
Electrochemical behavior and morphology of LiB compound anode materials
LIU Zhi-jian(刘志坚)1, YIN Jian(尹 健)1, MENG Zhen-qiang(孟振强)1,
CHONG Jin(种 晋)1, 2, ZHOU De-bi(周德壁)3
1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
3. Tianjin Institute of Power Sources, Tianjin 300381, China
Received 3 March 2005; accepted 18 December 2005
Abstract:
Electrochemical behavior of LiB compound in LiPF6-DMC/EMC/EC was studied. And morphology of the compound was investigated by SEM. The results show that there are three discharge potential plateaus for the LiB compound, corresponding to 0.46, 0.69 and 0.8 V (vs Li+/Li) respectively, its total discharge specific capacity is up to 660 mA·h/g, only first two potential plateau can be charged, its specific capacity is 274 mA·h/g. After discharging, the morphology of the Li compound is still fibrous shape.
Key words:
LiB compound; electrochemical behavior; morphology; discharge; anode;
1 Introduction
A LiB compound with chemical composition ratio 1∶1 was discovered at 1970s, when it was used as refractory frame in anode materials, Li-30%B alloy, used for thermal battery [1-3]. Structure of LiB compound is hexagonal, with space group No.194 P63/mmc[4, 5]. In the crystal of LiB compound, after acquiring electrons from Li atoms, the boron atoms form a covalent values bond chain along 001 direction. The compound has a fibrous shape growing along 001 direction[4, 5]. The Li-B alloy used for thermal battery is composed of LiB compound and pure lithium[3, 6, 7]. Discharging curves of the alloy in both high temperature molten salt electrolyte[8] and room temperature nonaqueous electrolyte[9] show that the compound can discharge at the potential near that of metal lithium. Recently, many experiments discovered that when the compound is in Li deficient state, its lattice constants will also change step by step[4, 6, 10], that is similar to the phenomenon happening in carbon negative materials of lithium ion battery. Then the LiB compound is similar to graphite carbon negative materials in many ways. In this paper, the aim is to investigate its electrochemical characteristics and explore the possibility of using it as anode materials of lithium ion battery.
2 Experimental
2.1 Preparation of LiB compound
Metal lithium ingot, purity 99.99%, and crystalline boron, purity 99% were used, as raw materials, in which the boron piece was broken into powder passing 0.15 mm screen. The preliminary Li-B alloy was pre- pared directly through thermal reaction synthesis [1, 11] in about 1 g a batch, according to the composition Li- 40%B under argon atmosphere. The pure lithium phase in the alloy is eliminated in the discharging course.
2.2 Charge and discharge experiments
At first, the Li-B alloy was rolled into foil with thickness 0.15 mm, then about 1 cm2 of Li-B alloy round flake was cut. An apparatus was designed to assemble the test battery (Fig.1), in which the Li-B alloy was the negative electrode, the lithium the both positive and reference electrode, the celgard 2400 the separate layer. The electrolyte was the solution of 1 mol/L LiPF6 in DMC∶EMC∶EC=1∶1∶1(mass ratio).
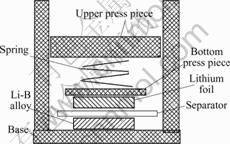
Fig.1 Illustration of cell discharge
2.3 Morphology
The discharged LiB compound was washed in tetrahydrofuran(THF) and then broken into powders. Its morphology was observed by scanning electron microscope(SEM) JEOL JSM-6360LV. The operation in air was as short as possible, to avoid the affection of humid air.
3 Results and discussion
3.1 Discharge
Fig.2 shows that there are two parts for the discharging curve of Li-B alloy. The potential of the first part is just a little higher than 0 V(vs Li+/Li), which should correspond to the discharging of metal lithium. The second part includes several stages of potential, which should correspond to the discharge of the LiB compound. Deducting the mass of metal lithium according to calculation of the first part of discharging curve, the mass ratio of the left lithium to boron is 0.758∶1, in other words, it is Li7.08B6, which is near the Li7B6[12]. There are several view points about the composition ratio of LiB compound. One is the Li7B6 by differential thermal analysis(DTA) and differential scanning calorimetry(DSC), the other is LiB[5] or LiB0.88[4] through calculation of XRD patterns. Here, a composition ratio from electro- chemical experiment was put forward.
Fig.2 shows that there are three discharging plateaus, 0.46, 0.69 and 0.80 V (vs Li+/Li), for the part of LiB compound discharge.
Calculation of the discharging curve shows that total discharging specific capacity of the LiB compound is up to 660 mA·h/g, which exceeds the theoretical specific capacity of the graphite carbon anode materials (LiC6) 372 mA·h/g[13]. The capacity ratio of the three stage discharging plateaus is Ⅰ∶Ⅱ∶Ⅲ=0.8∶0.2∶1, half of the total capacity comes from contribution of 0.8 V discharging plateau.
We ever prepared the lithium deficient LiB compound by selective dissolution and vacuum thermal evaporation. It is found that the decrease of Li content in LiB compound causes the lattice constant c to increase step by step[10]. In this paper extraction lithium by electrochemical method also makes the discharging curve produce several steps. What is their relationship, it needs detailed investigation.
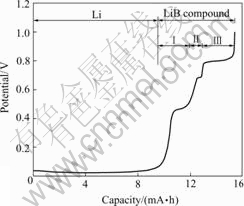
Fig.2 Discharge curve of Li-40%B alloy in LiPF6-DMC/EMC/ EC
The discharging experiment of Li-B alloy in LiClO4-PC or in LiCl/KCl molten salt had been made by different researchers[3, 8, 9]. Their results show that part of the lithium in the LiB compound can also take part in discharging. But no investigation on this phenomenon was presented in their reports. Knowing the crystal structure model of LiB compound, we had determined the density of the compound[14]. Now we can calculate the discharging specific capacity of LiB compound just according to the discharging curve of the Li-B alloy presented in the references. For the result of Li-B alloy discharging in LiClO4-PC by James[9](Fig.3), there is no discharging plateau 0.46 V. Calculation result shows the specific capacity of the compound is 666.7 mA·h/g, which is on the same level as presented in this paper. For the discharging curves of Li-B compound in LiCl/KCl molten salt by SZWARC and DALLEK [3], there is only one discharging plateau for LiB compound, with the potential 0.2 V higher than that of pure lithium. Calculation shows that the specific capacity of the LiB compound in the case is about 850 mA·h/g, which is much higher than that of the LiB compound at room temperature discharge. Then both discharging processes of LiB compound at room temperature and high temperature (i.e. in molten salt electrolyte) should have different mechanisms.
3.2 Discharge and charge cycle
Three discharging and charging experiments have
been completed for the three discharging plateaus mentioned in Fig.1, respectively. Fig.4 shows the first
cycle curves of the three discharging plateaus, in the volts range, (a) 0-0.60 V, (b) 0-0.75 V and (c) 0-1.0 V (vs Li+/Li) respectively. Fig.5 shows the second and the third cycle curves of them. Calculation shows that for curve (a) 0-0.6 V, the discharging capacity is 220 mA·h/g, the charging capacity 210 mA·h/g; for the curve (b) 0-0.75 V, the discharging capacity is 293 mA·h/g, the charging capacity is 274 mA·h/g; for the curve (c) 0-1.0 V, the discharging capacity is 660 mA·h/g, the charging capacity is only 274mA·h/g. The reversible percent capacity is 95.4% for curve (a), 93.5% for curve (b) and less than 50% for curve (c). These curves show that the 0.69 V discharging plateau has good reversibility, the 0.46 V discharging plateau has weak reversibility, but the 0.8 V discharging plateau has no reversibility. Because the cycle ability is related to not only the stability of the crystal structure of the compound , but also the manufacture of the electrode and matching of the electrolyte. Because the experiment here is not on the best and standard state, so the determined evaluation of the LiB compound about the cycle ability can not be made in this paper.
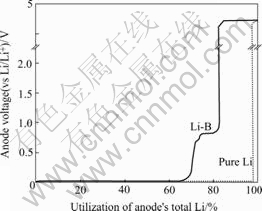
Fig.3 Discharge curve of Li-B alloy and Li in LiClO4-PC
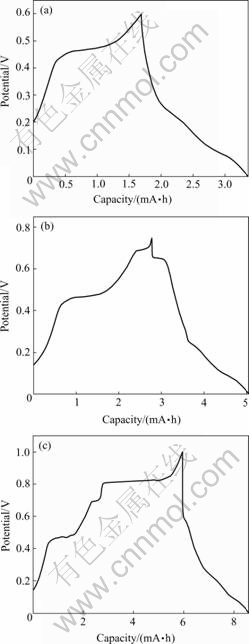
Fig.4 First cycle curves of discharge and charge for three potential ranges: (a) 0-0.60 V; (b) 0-0.75 V; (c) 0-1.0 V
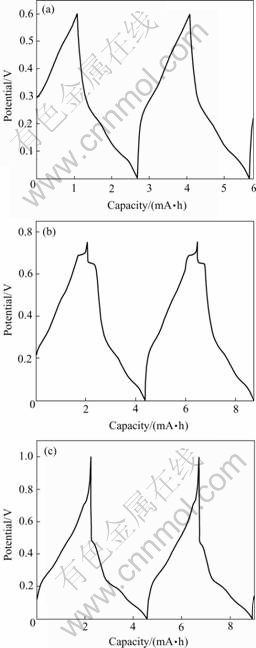
Fig.5 Second and third cycle curves of discharge and charge for three potentials: (a) 0-0.60 V; (b) 0-0.75 V; (c) 0-1.0 V
3.3 Morphology
Fig.6 shows the SEM photographs of the LiB compound before and after discharge. Fig.6(a) shows the picture of LiB compound just after the end of the metal lithium discharge in the Li-B alloy electrode. The compound is in the fibrous with the diameter about 1.5 μm, which is the same as that when metal lithium is taken off by other ways[4, 5, 10]. Figs.6(b)-(d) corresponding to the states just after discharge for stage I, Ⅱand Ⅲ respectively. In Figs.6(b) and (c), the Li fibers are bond together by something like glue. In Fig.6(d), the binding of the glue is weak, and the fiber is dispersing and shrinking. These microcosmic morphology characteristics also affect the macrocosmic properties of the materials. In preparing the SEM specimens for Figs.6, it is found that the specimens for Figs.6(a), (b) and (c) still have some strength, they still keep apparent electrode shape when they are taken from the test battery and a break process is needed to transform them into powders. The specimen for Fig.6(d) has no strength, it is loose when it is taken from the test battery and easy to disperse into powders when it is washed in the tetrahydrofuran(THF).
For the specimen of Fig.6(d), many properties are different from those of the specimens of Figs.6(b) and (c), the original factors should come from the lack of the something like glue and the shrink of the LiB compound. There are many ways which can compare for both graphite structure carbon anode used in Li ion battery and the LiB compound. In the Li ion battery, there is a SEI (solid electrolyte interface) film forming on the surface of the anode, which can prevent the co-intercalation of the solvent molecule into the crystal of the graphite with the Li ion[15]. For the experiment here, the electrochemical reactions also takes place at the potential below 0.8 V, the SEI film should also form on the surface of the LiB compound. Then the so called something like glue should be the SEI film. In the crystal of graphite, the interface space is 0.3369 nm[15]. For the LiB compound the space between the covalent boron chain is 0.4022 nm[5], the interface space of 100 face is 0.3483 nm, which is on the same level as that of the graphite. Then the LiB compound is also needed the protection of the SEI film in charge course. Therefore the lack of the SEI film in the specimen of Fig.6(d) is one of the reasons that make the charging capacity of the specimen of Fig.6(d) decrease greatly.
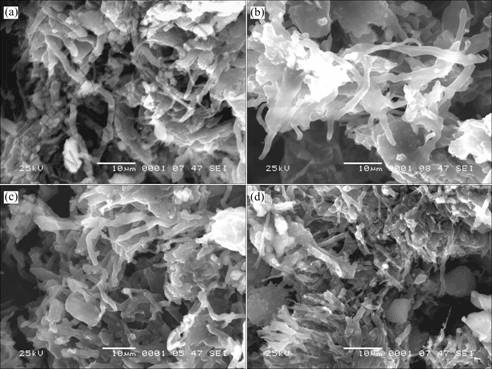
Fig.6 SEM photographs of LiB compound before and after discharge: (a) Before discharge; (b) After first stage (0.46 V); (c) After second stage(0.69 V); (d) After third stage(0.80 V)
Compared with LiC6, LiB compound has the discharging capacity up to 660 mA·h/g, which is much higher than that of the theoretical capacity of LiC6, 372 mA·h/g, but the charging capacity is only 274 mA·h/g. The disappear of the o.8 V discharging plateau in the charge and followed cycle test can not be explained on the moment just by the experiments here. More deep investigation is needed. Once the problem is solved, a new anode with large capacity used in Li ion battery will be presented. The discharging potential of the LiB compound is 0.46-0.8 V, which is higher than that of LiC6, 0-0.22 V[11]. higher potential can effectually prevent the metal lithium from precipitating on the anode. Therefore LiB would be a special anode materials of unique characters for Li ion battery.
4 Conclusions
1) There are three stages for discharge of LiB compound in LiPF6-DMC/EMC/EC electrolyte, their discharging potential are 0.46, 0.69 and 0.80 V (vs Li+/Li) respectively.
2) After discharge, the lithium deficient LiB compound is still fibrous, just after 0.8 V discharging the fibers are loose and shrinking.
3) The discharging specific capacity of the LiB compound is 660 mA·h/g, but the reversible capacity is only 274 mA·h/g in the potential region 0-0.75 V. The discharging plateau at 0.8 V has no charging ability.
References
[1] Wang F E. Metal Alloy and Method of Preparation Therof[P]. US, 1978. 4, 110, 111.
[2] Guidotti R A. Thermal Batteries: A Technology Review and Future Direction [R]. DE95014859.
[3] Szwarc r, dallek s. The Li(B) Ingot Preparation Scale-Up Study Final Report [R]. DE 82015798.
[4] Worle M, Nesper R. Infine, unbranched borynide chains in LiBx-isoelectronic to ployyne and ploycumulene[J]. Angew Chem Int Ed, 2000, 39(13): 2349.
[5] LIU Zhi-jian, QU Xuan-hui, HUANG Bai-yun, LI Zhi-you. Crystal structure and morphology of a new compound LiB[J]. Journal of Alloys and Compound, 2000, 311: 256.
[6] WANG F E. An unusual phenomenon in the formation of Li5B4 compound alloy[J]. Metal Trans A, 1979, 10A: 343.
[7] LIU Zhi-jian, LI Zhi-you, QU Xuan-hui, HUANG Bai-yun. Preparation, properties and application of the new type anode materials Li-B alloy for thermal battery[J]. Journal of Materials Science and Engineering, 2002, 20(2): 263-267.(in Chinese)
[8] Sanchez P, Belin C, Crepy G, De Guibert A J. Preparation and characterization of lithium-boron alloys: Electrochemical studies as anodes in molten salt media, and comparison with pure lithium involving systems[J]. Materials Science, 1992, 27: 240-246.
[9] JAMES S D. Comparison of anodic discharge ability of Li-B alloy with pure lithium in LiClO4-propylene carbonate[J]. J Appl Electrochem, 1982, 12: 317-321.
[10] LIU Z J, HUANG BY, LI Z Y. Preparation and XRD study of Lithium deficient LiB compound[J]. Acta Metallurgica Sinica(English Letters), 2004, 17(5): 667-671.
[11] LIU Zhi-jian, LI Zhi-you, DUAN Wei, QU Xuan-hui, HUANG Bai-yun, ZHANG Si-qi. Preparation of Li-B alloy and study of its microstructure and discharge characteristics[J]. J Mater Sci Technol, 2000, 16(6): 581-584.
[12] Dallek S, Ernst D W, LARICK B F. Thermal analysis of lithium-boron alloys[J]. J Electrochem Soc Solid-State Science and Technology, 1979, 126(5): 866-870.
[13] WU Yu-ping, WAN Rong-chun, JIANG Chang-yin. Li Ion Battery[M]. Beijing: Chemical Industry Press, 2002. (in Chinese)
[14] LIU Zhi-jian, QU Xuan-hui, LI Zhi-you, HUANG Bai-yun. Density of Li-B alloy used for anode materials of thermal battery[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(1): 99-102. (in Chinese)
[15] Yazami R. Carbon-lithium negative electrode for lithium ion batteries: main characteristics and features[A]. Proceedings of the NATO Advanced Study Institute on Materials for Lithium-Ion Batteries, Design and Optimization [C]. Netherlands: Kluwer Academic Publishers, 2000.
Foundation item: Project(50172062) supported by the National Natural Science Foundation of China
Corresponding author: LIU Zhi-jian; Tel: +86-731-8830464; E-mail: lzpm@mail.csu.edu.cn
(Edited by LI Xiang-qun)


