mol/L时,钛浸出率小于10%,当盐酸浓度大于5 mol/L时,钛浸出率增长明显。由图2(b)可知,随着酸浸温度升高,赤泥中钠、钙、铝、铁的浸出率增长明显,而钛浸出率增长较缓慢,这是由二氧化钛的酸溶解性差所致。当浸出温度为80 ℃时,铝、铁、钠、钙和钛的浸出率分别为81.2%、76.3%、99.2%、99.3%和3.2%。由图2(c)可知,增加浸出液固比,钠、钙、钛浸出率变化不明显,而铝和铁的浸出率呈明显的增长趋势。由图2(d)可知,随着浸出时间的延长,钠、钙和钛浸出率增长不明显,而铝和铁的浸出率增长平缓。
在盐酸浓度为5 mol/L、浸出温度为 80 ℃、液固比为6 mL/g和浸出时间为90 min的条件下,赤泥中钒、钪和钇浸出效果见图3。由图3可知,赤泥中超过95%以上的钒、钪、钇可溶解于盐酸溶液中,该浸出液通过离子交换和溶剂萃取的方法可回收其中的钒、钪和钇,采用聚合的方法回收铝、铁以制备聚合氯化铝铁。
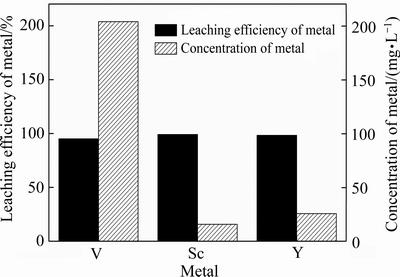
图3 赤泥中钒、钪、钇的浸出率
Fig. 3 Leaching efficiency and concentration of Sc, V and Y
2.2 活化焙烧-水浸参数影响
赤泥盐酸浸出渣的主要化学成分见表2。
表2 赤泥酸浸渣主要化学成分
Table 2 Main chemical composition of acid leaching residue (mass fraction, %)
由表2可知,赤泥经盐酸浸出后,实现了钪、钒、钇与钛的选择性浸出分离,酸浸渣中主要含有钛和硅的氧化物。为了进一步提高二氧化钛的品位,对浸出渣进行了活化焙烧和水浸作业。考察焙烧温度、碱渣比、水浸温度、液固比、水浸时间对酸浸渣中硅浸出率的影响,其结果如图4所示。
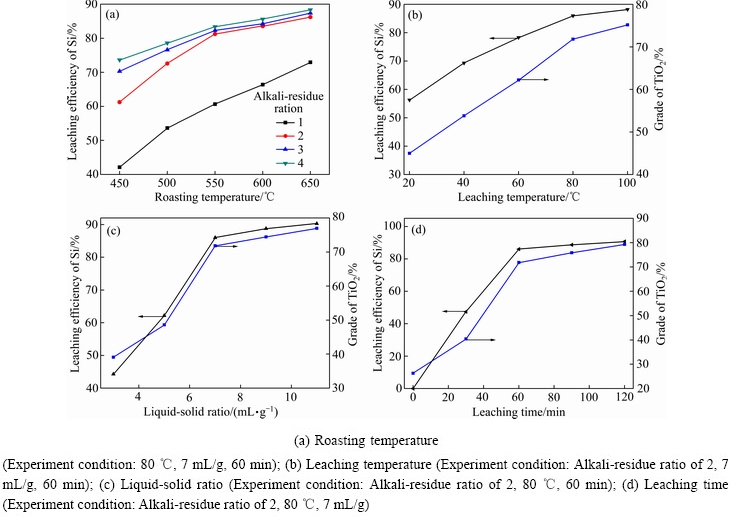
图4 活化焙烧-水浸参数对硅浸出率的影响
Fig. 4 Effects of active roasting and water leaching parameters on leaching efficiency of silicon
由图4(a)可知,焙烧温度和碱渣比对硅浸出率影响明显。当碱渣比为2时,焙烧温度从450 ℃升高至650 ℃,硅浸出率从61.3%提高至86.2%。在550 ℃条件下,碱渣比从1提高至4时,硅浸出率从60.7%提高至83.4%。为了充分生成可溶性硅酸钠并降低药剂能耗,选择合适的焙烧温度和碱渣比分别为550 ℃和2,后续分析样品的焙烧温度均为550 ℃。由图4(b)可知,随着水浸温度的提高,硅浸出率和二氧化钛品位均呈增长趋势。水浸温度从20 ℃提高至80 ℃,硅浸出率从 56.3% 提高至85.76%,而富钛料中二氧化钛品位可从44.9%提高至71.8%;继续提高水浸温度,硅浸出率和二氧化钛品位增长不显著。由于硅酸钠易溶于水,而二氧化钛和钛酸钠不溶于水,导致水浸过程硅与钛的分离。由图4(c)可知,液固比由3 mL/g增加至7 mL/g时,硅浸出率由 44.3%提高至85.8%,二氧化钛品位由39.1%提高至71.8%;继续提高液固比,硅浸出率和二氧化钛品位增长不明显。液固比较小时,硅酸钠水溶液呈乳状并黏滞,导致固体中硅酸钠溶解不充分;增加液固比,硅酸钠水溶液黏度变小,溶解更充分。由图4(d)可知,随着水浸时间的延长,硅浸出率和二氧化钛的品位呈现增长趋势。在小于60 min的条件下,增长趋势更为明显,当高于60 min后,增长趋势趋于平缓。赤泥经过盐酸浸出、活化焙烧、水浸作业后得到富钛料,其主要化学成分见表3。
表3 富钛料的主要化学成分
Table 3 Main chemical composition of titanium-rich materials (mass fraction, %)

3 机理分析
3.1 浸出渣物相分析
赤泥在盐酸浓度为5 mol/L、浸出温度为80 ℃、液固比为6 mL/g和酸浸时间为90 min条件下搅拌浸出,得到酸浸渣的XRD谱如图5所示。
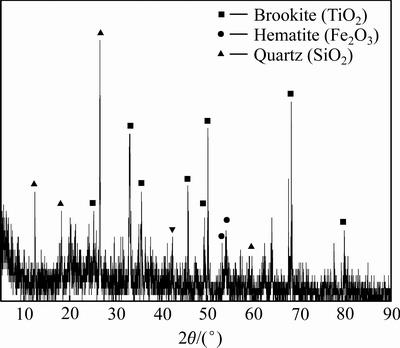
图5 赤泥酸浸渣XRD谱
Fig. 5 XRD pattern of acid leaching residue from red mud
由图5可知,赤泥中的钙霞石和方解石衍射峰消失,赤铁矿衍射峰减弱,石英和板钛矿衍射峰几乎没有改变。盐酸浸出赤泥过程中的化学反应和吉布斯自由能变如下所示。
Al2O3(s)+6H+(q)→2Al3+(q)+3H2O(q),
ΔG = -112.63 kJ/mol (2)
Fe2O3(s)+6H+(q)→2Fe3+(q)+3H2O(q),
ΔG = -4.13662 kJ/mol (3)
TiO2(s)+2H+(q)→TiO2+(q)+H2O(q),
ΔG = 73.87 kJ/mol (4)
CaO(s)+2H+(q)→Ca2+(q)+H2O(q),
ΔG = -185.93 kJ/mol (5)
Na2O(s)+2H+(q)→2Na+(q)+H2O(q),
ΔG = -383.331 kJ/mol (6)
由此可知,赤泥中钙、钠、铝氧化物的酸浸反应更容易进行,而氧化铁的酸浸过程能够发生,二氧化钛的酸浸溶解较难发生。该赤泥酸浸渣在焙烧温度为550 ℃、碱渣比为2、水浸温度为80 ℃、液固比为7 mL/g和水浸时间为60 min的条件下,得到富钛料的XRD谱如图6所示。
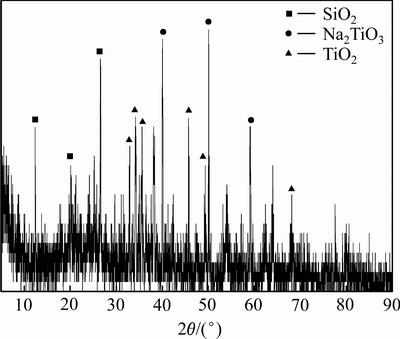
图6 富钛料XRD谱
Fig. 6 XRD pattern of titanium-rich materials
由图6可知,采用氢氧化钠活化焙烧,相应的钠盐会形成并存在于焙烧熟料中,经过水浸作业后,硅酸钠等水溶性的钠盐溶解于水溶液中,而非水溶性的TiO2和Na2TiO3存在于富钛料中,同时,富钛料中还含有一定量未反应的二氧化硅。
3.2 溶液热力学研究
φ-pH图能够判断离子的平衡状态和反应的可行性,选择赤泥中4种主要成分铁、铝、硅和钛进行溶液热力学分析。依据HSC分析软件,查阅Ti-H2O、Fe-H2O、Al-H2O和Si-H2O体系中存在物种的相关热力学数据,绘制标准状态下4种元素的φ-pH曲线,其结果如图7所示。
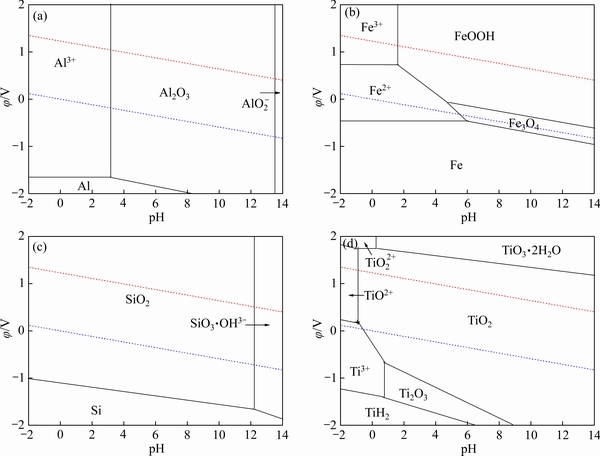
图7 铁、铝、硅和钛的φ-pH曲线
Fig. 7 φ-pH diagrams of Al(a), Fe(b), Si(c) and Ti(d)
由图7(a)可知,Al-H2O体系中,Al3+能够稳定存在于pH值小于3的酸性溶液中,强碱性条件下,铝会以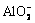 形式存在。由图7(b)可知,在Fe-H2O体系中,Fe2+和Fe3+能够稳定存在于酸性条件下,pH值高于1.9时,Fe(III)会形成沉淀物。由图7(c)可知,在Si-H2O体系中,当溶液pH<12时,SiO2能够稳定存在,在强碱性条件下,SiO3·OH3-能够稳定存在。由图7(d)可知,在Ti-H2O体系中,仅当强酸性条件下(pH<0时),二氧化钛才会以TiO2+的形式存在,随着pH值的升高,含钛沉淀物会形成。因此,赤泥在盐酸浸出、活化焙烧和水浸工艺过程中,Fe3+、Al3+和
形式存在。由图7(b)可知,在Fe-H2O体系中,Fe2+和Fe3+能够稳定存在于酸性条件下,pH值高于1.9时,Fe(III)会形成沉淀物。由图7(c)可知,在Si-H2O体系中,当溶液pH<12时,SiO2能够稳定存在,在强碱性条件下,SiO3·OH3-能够稳定存在。由图7(d)可知,在Ti-H2O体系中,仅当强酸性条件下(pH<0时),二氧化钛才会以TiO2+的形式存在,随着pH值的升高,含钛沉淀物会形成。因此,赤泥在盐酸浸出、活化焙烧和水浸工艺过程中,Fe3+、Al3+和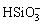 能够与TiO2 稳定共存于同一区间内,从而实现赤泥中铝、铁、硅与钛的分离。
能够与TiO2 稳定共存于同一区间内,从而实现赤泥中铝、铁、硅与钛的分离。
3.3 浸出动力学研究
赤泥浸出过程均属于典型固液两相化学反应,可由未反应核收缩模型 (SCM) 阐述浸出动力学。当浸出过程受内扩散步骤控制时,可以由以下方程表示:
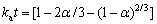 (7)
(7)
当浸出过程受化学反应步骤控制时,可由以下方程表示:
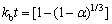 (8)
(8)
式中:t为浸出时间(min);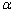 为时间t时金属浸出率(%);ka为内扩散控制速率常数;kb为化学反应控制速率常数。
为时间t时金属浸出率(%);ka为内扩散控制速率常数;kb为化学反应控制速率常数。
由于酸浸过程中铝和铁的浸出规律相似,而钒、钪和钇的浸出规律相似,因此选取铁和钒进行浸出动力学理论分析。不同盐酸浓度条件下,铁浸出的两种关键控制步骤线性拟合见图8。
通过对比图8(a)和(b),在高浓度盐酸(5 mol/L和6 mol/L)条件下,内扩散模型控制线性拟合好,低浓度盐酸(3 mol/L和4 mol/L)条件下,化学反应控制模型的线性拟合好。当浸出过程受内扩散控制时,盐酸向颗粒内部的扩散速率是关键控制步骤;当浸出过程受化学反应控制时,盐酸与赤泥中氧化铁的化学反应速率时关键控制步骤。不同温度条件下,铁浸出的内扩散控制步骤线性拟合和ln ka与温度的线性关系曲线见图9。
由图9 (a)可知,赤泥中铁浸出过程,在不同温度条件下受内扩散关键步骤控制,线性相关系数 (R2)大于0.98。在20 ℃、40 ℃、60 ℃和80 ℃条件下,扩散速率常数分别为0.0003、0.0006、0.0010 和0.0016。由图9(b)可知,ln ka与温度的关系曲线中线性相关系数大于0.99,经计算得到铁浸出的表观活化能(Ea)为23.86 kJ/mol,这与之前学者研究赤泥盐酸浸出过程中铁动力学表观活化能(22.35 kJ/mol)相吻合[29]。
在不同盐酸浓度条件下,对钒的浸出过程进行两种关键控制步骤的线性拟合,结果见图10。
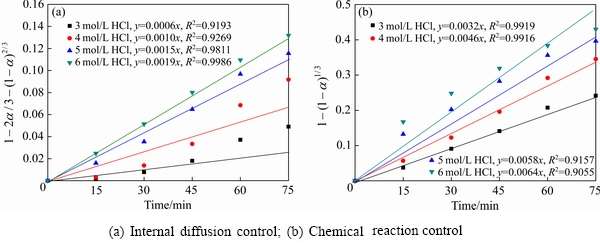
图8 不同盐酸浓度条件下铁的浸出动力学
Fig. 8 Leaching kinetic of iron at different hydrochloric acid concentraction
图9 不同温度条件下铁的浸出动力学
Fig. 9 Leaching kinetic of iron at different leaching temperature
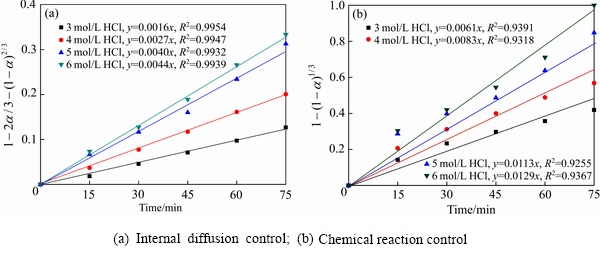
图10 不同盐酸浓度条件下钒的浸出动力学
Fig. 10 Leaching kinetic of vanadium at different hydrochloric acid concentraction
通过对比图10(a)和(b)可知,不同浓度盐酸条件下,钒浸出过程主要受内扩散关键步骤控制,线性相关系数大于0.98。不同温度条件下,钒浸出的内扩散控制步骤线性拟合和ln ka与温度的线性关系曲线见图11。
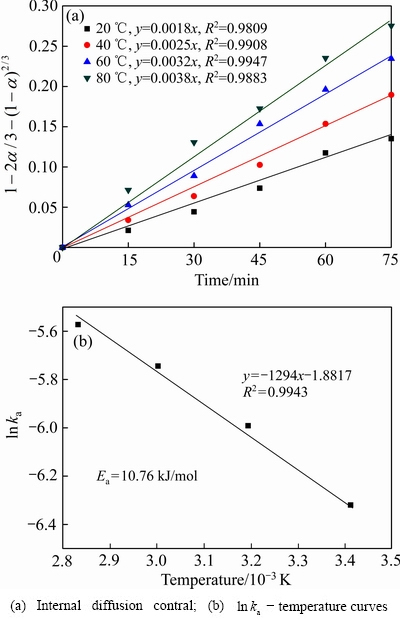
图11 不同温度条件下钒的浸出动力学
Fig. 11 Leaching kinetic of vanadium at different leaching temperature
由图11(a)可知,不同浸出温度条件下,钒的浸出过程主要受内扩散关键步骤控制,线性相关系数大于0.98。在20 ℃、40 ℃、60 ℃和80 ℃条件下,扩散速率常数分别为0.0018、0.0025、0.0032和0.0038。由图11(b)可知,钒浸出的ln ka与温度的线性相关系数(R2)大于 0.99,经计算钒浸出过程的表观活化能(Ea)为10.76 kJ/mol。根据浸出动力学理论,表观活化能越低,反应速率越快,反应也越容易进行[29]。因此,在赤泥酸浸过程中钒的浸出反应比铁的浸出反应更容易进行。
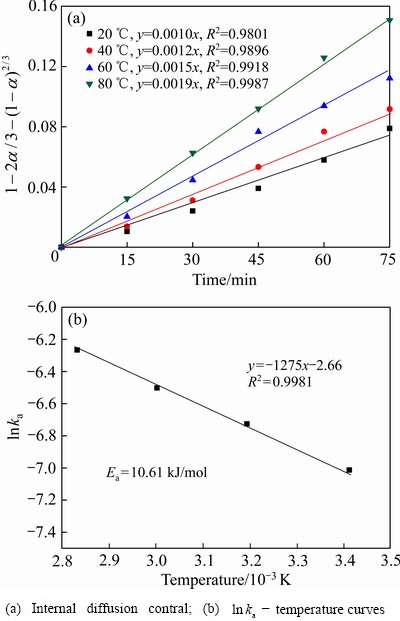
图12 不同温度条件下硅的浸出动力学
Fig. 12 Leaching kinetic of silicon at different leaching temperature
在不同温度条件下,硅水浸的动力学研究结果见图12。由图12(a)可知,硅的水浸过程也主要受内扩散步骤控制,且线性相关系数(R2)大于 0.98。在20 ℃、40 ℃、60 ℃和80 ℃条件下,扩散速率常数分别为0.0010、0.0012、0.0015和0.0019。由图12(b)可知,硅浸出的ln ka与温度的线性相关系数(R2)大于 0.99,经计算硅浸出过程的表观活化能(Ea)为10.61 kJ/mol。
4 结论
1) 在盐酸浓度为5 mol/L,液固比为6 mL/g,浸出温度为80 ℃和浸出时间为90 min的条件下,赤泥中铝、铁、钠、钙和钛的浸出率分别为81.2%、76.3%、99.2%、99.3%和3.2%,钒、钪和钇的浸出率均超过95%。酸浸渣在焙烧温度为550 ℃、碱渣比为2、焙烧时间为40 min、水浸温度为80 ℃、液固比为7 mL/g、浸出时间为60 min的条件下,硅浸出率为85.7%,富钛料的TiO2品位为71.8%。
2) 酸浸过程中含钠、钙、铝和稀有金属的钙霞石矿物衍射峰消失,赤铁矿衍射峰减弱,而板钛矿和石英衍射峰更加明显。
3) 通过活化焙烧-水浸作业后,富钛料中主要包含钛酸钠和少量未反应的石英。Fe3+和Al3+能够稳定存在于弱酸性条件,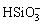 能够稳定存在于强碱性条件,TiO2+仅能稳定存在于pH值小于0的强酸性溶液中,赤泥在活化焙烧-联合浸出过程,其中Fe3+、Al3+和
能够稳定存在于强碱性条件,TiO2+仅能稳定存在于pH值小于0的强酸性溶液中,赤泥在活化焙烧-联合浸出过程,其中Fe3+、Al3+和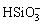 能够与TiO2稳定共存于同一区间内,可以实现铝、铁、硅与钛的分离。
能够与TiO2稳定共存于同一区间内,可以实现铝、铁、硅与钛的分离。
4) 赤泥中铁的酸浸过程严重依赖于盐酸浓度,低浓度盐酸条件下受化学反应控制,高浓度盐酸条件下受内扩散控制,而钒和硅的浸出过程均受内扩散控制。铁、钒、硅的浸出表观活化能分别为23.86 kJ/mol、10.76 kJ/mol和10.61 kJ/mol。
REFERENCES
[1] 朱 锋, 韩附送, 薛生国, 等. 氧化铝赤泥堆场团聚体的分形特征[J]. 中国有色金属学报, 2016, 26(16): 1316-1323.
ZHU Feng, HAN Fu-song, XUE Sheng-guo, et al. Fractal characteristics of bauxite residue aggregates in red mud yard[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(16): 1316-1323.
[2] 李 彬, 张宝华, 宁 平, 等. 赤泥资源化利用和安全处理现状与展望[J]. 化工进展, 2018, 37(2): 714-723.
LI Bin, ZHANG Bao-hua, NING Ping, et al. Present status and prospect of red mud resource utilization and safety treatment[J]. Chemical Industry and Engineering Process, 2018, 37(2): 714-723.
[3] KLAUBER C, GR FE M, POWER G. Bauxite slag issues: Ⅱ. Options for slag utilization[J]. Hydrometallurgy, 2011, 108: 11-32.
FE M, POWER G. Bauxite slag issues: Ⅱ. Options for slag utilization[J]. Hydrometallurgy, 2011, 108: 11-32.
[4] KONG X F, JIANG X X, XUE S G, et al. Migration and distribution of saline ions in bauxite residue during water leaching[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(3): 534-541.
[5] LI X F, YE Y Z, XUE S G, et al. Leaching optimization and dissolution behavior of alkaline anions in bauxite residue [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(6): 1248-1255.
[6] LI Y W, JIANG J, XUE S G, et al. Effect of ammonium chloride on leaching behavior of alkaline anion and sodium ion in bauxite residue[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(10): 1248-1255.
[7] 刘晓明, 唐彬文, 尹海峰, 等. 赤泥-煤矸石基公路路面基层材料的耐久与环境性能[J]. 工程科学学报, 2018, 40(4): 438-445.
LIU Xiao-ming, TANG Bin-wen, YIN Hai-feng, et al. Durability and environmental performance of Bayer red mud-coal gangue-based road base material[J]. Chinese Journal of Engineering, 2018, 40(4): 438-445.
[8] 仇雅丽, 李长明, 王德亮, 等. 赤泥/煤基铁炭材料的制备及其脱除废水Cr(Ⅵ)的性能[J]. 化工学报, 2018, 69(7): 3216-3225.
QIU Ya-li, LI Chang-ming, WANG De-liang, et al. Preparation of red mud/coal based material and its performance to remove Cr(Ⅵ) in waste water[J]. Journal of Chemical Industry and Engineering, 2018, 69(7): 3216-3225.
[9] KIMA S C, NAHMA S W, PARK Y K. Property and performance of red mud-based catalysts for the complete oxidation of volatile organic compounds[J]. Journal of Hazardous Materials, 2015, 300: 104-113
[10] INDRANI G, SAUMYEN G, BALASUBRAMANIAM R, et al. Leaching of metals from fresh and sintered red mud[J]. Journal of Hazardous Materials, 2011, 185: 662-668.
[11] 王 璐, 郝彦忠, 郝增发. 赤泥中有价金属提取与综合利用进展[J]. 中国有色金属学报, 2018, 28(8): 1697-1710.
WANG Lu, HAO Yan-zhong, HAO Zeng-fa. Process in valuable metal element recovery and utilization of red mud—A review[J]. The Chinese Journal of Nonferrous Metals, 2016, 2018, 28(8): 1697-1710.
[12] 柳 晓, 高 鹏, 韩跃新, 等. 山东某高铁赤泥工艺矿物学研究[J]. 东北大学学报(自然科学版), 2019, 40(11): 1611-1616.
LIU Xiao, GAO Peng, HAN Yue-xin, et al. Study on process mineralogy of a high-iron red mud from Shandong province[J]. Journal of Northeastern University (Natural Science), 2019, 40(11): 1611-1616.
[13] POWER G, GR FE M, KLAUBER C. Bauxite slag issues: Ⅰ. Current management, disposal and storage practices[J]. Hydrometallurgy, 2011, 108: 33-45.
FE M, KLAUBER C. Bauxite slag issues: Ⅰ. Current management, disposal and storage practices[J]. Hydrometallurgy, 2011, 108: 33-45.
[14] CENGLOGLU Y, KIR E, ERSON M. Recovery and concentration of Al(Ⅲ), Fe(Ⅲ), Ti(Ⅳ), Na(Ⅰ) from red mud[J]. Journal of Colloid and Interface Science, 2001, 244: 342-346.
[15] LI X B, ZHOU Z Y, WANG Y L, et al. Enrichment and separation of iron minerals in gibbsitic bauxite residue based on reductive Bayer[J]. Transactions of Nonferrous Metals Society of China, 2020, 30(7): 1980-1990.
[16] LI G H, LIU M X, RAO M J, et al. Stepwise extraction of valuable components from red mud based on reductive roasting with sodium salts[J]. Journal of Hazardous Materials, 2014, 280: 774-780.
[17] HUANG Y F, CHAI W C, HAN G H, et al. A perspective of stepwise utilisation of Bayer red mud: Steptwo-Extracting and recovering Ti from Ti-enriched tailing with acid leaching and precipitate flotation[J]. Journal of Hazardous Materials, 2016, 307: 318-327.
[18] RUBISOV D H, KROWINKEL J M, PAPANGELAKIS V G. Sulphuric acid. pressure leaching of laterites universal kinetics of nickel dissolution for limonites and limonitic/saprolitic blends[J]. Hydrometallurgy, 2000, 58: 1-11.
[19] SMIRNOV D I, MOLCHANOVA T V. The investigation of sulfuric acid sorption recovery of scandium and uranium from the red mud of alumina production[J]. Hydrometallurgy, 1997, 45: 249-259.
[20] BORRA C R, PONTIKES Y, BINNEMANS K, et al. Leaching of rare earths from bauxite slag (red mud)[J]. Minerals Engineering, 2015, 76: 20-27.
[21] 肖军辉, 梁冠杰, 黄雯孝, 等. 含钪赤泥氯化钠离析焙烧-弱磁选-盐酸浸出分离铁、钪试验研究[J]. 工程科学与技术, 2019, 51(4): 199-209.
XIAO Jun-hui, LIANG Guan-jie, HUANG Wen-xiao, et al. Research on separation iron and scandium-contained red mud using sodium chloride segregation roasting-low intensity magnetic separation-hydrochloric acid leaching[J]. Advanced Engineering Science, 2019, 51(4): 199-209.
[22] 周秋生, 范旷生, 李小斌, 等. 采用烧结法处理高铁赤泥回收氧化铝[J]. 中南大学学报(自然科学版), 2008, 39(1): 92-97.
ZHOU Zhi-hong, FAN Kuang-sheng, LI Xiao-bin, et al. Alumina recovery from red mud with high iron by sintering process[J]. Journal of Central South University (Science and Technology), 2008, 39(1): 92-97.
[23] 宁凌峰, 何德文, 陈 伟, 等. 赤泥中硫酸选择性浸出铁、钪及动力学研究[J]. 矿冶工程, 2019, 39(3): 81-84.
NING Li-feng, HE De-wen, CHEN Wei, et al. Sulfuric acid leaching and kinetics study for separation of iron and scandium from red mud[J]. Mining and Metallurgical Engineering, 2019, 39(3): 81-84.
[24] ZHU X B, LI W, GUAN X M. Kinetics of titanium leaching with citric acid in sulfuric acid from red mud[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(9): 3139-3145.
[25] WANG W W, PRANOLO Y, CHENG C Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA[J]. Separation and Purification Technology, 2013, 108: 96-102.
[26] ZHU X B, LI W, GUAN X M. An active dealkalization of red mud with roasting and water leaching[J]. Journal of Hazardous Materials, 2015, 286: 85-91.
[27] ZHU X B, LI W, TANG S. Selective recovery of vanadium and scandium by ion exchange with D201 and solvent extraction using P507 from hydrochloric acid leaching solution of red mud[J]. Chemosphere, 2017, 175: 365-372.
[28] 赵 恒, 李 望, 牛泽鹏, 等. 赤泥制备聚合氯化铝铁及其吸附性能研究[J]. 稀有金属与硬质合金, 2019, 47(5): 64-68.
ZHAO Heng, LI Wang, NIU Ze-peng, et al. Preparation and adsorption properties of polyaluminum ferric chloride from red mud[J]. Rare Metals and Cemented Carbides, 2019, 47(5): 64-68.
[29] 薛 真, 薛彦辉, 王 力. 拜耳法赤泥中铝铁的盐酸浸出过程研究[J]. 矿产综合利用, 2018, 39(6): 139-143.
XUE Zhen, XUE Yan-hui, WANG Li. Study on leaching of aluminum and iron from Bayer red mud with hydrochloric acid[J]. Multipurpose Utilization of Mineral Resources, 2018, 39(6): 139-143.
Preparation of titanium-rich materials and dissolution behavior of components from red mud by active roasting and combined leaching
ZHU Xiao-bo, GONG Wen-hui, LI Wang
(School of Chemistry and Chemical Engineering, Henan Polytechnic University, Jiaozuo Henan, 454000, China)
Abstract: Titanium-rich materials were prepared and valuable components were separated from red mud by active roasting and combined leaching. The effects of parameters on the leaching efficiency of aluminum, iron, sodium, calcium and titanium were investigated. The dissolution behavior of components in the process of active roasting and combined leaching was theoretically analyzed. The results show that the leaching efficiencies of aluminum, iron, sodium, calcium and titanium are 81.2%, 76.3%, 99.2%, 99.3%, respectively, under the conditions of HCl concentration of 5 mol/L, liquid-solid ration of 6 mL/g, leaching temperature of 80 ℃ and leaching time of 90 min, respectively. The leaching efficiencies of vanadium, scandium and yttrium are more than 95%. The leaching efficiency of silicon reaches 85.7% and the titanium-rich material with TiO2 grade of 71.8% is obtained by roasting with alkali slag ratio of 2 at 550 ℃ for 40 min and liquid-solid ratio of 7 mL/g at 80 ℃ for 60 min. The sodium titanate and some unreacted quartz exist in the titanium-rich material. TiO2 exists stably in the solution system of Fe3+, Al3+ and 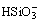 . The leaching processes of vanadium and silicon are controlled by internal diffusion. The apparent activation energies of vanadium, silicon and iron are 10.76 kJ/mol, 10.61 kJ/mol and 23.86 kJ/mol, respectively.
. The leaching processes of vanadium and silicon are controlled by internal diffusion. The apparent activation energies of vanadium, silicon and iron are 10.76 kJ/mol, 10.61 kJ/mol and 23.86 kJ/mol, respectively.
Key words: red mud; titanium-rich material; active roasting; combined leaching; valuable component
Foundation item: Projects(51804103, 51904097) supported by the National Natural Science Foundation of China; Project(2019GGJS056) supported by the Training Program for Young Backbone Teachers in Colleges and Universities of Henan Province, China; Project(202102310548) supported by the Scientific and Technological Project of Henan Province, China; Project(HB201905) supported by the Open Foundation of State Environmental Protection Key Laboratory of Mineral Metallurgical Resources Utilization and Pollution Control, China; Project(21IRTSTHN006) supported by the Program for Innovative Research Team in the University of Henan Province, China
Received date: 2020-08-12; Accepted date: 2020-11-26
Corresponding author: LI Wang; Tel: +86-18639104027; E-mail: liwang0805@126.com
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(51804103,51904097);河南省高等学校青年骨干教师培养计划项目(2019GGJS056);河南省科技攻关项目(202102310548);国家环境保护矿冶资源利用与污染控制重点实验室开放基金课题(HB201905);河南省高校科技创新团队(21IRTSTHN006)
收稿日期:2020-08-12;修订日期:2020-11-26
通信作者:李 望,副教授,博士;电话:18639104027;E-mail:liwang0805@126.com
摘 要:赤泥活化焙烧-联合浸出制备富钛料并分离有价组分,考察参数对各金属浸出率的影响,并对活化焙烧-联合浸出过程中组分溶解行为进行了理论分析。结果表明:在盐酸浓度为5 mol/L、液固比为6 mL/g、浸出温度为80 ℃和浸出时间为90 min的条件下,铝、铁、钠、钙和钛的浸出率分别为81.2%、76.3%、99.2%、99.3%和3.2%,同时稀有金属钒、钪和钇的浸出率均超过95%。在焙烧温度为550 ℃、碱渣比为2、焙烧时间为40 min、水浸温度为80 ℃、液固比为7 mL/g、浸出时间为60 min的条件下,硅浸出率可达85.7%,可获得TiO2品位为71.8%的富钛料。钛酸钠和少量未反应的石英存在于富钛料中,TiO2能够稳定存在于Fe3+、Al3+和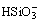 的溶液体系中。钒和硅的浸出过程均受内扩散控制,浸出表观活化能分别为10.76 kJ/mol和10.61 kJ/mol,而铁的酸浸表观活化能达到23.86 kJ/mol。
的溶液体系中。钒和硅的浸出过程均受内扩散控制,浸出表观活化能分别为10.76 kJ/mol和10.61 kJ/mol,而铁的酸浸表观活化能达到23.86 kJ/mol。
[1] 朱 锋, 韩附送, 薛生国, 等. 氧化铝赤泥堆场团聚体的分形特征[J]. 中国有色金属学报, 2016, 26(16): 1316-1323.
[2] 李 彬, 张宝华, 宁 平, 等. 赤泥资源化利用和安全处理现状与展望[J]. 化工进展, 2018, 37(2): 714-723.
[7] 刘晓明, 唐彬文, 尹海峰, 等. 赤泥-煤矸石基公路路面基层材料的耐久与环境性能[J]. 工程科学学报, 2018, 40(4): 438-445.
[8] 仇雅丽, 李长明, 王德亮, 等. 赤泥/煤基铁炭材料的制备及其脱除废水Cr(Ⅵ)的性能[J]. 化工学报, 2018, 69(7): 3216-3225.
[11] 王 璐, 郝彦忠, 郝增发. 赤泥中有价金属提取与综合利用进展[J]. 中国有色金属学报, 2018, 28(8): 1697-1710.
[12] 柳 晓, 高 鹏, 韩跃新, 等. 山东某高铁赤泥工艺矿物学研究[J]. 东北大学学报(自然科学版), 2019, 40(11): 1611-1616.
[21] 肖军辉, 梁冠杰, 黄雯孝, 等. 含钪赤泥氯化钠离析焙烧-弱磁选-盐酸浸出分离铁、钪试验研究[J]. 工程科学与技术, 2019, 51(4): 199-209.
[22] 周秋生, 范旷生, 李小斌, 等. 采用烧结法处理高铁赤泥回收氧化铝[J]. 中南大学学报(自然科学版), 2008, 39(1): 92-97.
[23] 宁凌峰, 何德文, 陈 伟, 等. 赤泥中硫酸选择性浸出铁、钪及动力学研究[J]. 矿冶工程, 2019, 39(3): 81-84.
[28] 赵 恒, 李 望, 牛泽鹏, 等. 赤泥制备聚合氯化铝铁及其吸附性能研究[J]. 稀有金属与硬质合金, 2019, 47(5): 64-68.
[29] 薛 真, 薛彦辉, 王 力. 拜耳法赤泥中铝铁的盐酸浸出过程研究[J]. 矿产综合利用, 2018, 39(6): 139-143.


