DOI:10.19476/j.ysxb.1004.0609.2019.10.14
BiVO4/WO3薄膜的原位相转换法制备及其光电化学性能研究
刘 鑫1,刘灿军1,陈 述1,杨亚辉2
(1. 湖南科技大学 化学化工学院 理论有机化学与功能分子教育部重点实验室,湘潭 411201;
2. 湖南农业大学 资源环境学院,长沙 410128)
摘 要:
BiVO4/WO3异质结薄膜因其优异的光电催化活性,已成为光电催化领域的研究热点。然而,目前制备BiVO4/WO3异质结薄膜通常采用简单的沉积方法,制备的薄膜存在大量的晶隙和界面缺陷,不利于载流子在界面处的传输。本文利用WO3→Bi2WO6→BiVO4原位相转换的原理,成功制备BiVO4/WO3异质结薄膜。通过XRD和TEM等手段表征BiVO4/WO3异质结薄膜的结构,发现制备的薄膜存在晶隙和界面缺陷少的特点。以制备的薄膜为光阳极,通过光电化学测试,表明原位生长法制备的BiVO4/WO3薄膜的光电化学性能优于沉积法制备的BiVO4/WO3薄膜的,原位生长法制备的BiVO4/WO3薄膜的光电流密度达到0.32 mA/cm2 (φ=1 V (vs. Ag/AgCl))。
关键词:
文章编号:1004-0609(2019)-10-2334-07 中图分类号:O644 文献标志码:A
太阳能光催化技术是一种在能源和环境领域有着重要应用前景的绿色技术,它不仅能实现太阳能向化学能的清洁转换,还可以有效地处理各种工业有机污染物和重金属废水[1-2]。然而,目前太阳能光催化技术仍然存在可见光催化活性不高、光生载流子易复合和光化学稳定性差等问题,导致太阳光利用率低,限制了太阳能光催化技术的实际应用[3-5]。
目前提高半导体光催化剂材料的性能可采用的方法主要包括:元素掺杂、窄带隙半导体敏化、贵金属修饰和异质结构筑等[6-11]。其中,异质结构筑由于能明显提高光催化性能效果,并且其技术较成熟、易操作,被认为是提高光催化剂性能最有效的途径之一[12-15]。半导体异质结是由两种不同的半导体材料结合形成的,其中,Ⅱ型异质结半导体光催化剂能综合两种半导体材料的物化性质方面的优势。例如,若一种为窄带隙、高吸光系数的半导体,则能有效地扩展光催化剂材料的可见光响应范围;另一种为电子(空 穴)传输性能优异的半导体,则可作为载流子的传输载体,能有效改善光生载流子的传输效率,减少光生载流子的复合[16-19]。众多异质结光催化材料中,BiVO4/ WO3异质结薄膜材料因其具有优异的可见光光催化活性在光电催化领域被广泛地研究[20-22]。然而,目前制备BiVO4/WO3异质结薄膜通常采用简单的表面沉积技术,这导致异质结界面结合程度较差,不利于电荷在交界面处的传输。
针对上述问题,本文拟采用原位相转换法制备交界面结合紧密的BiVO4/WO3异质结薄膜。首先利用在高温下Bi(NO3)3能与表层的WO3反应,形成Bi2WO6/WO3薄膜;再以V2O5作为钒源,在水热条件下将Bi2WO6原位地转换成BiVO4,从而形成原位生长的BiVO4/WO3异质结薄膜。采用TEM、XRD和UV-vis等手段分析和考察薄膜的表面形貌、物相组成和光吸收性能,并用制备的BiVO4/WO3异质结薄膜作为光电化学池的光阳极,研究其光电化学性能。
1 实验
1.1 WO3薄膜的制备
将2.0 g聚乙烯吡咯烷酮(PVP K-30)溶于15 mL去离子水,得到PVP溶液。将1.5 g偏钨酸铵分散在10 mL去离子水中,得到偏钨酸铵溶液。随后将偏钨酸铵溶液缓慢地滴加于PVP溶液中,并不断搅拌。搅拌2 h后,加入2.0 g PEG2000,充分溶解后,将溶液置于80 ℃下干燥,最后在600 ℃下煅烧1 h,获得WO3纳米晶颗粒。
称取0.18 g WO3纳米晶颗粒于玛瑙球磨罐中,随后依次加入0.051 g聚乙二醇20000、0.025 g乙基纤维素、0.8 mL松油醇、0.2 mL乙酰丙酮和0.2 mL曲拉通,采用球磨机球磨4 h,得到分散均匀的WO3浆料。
采用涂敷法制备WO3纳米薄膜电极。首先,在洗净的FTO玻璃导电面贴上3 mol/L Scotch透明胶带,以控制薄膜的厚度和面积。随后,将WO3浆料涂敷在FTO基底上,待自然干燥,500 ℃煅烧1 h,自然冷却,即得到WO3薄膜。
1.2 Bi2WO6/WO3薄膜的制备
将制备的WO3薄膜置于0.30 mol/L Bi(NO3)3的醋酸溶液中,浸泡12 h。为了保证每个样表面吸附的Bi3+的量相同,WO3薄膜以2 mm/s的速度被取出,随后在25 ℃下放置1 h,挥发表面的溶剂。最后将样品薄膜置于马弗炉中,520 ℃煅烧4 h,自然冷却后即得到Bi2WO6/WO3薄膜。
1.3 BiVO4/WO3薄膜的制备
先将0.0050 g V2O5溶解在50 mL去离子水中,并加入100 μL 3 mol/L的HCl溶液,然后把制备的Bi2WO6/WO3薄膜和V2O5溶液置于80 mL反应釜中,密封反应釜,在160 ℃条件下,水热反应4 h。等反应釜自然冷却至室温时,取出样品,用去离子水反复洗涤,随后在500 ℃下热处理1 h,得到BiVO4/WO3异质结薄膜。
为了比较不同方法制备的BiVO4/WO3异质结薄膜的光电化学性能,也采用简单沉积法制备BiVO4/WO3异质结薄膜。先称取0.18 g五水合硝酸铋和0.10 g乙酰丙酮氧矾分散于含2 mL冰醋酸和8 mL乙酰丙酮混合溶剂中。然后将制备WO3薄膜浸入上述混合溶液中,浸泡12 h,取出,500 ℃煅烧1 h,获得BiVO4/WO3异质结薄膜。必须指出,上述实验条件已被优化。
1.4 材料的表征
薄膜电极材料的结构、形貌特征和光吸收性能分别采用X射线全自动衍射仪(XRD,SIMENS D500)、透射电子显微镜(TEM,Titan G2 60-300)和紫外-可见分光光度计(UV-vis,TU-1901)进行表征。
1.5 光电化学测试
采用150 W氙灯作为模拟太阳光源,外加400 nm滤光片滤掉紫外光部分,并调节光强至100 mW/cm2,使其接近于1个太阳。使用德国ZAHNER公司生产的电化学工作站,以三电极体系对样品的光电化学性能进行测试,其中,以所制备的薄膜为工作电极,铂电极为对电极,Ag/AgCl(饱和KCl)电极为参比电极。光电流密度测试的电位范围为0~1.4 V (vs. Ag/AgCl),扫描速率为20 mV/s,电解质为0.5 mol/L的KH2PO4溶液。
2 结果与讨论
2.1 材料表征
图1(a)所示为原位合成BiVO4/WO3薄膜的技术路线图。首先以WO3粉末为原料,采用涂敷法制备WO3薄膜。然后,采用简单的浸泡和热处理方法将表层的WO3原位地转换成Bi2WO6,形成Bi2WO6/WO3薄膜。最后,以V2O5作为钒源,采用水热法处理Bi2WO6/WO3薄膜,即可得到BiVO4/WO3薄膜。图1(b)所示为WO3、Bi2WO6/WO3和BiVO4/WO3薄膜的XRD谱。由图可知,与WO3薄膜相比,Bi2WO6/WO3薄膜在2θ为28.4°和33.0°处均出现了Bi2WO6的特征衍射峰,这说明了 Bi2WO6的形成(JCPDS 73-1126)。由BiVO4/WO3薄膜的XRD谱易知,在衍射角为19.3°、30.6°、36.5°和46.0°处出现较弱衍射峰,与之对应的是单斜BiVO4特征衍射峰(JCPDS 75-2480),这说明如此的原位转换法能制备出BiVO4/WO3薄膜。
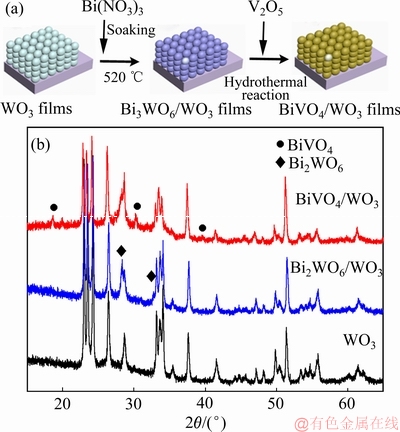
图1 原位合成BiVO4/WO3薄膜路线图和WO3、Bi2WO6/ WO3和BiVO4/WO3薄膜的XRD谱
Fig. 1 Synthetic process by in-situ transformation(a) and XRD patterns of WO3,Bi2WO6/WO3 and BiVO4/WO3 films(b)
为了观察BiVO4/WO3薄膜表面微观结构和确定BiVO4在WO3颗粒表面的形成,对BiVO4/WO3薄膜进行了透射电子显微镜(TEM)测试。图2(a)~(b)分别为BiVO4/WO3纳米晶样品的低分辨和高分辨TEM像。从样品的低分辨TEM像可知,样品颗粒的粒径大约在100~200 nm范围内。通过对样品边缘进行局部放大,可以发现两种不同的晶格条纹(见图2(b))。外层晶格条纹的间距约为0.308 nm,对应于BiVO4的(113)的晶面间距;内层晶格条纹的间距约为0.385 nm,匹配于单斜相WO3的(002)晶面间距。因此,样品的TEM像证实了BiVO4在WO3颗粒表面形成。更重要的是,BiVO4/WO3界面结合紧密,BiVO4层无明显的晶隙。
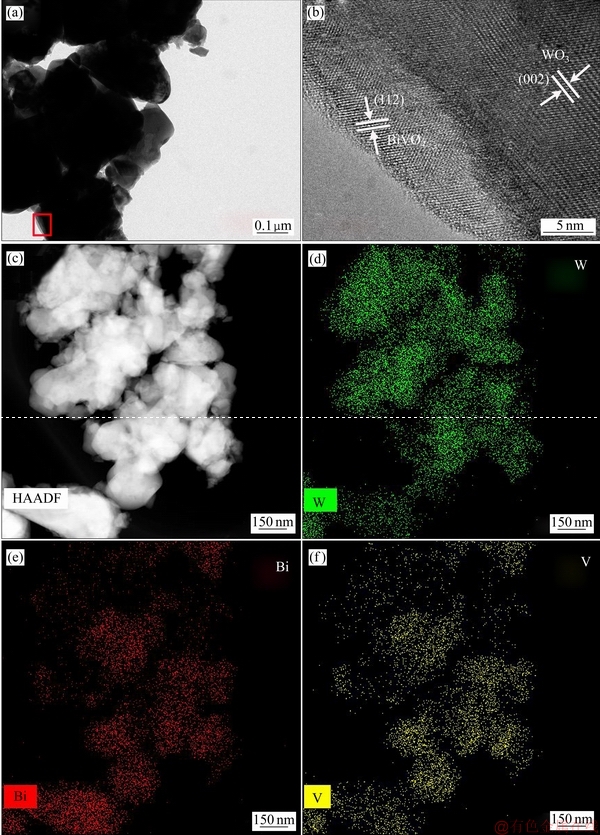
图2 BiVO4/WO3纳米晶的LR-TEM,HR-TEM和元素分布图
Fig. 2 LR-TEM(a), HR-TEM(b) and elemental mapping images((c)-(f)) of BiVO4/WO3 nanoparticles
通过空间元素分布图可以直观表征材料表面各元素分布。图2(c)所示为BiVO4/WO3纳米晶颗粒的元素分布图。从图中可知,纳米晶表面均匀地分布着W、Bi和V元素。结合SEM和TEM数据进一步说明BiVO4统一地覆盖在纳米晶颗粒表面,形成以WO3晶颗粒为核和以BiVO4为壳的核壳结构。
为研究薄膜光吸收性能的变化,对样品进行了UV-vis 吸收测试。图3所示为WO3、Bi2WO6/WO3和BiVO4/WO3薄膜的UV-vis 吸收光谱。从图中可以看出,纯WO3 薄膜只对波长λ≤460 nm 的光有吸收。表面原位形成Bi2WO6后,薄膜的光吸收范围没有发生明显的变化,这是因为Bi2WO6与WO3有相近的带隙。从实物照片图中可以看出,它们的颜色没有明显的区别。相比之下,BiVO4/WO3 薄膜的光吸收曲线则有显著的变化,其吸收边缘大约在510 nm,与文献报道的相符。从它的照片图也可以看出,薄膜的颜色明显变成黄色,这符合于BiVO4/WO3薄膜的UV-vis吸收曲线。
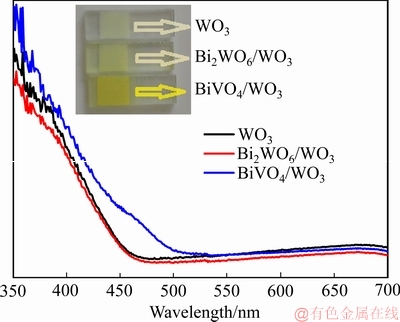
图3 WO3、Bi2WO6/WO3和BiVO4/WO3薄膜的UV-vis吸收光谱
Fig. 3 UV-vis absorbance spectra of WO3, Bi2WO6/WO3 and BiVO4/WO3 film
2.2 光电化学性能研究
为考察电极的光电化学性能,以制备的样品电极作为光阳极,在相同条件下进行了光电流测试,结果如图4(a)所示。由图4可知,BiVO4/WO3电极的光电流密度明显高于纯WO3和Bi2WO6/WO3电极的,这是因为BiVO4在WO3表面形成,构成的BiVO4/WO3异质结不但能扩展电极的可见光响应,还能改善光生载流子的分离和传输效率。然而,在暗光条件下,BiVO4/WO3电极的光电流密度在整个测试电势范围都几乎为0,表明BiVO4/WO3电极在无光照条件下不能发生电子与空穴的分离。为比较不同方法制备的BiVO4/WO3薄膜的光电化学性能,也采用简单沉积法制备了BiVO4/WO3薄膜。图4(b)所示为采用原位和简单沉积法制备的BiVO4/WO3薄膜光电流曲线图。为了方便区别,以1-BiVO4/WO3和2-BiVO4/WO3分别表示采用原位和简单沉积法制备的BiVO4/WO3薄膜。从图中可知,1-BiVO4/WO3电极的光电流密度明显要高于2-BiVO4/WO3电极的。
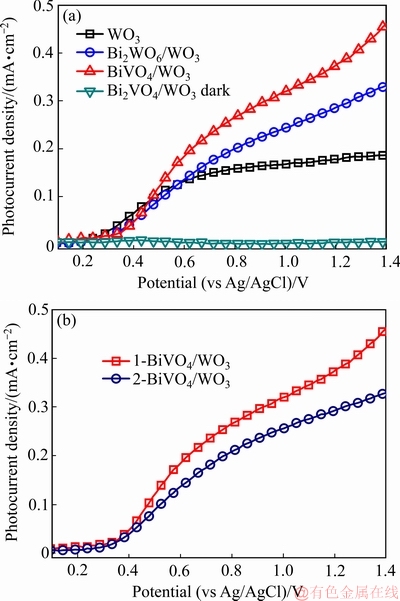
图4 WO3、Bi2WO6/WO3和BiVO4/WO3薄膜光电流密度图以及1-BiVO4/WO3和2-BiVO4/WO3薄膜的光电流图
Fig. 4 Photocurrent density curves of WO3, Bi2WO6/WO3 and BiVO4/WO3 films (a) and 1-BiVO4/WO3 and 2-BiVO4/WO3 films (b)
为了更直观地理解原位相转换法制备的薄膜电极具有更高光电化学性能的原因,光生电子和空穴在BiVO4/WO3电极中输运机制被描绘在图5中。在光照条件下,BiVO4和WO3能同时俘获光能,其价带中电子被激发到导带,由于BiVO4的导带位置(+0.02 VRHE)高于WO3的导带位置(+0.41 VRHE),BiVO4导带中的电子将迁移到WO3的导带,然后传输到FTO基底上,再通过外电流传输到阴极,参与析氢反应[21-22]。同时,电子激发后在价带上留下了空穴,由于BiVO4具有更高的价带位置,WO3价带中的空穴将迁移到BiVO4价带上,然后传输到电极表面,参与析氧反应。如此的异质结Ⅱ型传输方式能促进光生载流子在BiVO4与WO3相间的分离和传输。然而,若BiVO4/WO3异质结是由简单的表面沉积而形成,BiVO4与WO3间很难形成紧密接触的异质结,将增加光生载流子在BiVO4/WO3界面处的阻力,导致光生载流子的复合几率增加。两相间的良好接触是形成有效异质结和保证光生载流子快速分离与传输的基础条件。采用原位转换法(WO3→Bi2WO6→BiVO4)制备BiVO4/WO3异质结薄膜,能在两相间形成紧密接触,有利于光生载流子在BiVO4/WO3界面处传输,减少光生载流子的复合几率。因此,相比较于简单沉积法制备的BiVO4/WO3薄膜,原位法制备的BiVO4/WO3薄膜具有更高的光电催化性能。
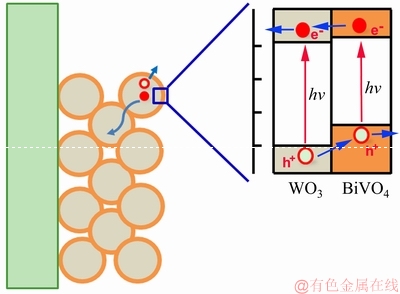
图5 BiVO4/WO3电极中光生电子和空穴的传输的机制图
Fig. 5 Schematic diagram of photogenerated charge transport mechanism in BiVO4/WO3 electrode
3 结论
1) 针对BiVO4/WO3异质结薄的制备通常采用简单的表面沉积技术,制备的异质结薄膜存在交界面结合不紧密和BiVO4层晶隙较多的缺点,本文研究了BiVO4/WO3异质结薄膜的一种原位生长的制备方法,有望克服上述不足。
2) 通过XRD和TEM等手段表征了制备的BiVO4/WO3薄膜的结构,表明制备的薄膜界面结合紧密和晶隙小;通过光电化学测试,表明了原位相转换法制备的BiVO4/WO3薄膜光阳极的光电流密度大于简单沉积法制备的光阳极。
REFERENCES
[1] HISATOMI T, KUBOTA J, DOMEN K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting[J]. Chemical Society Reviews, 2014, 43(22): 7520-7535.
[2] YUE Dong-ting, QIAN Xu-fang, ZHAO Yi-xin. Photocatalytic remediation of ionic pollutant[J]. Science Bulletin, 2015, 60(21): 1791-1806.
[3] 闫世成, 邹志刚. 高效光催化材料最新研究进展及挑战[J]. 中国材料进展, 2015, 34(9): 652-658.
YAN Shi-cheng,ZOU Zhi-gang. Recent progress and challenge in research of novel photocatalytic materials[J]. Materials China, 2015, 34(9): 652-658.
[4] 郑 云, 潘志明, 王心晨. 国内光催化研究进展简述[J]. 催化学报, 2013, 34(3): 524-535.
ZHENG Yun, PAN Zhi-ming, WANG Xin-chen. Advances in photocatalysis in China[J]. Chinese Journal of Catalysis, 2013, 34(3): 524-535.
[5] 陈 香, 李 洁, 李文章, 陈启元. 纳米多孔 WO3 薄膜电极的制备及其光电化学性质[J]. 中国有色金属学报, 2012, 22(12): 3487-3494.
CHEN Xiang, LI Jie, LI Wen-zhang, CHEN Qi-yuan. Preparation and photoelectrochemical properties of nano- porous tungsten trioxide films electrode[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(12): 3487-3494.
[6] PU Y C, WANG G, CHANG K D, LING Y, LIN Y K, FITZMORRIS B C, LIU C M, LU X, TONG Y, ZHANG J Z, HSU Y J, LI Y. Au nanostructure-decorated TiO2 nanowires exhibiting photoactivity across entire UV-visible region for photoelectrochemical water splitting[J]. Nano Letters, 2013, 13(8): 3817-23.
[7] SUBRAMANIAN A, ANNAMALAI A, LEE H H, CHOI S H, RYU J, PARK J H, JANG J S. Trade-off between Zr passivation and Sn doping on hematite nanorod photoanodes for efficient solar water oxidation: Effects of a ZrO2 underlayer and FTO deformation[J]. ACS Applied Materials & Interfaces, 2016, 8(30): 19428-19437.
[8] SIVULA K, FORMAL F L, GRATZEL M. WO3-Fe2O3 photoanodes for water splitting: A host scaffold, guest absorber approach[J]. Chemistry of Materials, 2009, 21(13): 2862-2867.
[9] PARK J H, KIM S, BARD A J. Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting[J]. Nano Letters, 2005, 6(1): 24-28.
[10] LEE Yuh-Lang, CHI Ching-Fa, LIAU Shih-Yi. CdS/CdSe Co-sensitized TiO2 photoelectrode for efficient hydrogen generation in a photoelectrochemical cell[J]. Chemistry of Materials, 2009, 22(3): 922-927.
[11] QIAO Li-Ying, XIE Feng-Yu, XIE Ming-Hui, GONG Cai-Hua, WANG Wei-Lang, GAO Jia-Cheng. Characterization and photoelectrochemical performance of Zn-doped TiO2 films by sol-gel method[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(8): 2109-2116.
[12] WANG Ya-jun, WANG Qi-sheng, ZhAN Xue-ying, WANG Feng-mei, SAFDAR M, HE Jun. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review[J]. Nanoscale, 2013, 5(18): 8326-8339.
[13] LIU Can-jun, YANG Ya-hui, LI Jie, CHEN Shu. Phase transformation synthesis of TiO2/CdS heterojunction film with high visible-light photoelectrochemical activity[J]. Nanotechnology, 2018, 29(26): 265401.
[14] LIU Can-jun, YANG Ya-hui, LI Wen-zhang, LI Jie, SHI Qi-lin, CHEN Qi-yuan. Highly Efficient photoelectrochemical hydrogen generation using ZnxBi2S3+x sensitized platelike WO3 photoelectrodes[J]. ACS Applied Materials & Interfaces, 2015, 7(20): 10763-10770.
[15] YANG Ya-Hui, XIE Ren-Rui, HANG Li, LIU Can-Jun, LIU Wen-Hua, ZHAN Fa-Qi. Photoelectrocatalytic reduction of CO2 into formic acid using WO3–x/TiO2 film as novel photoanode[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(9): 2390-2396.
[16] YAN Lu, ZHAO Wei, LIU Zhi-feng. 1D ZnO/BiVO4 heterojunction photoanode for efficient photoelectrochemical water splitting[J]. Dalton Transactions, 2016, 45(28): 11346-11352.
[17] SU Jin-zhan, GUO Lie-jin, BAO Ning-zhong, GRIMES C A. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting[J]. Nano Letters, 2011, 11(5): 1928-1933.
[18] JUNG H, CHAE S Y, SHIN C, MIN B K, JOO O S, HWANG Y J. Effect of the Si/TiO2/BiVO4 heterojunction on the onset potential of photocurrents for solar water oxidation[J]. ACS Applied Materials & Interfaces, 2015, 7(10): 5788-5796.
[19] 唐建军, 陈益清, 李文龙. 光电化学协同催化降解水中的扑草净[J]. 中国有色金属学报, 2014, 24(7): 1915-1920.
TANG Jian-jun, CHEN Yi-qing, LI Wen-long. Synergetic degradation of prometryn by photo-electro-chemical catalytic method[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(7): 1915-1920.
[20] ZENG Qing-yi, LYU Lai, GAO Yao-wen, CHANG Sheng, HU Chun. A self-sustaining monolithic photoelectrocatalytic /photovoltaic system based on a WO3/BiVO4 photoanode and Si PVC for efficiently producing clean energy from refractory organics degradation[J]. Applied Catalysis B: Environmental, 2018, 238: 309-317.
[21] BAEK J H, KIM B J, HAN G S, HWANG S W, KIM D R, CHO I S, JUNG H S. BiVO4/WO3/SnO2 double- heterojunction photoanode with enhanced charge separation and visible-transparency for bias-free solar water-splitting with a perovskite solar cell[J]. ACS Applied Materials & Interfaces, 2017, 9(2): 1479-1487.
[22] XIA Li-gang, BAI Jing, LI Jin-hua, ZENG Qing-yi, LI Xue-jin, ZHOU Bao-xue. A highly efficient BiVO4/WO3/W heterojunction photoanode for visible-light responsive dual photoelectrode photocatalytic fuel cell[J]. Applied Catalysis B: Environmental, 2016, 183: 224-230.
In situ phase transformation synthesis of BiVO4/WO3 films and its photoelectrochemical performance
LIU Xin1, LIU Can-jun1, CHEN Shu1, YANG Ya-hui2
(1. Key Laboratory of Theoretical Chemistry and Molecular Simulation, Ministry of Education, School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
2. College of Resources and Environment, Hunan Agricultural University, Changsha 410128, China)
Abstract: BiVO4/WO3 heterojunction films have attracted much attention in the field of photoelectrocatalysis due to its excellent photoelectrochemical activity. However, the BiVO4/WO3 films prepared by the simple deposition methods at present exhibited numerous grain boundaries and interface defects, which were unfavorable to the charge transfer in the BiVO4/WO3 interface. In this paper, BiVO4/WO3 films were prepared based on the principle of in-situ transformation (WO3→Bi2WO6→BiVO4). The prepared BiVO4/WO3 films were characterized by XRD and TEM. The results show that the prepared BiVO4/WO3 films have less grain boundaries and interface defects. The photoelectrochemical (PEC) measurements indicate that the BiVO4/WO3 films prepared by the in-situ method have a photocurrent density as high as 0.32 mA/cm2 (φ=1 V (vs. Ag/AgCl)), present a higher PEC activity than those prepared by the deposition method.
Key words: BiVO4/WO3; photoelectrochemical; in situ; photoanode; heterojunction
Foundation item: Project(21808051) supported by the National Natural Science Foundation of China; Project (2017JJ3079) supported by the Hunan Provincial Natural Science Foundation, China; Project (17C0628) supported by the Scientific Research Fund of Hunan Provincial Education Department, China; Project(E51757) supported by the Doctoral Foundation of Hunan University of Science and Technology, China
Received date: 2018-11-06; Accepted date: 2019-03-11
Corresponding author: LIU Can-jun; Tel: +86-731-58240059; E-mail: liucanjun@hnust.edu.cn
(编辑 王 超)
基金项目:国家自然科学基金资助项目(21808051);湖南省自然科学基金资助项目(2017JJ3079);湖南省教育厅科学研究基金资助项目(17C0628);湖南科技大学博士启动基金资助项目(E51757)
收稿日期:2018-11-06;修订日期:2019-03-11
通信作者:刘灿军,讲师,博士;电话:0731-58290045;E-mail:liucanjun@hnust.edu.cn
摘 要:BiVO4/WO3异质结薄膜因其优异的光电催化活性,已成为光电催化领域的研究热点。然而,目前制备BiVO4/WO3异质结薄膜通常采用简单的沉积方法,制备的薄膜存在大量的晶隙和界面缺陷,不利于载流子在界面处的传输。本文利用WO3→Bi2WO6→BiVO4原位相转换的原理,成功制备BiVO4/WO3异质结薄膜。通过XRD和TEM等手段表征BiVO4/WO3异质结薄膜的结构,发现制备的薄膜存在晶隙和界面缺陷少的特点。以制备的薄膜为光阳极,通过光电化学测试,表明原位生长法制备的BiVO4/WO3薄膜的光电化学性能优于沉积法制备的BiVO4/WO3薄膜的,原位生长法制备的BiVO4/WO3薄膜的光电流密度达到0.32 mA/cm2 (φ=1 V (vs. Ag/AgCl))。


