
Synthesis of ZnO whiskers via hydrothermal decomposition route
SHI Wen-tao(史文涛), GAO Guo(高 国), XIANG Lan(向 兰)
Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Received 6 July 2009; accepted 23 March 2010
Abstract:
ZnO whiskers with a length of 30-40 mm and a diameter of about 1 mm were synthesized by co-precipitation of ZnSO4 and Na2CO3 solution at room temperature followed by hydrothermal treatment of the as-prepared Zn5(CO3)2(OH)6 precursor at 160 ℃ for 6 h. The increase of the initial solution pH promotes the hydrothermal conversion of the particulate Zn5(CO3)2(OH)6 to ZnO whiskers. The presence of minor amount of EDTA in the hydrothermal solution promotes the one dimensional growth of ZnO whiskers, leading to the formation of ZnO whiskers with a length of 50-60 mm and a diameter of 1-2 mm.
Key words:
basic zinc carbonate; zinc oxide; whiskers; hydrothermal decomposition;
1 Introduction
In recent years, one-dimensional (1D) oxides have attracted considerable interest due to their distinctive geometry characteristics and novel chemical and physical properties. As one of the 1D oxide materials, 1D ZnO materials can be used in many fields such as ceramics, electrics, solar cells, reinforcing rubbers and zinc alloys owing to their unique optical, electric and piezoelectric properties[1-3]. Many techniques have been employed to synthesize 1D ZnO materials. Among them, the vapor phase transport process with the assistance of noble metal catalysts and thermal evaporation are the two major methods to fabricate 1D ZnO nanostructures[4-5]. The solution-based hydrothermal methods have also been used for the synthesis of 1D ZnO. Compared with the vapor phase methods, the hydrothermal method has some distinctive advantages such as moderate reaction condition and easy control of the solution composition[7-11]. It was reported that ZnO whiskers with a diameter of around 4 mm and a length of up to 20 mm were synthesized at 110℃ for 12 h using zinc acetate dehydrate and ammonia as the raw materials[12]. The multi-pod-like ZnO whiskers with a length of 2-4 mm and a diameter of ~150 nm were formed using Zn(NO3)2 and ammonia as the raw materials[13]. Some surfactants have been adopted to obtain the 1D ZnO with high aspect ratio involving polyvinyl alcohol, polyethylene glycol, cetyltrimethyl ammonium bromide (CTAB)[14-15] and so on. In addition, the presence of some organic solvents, such as the n-hexane, n-decane and benzene [16], is favorable for the 1D growth of ZnO. Up to now, little work has been reported on the formation of 1D ZnO in ZnSO4-Na2CO3 system.
In the present work, a facile hydrothermal decomposition method was developed to synthesize ZnO whiskers in alkaline media, using ZnSO4 and Na2CO3 as the reactants. The influence of the reaction time and the solution pH on the morphology and composition of the hydrothermal products was investigated. The hydrothermal decomposition process was discussed primarily based on the experimental detections and the thermodynamic analysis.
2 Experimental
2.1 Synthesis of ZnO whiskers
In a typical experiment, certain amounts of 0.2 mol/L ZnSO4 and 0.25 mol/L Na2CO3 were mixed, keeping the molar ratio of ZnSO4 to Na2CO3 as 1:(1-4). The slurry formed at room temperature was then transferred to a Teflon-lined stainless steel autoclave with an inner volume of 80 mL, heated to 160 ℃ and kept under isothermal condition for 6-12 h. After the hydrothermal treatment, the product was filtrated, washed and dried at 60 ℃ in vacuum for 12 h.
2.2 Analysis
The morphology of the sample was examined with the field emission scanning electron microscopy (JSM7401F, JEDL, Japan). The structure and composition of the sample were identified by the X-ray powder diffractometer (D/max2500, Rigaku, Japan). The content of ZnO was analyzed by EDTA titration method, and the solution pH was detected by pH meter (DELTA 320, METTLER-TOLEDO, China).
3 Results and discussion
3.1 Formation and conversion of basic zinc carbonate
Fig.1 shows the morphology and XRD patterns of the precipitates before and after hydrothermal treatment (160 ℃, 6 h). The precipitate formed at room temperature was composed of basic zinc carbonate (PDF No.19-1458) with poor crystallinity. The precipitate after hydrothermal treatment was a mixture of the particles and the whiskers (a length of 30-40 mm and a diameter of about 1 mm). The XRD pattern shows that Zn5(CO3)2(OH)6 and ZnO coexisted in the hydrothermal product, implying that the whiskers may be composed of ZnO phase.
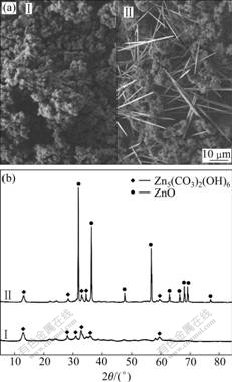
Fig.1 Morphology (a) and XRD patterns (b) of precipitates before (Ⅰ) and after (Ⅱ) hydrothermal treatment
Fig.2 shows the variation of the solution pH with the hydrothermal reaction time. The pH decreased from 9.56 to 8.49 with the increase of the reaction time from 0 to 6 h. The accumulation of H+ should be connected with the hydrothermal decomposition of Zn5(CO3)2(OH)6:
Zn5(CO3)2(OH)6=5ZnO+4H++2CO32-+H2O (1)
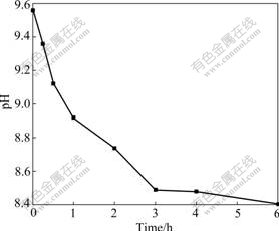
Fig.2 Variation of solution pH with hydrothermal reaction time
The co-existence of Zn5(CO3)2(OH)6 particles and ZnO whiskers in the hydrothermal product indicated the incomplete conversion of Zn5(CO3)2(OH)6 in the experimental condition. The gradual decrease of solution pH may be one of the major reasons for the inhibition of the conversion of Zn5(CO3)2(OH)6 to ZnO.
3.2 Influence of pH on hydrothermal conversion of basic zinc carbonate
Fig.3 shows the influence of initial solution pH on the morphology of hydrothermal products formed at 160℃ for 12 h. No Zn5(CO3)2(OH)6 was converted to ZnO at pH=6.5; Zn5(CO3)2(OH)6 and ZnO whiskers coexisted at pH=9.5; ZnO whiskers ( with a length of 40-50 mm and a diameter of about 1-2 mm) coexisted with minor amount of Zn5(CO3)2(OH)6 particles at pH=10.0. Only ZnO whiskers were detected in the case of pH 10.5. The solution with a comparatively high initial pH was favorable for the conversion of Zn5(CO3)2(OH)6 to ZnO, producing ZnO whiskers with low aspect ratio due to the quick dissolution of Zn5(CO3)2(OH)6 and the quick formation of ZnO.
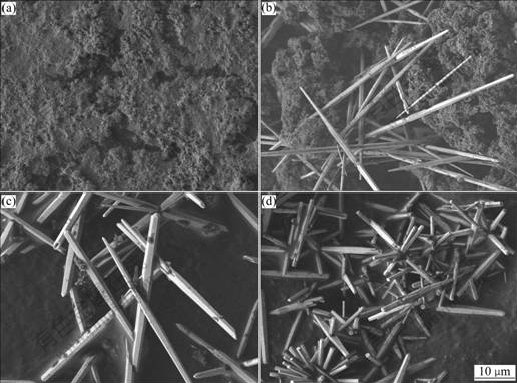
Fig.3 Influence of initial pH on morphology of hydrothermal products: (a) pH=6.5; (b) pH=9.5; (c) pH=10.0; (d) pH=10.5
Fig.4 shows the influence of the initial solution pH on the hydrothermal conversion of Zn5(CO3)2(OH)6 to ZnO. After 12 h of reaction, 8.6% and 87.3% (mass fraction) of ZnO were detected in the cases of initial pH 9.5 and 10.0, respectively, while Zn5(CO3)2(OH)6 was completely converted to ZnO within 2 h of reaction in the case of the initial pH 11.0, reconfirming that a high initial pH promoted the hydrothermal decomposition of Zn5(CO3)2(OH)6.
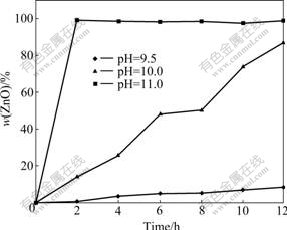
Fig.4 Influence of initial pH on conversion of Zn5(CO3)2(OH)6 to ZnO
3.3 Thermodynamic analysis on hydrothermal conversion of Zn5(CO3)2(OH)6 to ZnO
The hydrothermal decomposition of Zn5(CO3)2(OH)6 in acidic and alkaline media can be expressed as reaction (1) and reaction (2), respectively:
Zn5(CO3)2(OH)6+4OH-=5ZnO+2CO32-+5H2O (2)
The mechanical mixture model, in which the complex compound is considered as a combination of several simple compounds[7], was employed to estimate the ![]() for Zn5(CO3)2(OH)6:
for Zn5(CO3)2(OH)6:
![]() [Zn5(CO3)2(OH)6]=
[Zn5(CO3)2(OH)6]=
2![]() [ZnCO3]+3
[ZnCO3]+3![]() [Zn(OH)2] (3)
[Zn(OH)2] (3)
The basic thermodynamic data of the substances involved in the hydrothermal conversion of Zn5(CO3)2(OH)6 to ZnO were originated from the HSC software[8].
The variations of the ![]() for reactions (1) and (2) are shown in Fig.5. The positive
for reactions (1) and (2) are shown in Fig.5. The positive ![]() values for reaction (1) in the temperature range of 0-200℃ indicated the thermodynamic impossibility for the conversion of Zn5(CO3)2(OH)6 to ZnO in acidic media. The negative
values for reaction (1) in the temperature range of 0-200℃ indicated the thermodynamic impossibility for the conversion of Zn5(CO3)2(OH)6 to ZnO in acidic media. The negative ![]() values for reaction (2) and the gradual decrease tendency of the
values for reaction (2) and the gradual decrease tendency of the ![]() with the increase of temperature revealed possible conversion of Zn5(CO3)2(OH)6 to ZnO in the alkaline media and the enhanced decomposition tendency of Zn5(CO3)2(OH)6 at elevated temperatures.
with the increase of temperature revealed possible conversion of Zn5(CO3)2(OH)6 to ZnO in the alkaline media and the enhanced decomposition tendency of Zn5(CO3)2(OH)6 at elevated temperatures.
3.4 Influence of EDTA on hydrothermal product
Fig.6 shows the morphologies of the products formed in the absence and presence of EDTA, keeping the hydrothermal condition as follows: 160 ℃, 12 h and initial pH 10.5. Compared with the result without EDTA, the presence of minor amount of EDTA promoted the 1D growth of ZnO whiskers, leading to the formation of ZnO whiskers with a length of 50-60 mm and a diameter of about 1-2 mm. It was suggested that the chelating of EDTA with Zn2+ and the possible selective adsorption of EDTA on the specific planes of ZnO may be the main reasons for the enhanced preferential growth of ZnO along one direction and the details are still on research.
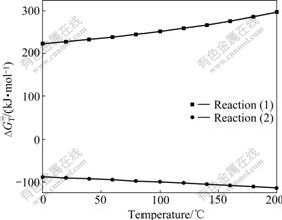
Fig.5 Variation of ![]() with temperature
with temperature
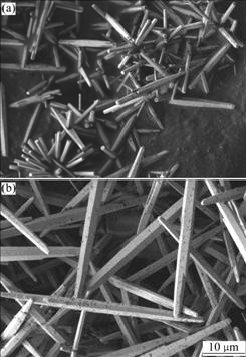
Fig.6 Influence of EDTA on morphology of products:(a) Without EDTA; (b) 5 mmol/L EDTA
4 Conclusions
1) A facile hydrothermal method is developed to synthesize ZnO whiskers in alkaline media, using ZnSO4 and Na2CO3 as the reactants.
2) The increase of the solution pH accelerates the hydrothermal decomposition of the particulate Zn5(CO3)2(OH)6 precursor but leads to the formation of ZnO whiskers with low aspect ratios.
3) The presence of 5×10-3 mol/L of EDTA promotes the hydrothermal growth of ZnO whiskers along one direction, producing ZnO whiskers with a length of 50-60 mm and a diameter of 1-2 mm.
References
[1] YANG R, WANG Z L. Interpenetrative and transverse growth process of self-catalyzed ZnO nanorods [J]. Solid State Communications, 2005, 134: 741-745.
[2] WANG Z L. Zinc oxide nanostructures: Growth, properties, and applications [J]. Journal of Physics: Condensed Matter, 2006, 16: 529-858.
[3] GREENE L E, YUHAS B D, LAW M, ZITOUN D, YANG P. Solution-grown zinc oxide nanowires [J]. Inorganic Chemistry, 2006, 45: 7535-7543.
[4] DAI Y, ZHANG Y, LI Q K, NAN C W. Synthesis and optical properties of tetrapod-like zinc oxide nanorods [J]. Chemical Physics Letters, 2002, 358: 83-86.
[5] SONG J H, WANG X D, RIEDO E, WANG Z L. Systematic study on experiment condition for large-scale growth of aligned ZnO nanowires on nitrides [J]. Journal of Physical Chemistry B, 2005, 109: 9869-9872.
[6] CHO S, JANG J W, JUNG S H, LEE B R, LEE K H. Precursor effects of citric acid and citrates on ZnO crystal formation [J]. Langmuir, 2009, 25: 3825-3831.
[7] HOU H W, XION Y J, XIE Y, LI Q, ZHANG J Y, TIAN X B. Structure-direct assembly of hexagonal pencil-like ZnO group whiskers [J]. Journal of Solid State Chemistry, 2004, 177: 176-180.
[8] HOU H W, XIE Y, LI Q. Structure-directing self-organized one-dimensional ZnO single-crystal whiskers [J]. Solid State Sciences, 2005, 7: 45-51.
[9] XIE J, LI P, WANG Y J, WEI Y. Synthesis of needle and flower like ZnO microstructures by simple aqueous solution route [J]. Journal of Physics and Chemistry of Solids, 2009, 70: 112-116.
[10] LIU B, ZENG H C. Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50 nm [J]. Journal of the American Chemical Society, 2003, 125: 4430-4431.
[11] LIU C Y, LI H Y, JIE W Q, ZHANG X Z, YU D P. Preparation of ZnO cluster and rod like whiskers through hydrothermal methods [J]. Materials Letters, 2006, 60: 1394-1398.
[12] JIMIN D, ZHIMIN L, HUANG Y, GAO Y, HAN B X, LI W, YANG G Y. Control of ZnO morphologies via surfactants assisted route in the subcritical water [J]. Journal of Crystal Growth, 2005, 280: 126-134.
[13] WANG J M, GAO L. Synthesis of uniform rodlike, multi-pod-like ZnO whiskers and their photoluminescence properties [J]. Journal of Crystal Growth, 2004, 262: 290-294.
[14] SHEN L, BAO N Z, YANAGISAWA K, DOMEN K, GRIMES C A, GUPTA A. Organic molecule-assisted hydrothermal self-assembly of size-controlled tubular ZnO nanostructures [J]. Journal of Physical Chemistry C, 2007, 111: 7280-7287.
[15] LI Z Q, XION Y J, XIE Y. Selected-control synthesis of ZnO nanowires and nanorods via a PEG-assisted route [J]. Inorganic Chemistry, 2003, 42: 8105-8109.
[16] NA AYUDHYA S, TONTO P, MEKASUWANDUMORNG O. Solvothermal synthesis of ZnO with various aspect ratios using organic solvents [J]. Crystal Growth and Design, 2006, 6: 2446- 2450.
Foundation item: Project(50874066) supported by the National Natural Science Foundation of China
Corresponding author: XIANG Lan; Tel: +86-10-62789643; E-mail: xianglan@mail.tsinghua.edu.cn
DOI: 10.1016/S1003-6326(09)60256-9
(Edited by LI Xiang-qun)
Abstract: ZnO whiskers with a length of 30-40 mm and a diameter of about 1 mm were synthesized by co-precipitation of ZnSO4 and Na2CO3 solution at room temperature followed by hydrothermal treatment of the as-prepared Zn5(CO3)2(OH)6 precursor at 160 ℃ for 6 h. The increase of the initial solution pH promotes the hydrothermal conversion of the particulate Zn5(CO3)2(OH)6 to ZnO whiskers. The presence of minor amount of EDTA in the hydrothermal solution promotes the one dimensional growth of ZnO whiskers, leading to the formation of ZnO whiskers with a length of 50-60 mm and a diameter of 1-2 mm.


