文章编号:1004-0609(2011)08-1893-11
Al2O3陶瓷基片电子封装材料研究进展
刘 兵,彭超群,王日初,王小锋,李婷婷,王志勇
(中南大学 材料科学与工程学院,长沙 410083)
摘 要:
总结微电子封装技术对封装基片材料性能的要求,介绍Al2O3的多晶转变和典型性能,讨论超细Al2O3粉体的3种制备方法:固相法、气相法和液相法;分析Al2O3陶瓷常用烧结助剂的基本作用和Al2O3陶瓷常用的6种烧结方法:常压烧结法、热压烧结法、热等静压烧结法、微波加热烧结法、微波等离子烧结法和放电等离子烧结法;指出Al2O3陶瓷基片的发展方向。
关键词:
电子封装材料;Al2O3陶瓷;超细粉体;烧结助剂;烧结方法;
中图分类号:TG174 文献标志码:A
Research progress in alumina ceramics substrate material for electronic packaging
LIU Bing, PENG Chao-qun, WANG Ri-chu, WANG Xiao-feng, LI Ting-ting, WANG Zhi-yong
(School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The elemental requirements for electronic packaging substrate materials were summarized. The polymorphic transformation and typical properties of alumina were introduced. Three synthesis methods of alumina ultrafine powder were discussed, namely solid phase, gas phase and liquid phase methods. The effect of alumina ceramics sintering additives and six kinds of sintering technologies were analyzed, namely pressureless sintering, hot pressing sintering, high temperature isostatic pressing, microwave sintering, microwave plasma sintering and spark plasma sintering. The development trends of alumina ceramics substrate materials were pointed out.
Key words: electronic packaging materials; alumina ceramics; ultrafine powder; sintering additives; sintering technologies
现代电子信息技术的飞速发展,电子产品向小型化、便携化、多功能化方向发展。电子封装正在与电子设计和电子制造一起,共同推动信息化社会的发展[1]。电子封装材料主要包括基片、布线、框架、层间介质和密封材料等。作为电子封装材料的一部分,电子封装基片材料应满足以下性能要求:1) 高的热导率,保证电子元件不受热破坏;2) 与芯片相匹配的线膨胀系数,确保芯片不因热应力而失效;3) 良好的高频特性,满足高速传输要求[2-3]。此外,电子封装基片还应具有力学性能高、电绝缘性能好、化学性质稳定(对电镀处理液、布线用金属材料的腐蚀而言)以及易于加工等特点[4]。电子封装基片材料的种类很多,包括陶瓷、环氧玻璃、金刚石、金属及金属基复合材料等[5]。陶瓷材料价格低廉、化学性能稳定、热导率高、介电常数低、耐热冲击性和电绝缘性好、高频特性优异、可靠性高、线膨胀系数与电子元器件非常相近[6],主要用于对热导率和气密性要求较高的场合,是电子封装中常用的基片材料[4]。当今已投入使用的陶瓷基片主要有Al2O3、BeO、Si3N4、SiC和AlN等。其中Al2O3基片价格低廉,强度、硬度、化学稳定性和耐热冲击性能高,绝缘性和与金属附着性良好,是目前电子行业中综合性能较好、应用最成熟的陶瓷材料,占 陶瓷基片总量的90%。但是Al2O3陶瓷热导率相对较低,热膨胀系数和Si不太匹配,难以在大功率集成电路中大量使用[5, 7]。本文作者总结Al2O3的多晶转变和典型性能,介绍超细Al2O3粉体的制备方法,探讨Al2O3陶瓷的常用烧结助剂和烧结方法,并指出Al2O3陶瓷基片的发展方向。
1 Al2O3的多晶转变和典型性能
Al2O3有许多同质多晶体,研究报道过的变体达十多种,α-Al2O3、β-Al2O3、γ-Al2O3、ζ-Al2O3是其中主要的4种晶型。这4种晶型之间的转变如图1所示[8]。α-Al2O3为刚玉型结构,属于六方晶系,单位晶胞是一个尖的菱面体。α-Al2O3结构最紧密,活性最低,是4种主要形态中最稳定的晶型,在所有温度下都能稳定存在。在自然界只存在α-Al2O3,当温度达到1 000~ 1 600 ℃时,其他变体都会不可逆地转变为α-Al2O3。Al2O3陶瓷的主晶相为α-Al2O3[9]。4种晶型的性质如表1所列[10]。
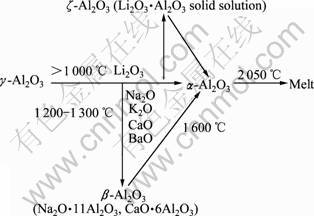
图1 Al2O3 4种主要晶型之间的转变
Fig.1 Transform of four main polymorphisms of alumina
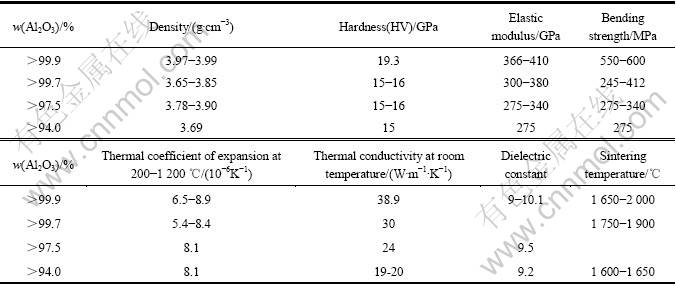
Al2O3的生成热为1 685 kJ/mol,键结合强度高,在氧化物陶瓷中具有最高的硬度[8]。除金刚石外,在所有天然产物中,只有少数人工合成的材料具有比Al2O3更高的硬度[11]。纯Al2O3陶瓷既可以耐酸腐蚀又可以耐碱腐蚀,性能优异。Al2O3陶瓷的机械强度、热性能、介电性能和化学稳定性等性能随着Al2O3含量的增加进一步提高[12]。不同Al2O3含量的Al2O3陶瓷基片典型性能如表2所列[11]。
2 超细Al2O3粉体的制备
Al2O3陶瓷的烧结主要靠表面和界面的扩散以及晶内扩散等机制来完成,烧结驱动力的大小取决于表面应力F=2γ/r(γ为表面能,r为表面曲率半径)的大小,粒子尺寸越小,F越大,烧结就越容易进行[13]。具体来讲,对于液相烧结,粒径越小,固体颗粒在液相中的溶解就越多,液相的传质效果就越好;对于固相烧结,粒径越小,原子扩散的距离就越短,扩散速率就越快[14]。总之,A12O3粉体的颗粒越细,活化程度越高,粉体就越容易烧结、烧结温度也就越低。制备超细、无团聚、分散均匀且具有良好烧结活性的粉体已经成为降低Al2O3陶瓷烧结温度的重要方法之一。AKIRA等[15]分别以颗粒尺寸为0.2 μm和1.8 μm的高纯A12O3粉体为原料,添加MgO和SiO2作为烧结助剂进行常压烧结,烧结温度为1 460 ℃。实验发现,颗粒尺寸为0.2 μm的Al2O3坯体在保温100 min后几乎完全致密(相对密度大于98%),而颗粒尺寸为1.8 μm的坯体相对密度只有85%。目前,制备超细Al2O3粉体的方法主要有固相法、液相法和气相法[16-18]。固相法包括机械粉碎法、化学热分解法和燃烧法等,其基本原理是采用几种单一成分的原料,经过配料、混合和锻烧得到组成一定的多组分化合物。固相法工艺过程简单、成本低廉、产量较高,但是固相法存在能耗大、制取过程易带入杂质、制取的Al2O3粒径分布范围较宽且难控制、最终粉末收集困难等缺点[19]。气相法包括物理气相沉积法(PVD)和化学气相沉积法(CVD)等,基本原理是利用各种方式将物质变成气体,然后使物质在气体状态下发生物理或化学变化,最后在冷却过程中凝聚长大从而形成超细粉体。气相法可制备出无团聚、粒径分布窄的粉体,但是气相法存在设备投资大,工艺操作复杂,粉末收集困难等缺点[20]。液相法种类众多,包括熔融法、沉淀法、水热法、溶液蒸发法和溶胶-凝胶法等[21]。液相法的基本原理是把铝盐配制成一定浓度的溶液,再选择一种合适的沉淀剂或用蒸发、升华、水解等操作将金属离子均匀沉淀或结晶出来,最后将沉淀或结晶物加热分解或者脱水,从而制得超细Al2O3粉体。液相法操作简单,可制取性能优良的粉末,但液相法制备工艺较复杂、生产成本高、效率低、不适合大规模生产[22]。溶胶-凝胶法又称胶体化学法,这是一种借助于胶体分散系的制粉方法[23],其原理是利用铝盐(醇盐[24]或无机 盐[25])的水解和聚合反应制备Al2O3水合物的均匀溶胶,然后浓缩成透明凝胶,再经过干燥和焙烧来得到超细Al2O3粉体。该方法具有纯度高、分散均匀、加工温度低、颗粒尺寸可控等优点,已经成为制备粒度均匀的高纯超细Al2O3粉体的重要手段[26]。
表1 Al2O3 4种主要晶型的性质
Table 1 Properties of four main polymorphisms of alumina
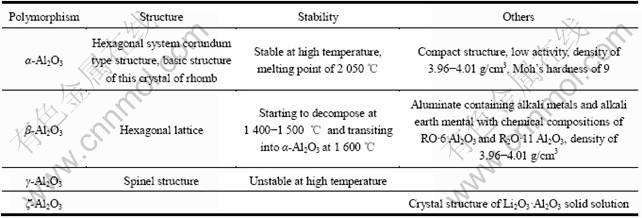
表2 不同Al2O3含量的Al2O3陶瓷基片的典型性能
Table 2 Typical properties of alumina ceramics substrate with different contents of alumina
此外,对于给定的粉料系统,存在一个最合适的粒度分布范围,在该粒度分布范围内,样品表现出最好的烧结性能,但如果极细的颗粒中间夹杂着少量大颗粒,烧结产品的性能会变差。SHIAU等[27]通过保持颗粒中位粒径不变、改变颗粒尺寸分布的标准偏差来考察粒径分布范围对烧结的影响,研究发现,宽的粒径分布会使烧结中期致密化速率加快;窄的粒径分布会减慢烧结中期的致密化速率,减少烧结后期晶粒粗化现象,提高最终的致密度。MA和LIM[28]对粒径分布范围的影响进行了更深入的研究,实验中固定中位粒径为0.4 μm,选取标准偏差为1.2、1.4、1.6、1.9和2.1的粉体样品分别进行实验,发现宽粒度分布的样品初始致密化速率大;研究认为这是由于级配的存在使样品颗粒之间接触点增多、扩散路径减小引起的。但是宽粒度分布会同时强化烧结中期晶粒的生长,由于晶粒生长与坯体致密化是两个相互竞争的过程,因此,宽粒度分布的样品烧结中期致密化速率比近单一尺寸样品的致密化速率小。在他们的实验中,最合适的粒度分布范围为标准偏差1.6~1.9。
3 Al2O3陶瓷烧结助剂的选择
烧结是Al2O3陶瓷制备过程中最为重要的环节之一。Al2O3陶瓷烧结主要通过扩散完成致密化,Al2O3晶格能较大,离子键较强,烧结激活能大,结构比较稳定,质点扩散系数低(Al3+在1 700 ℃时扩散系数仅10-11 cm-2/s),因此烧结温度较高[8]。99-Al2O3的烧结温度高达1 800 ℃,95-Al2O3的烧结温度为1 650~ 1 700 ℃,能耗大,生产成本高。如此高的烧结温度也将促使晶粒长大、气孔难以排除,导致陶瓷的气密性变差、力学性能降低,难以达到技术要求。在较低温度下烧结出质地致密、性能良好的Al2O3陶瓷基片材料一直是人们努力的方向。一般可在原料中添加适当的烧结助剂来降低Al2O3陶瓷的烧结温度,改善Al2O3陶瓷的微观组织结构,实现基片的高致密度和低气孔率,提高其热导率,降低其介电常数。传统烧结助剂按照促进烧结的作用机理可以分为两大类:一类是烧结过程中生成液相的烧结助剂,另一类是与Al2O3生成固溶体的烧结助剂[29]。近年来,稀土元素作为烧结助剂的作用越来越受到人们的重视,已成为Al2O3陶瓷最有前途的烧结助剂之一。
这类烧结助剂包括SiO2以及CaO、SrO、BaO等碱土金属氧化物,它们多为立方密堆、NaCl型晶体结构。由于晶体结构的差异,它们在Al2O3基体中的“溶解度”极小,大多以杂质方式聚集在晶界处。随着烧结的进行,晶界数量和晶界面积不断减少,晶界处这些烧结助剂含量相对增加,共熔温度下降,当温度达到共熔点时开始出现液相[30]。液相的表面润湿力和表面张力可方便A12O3颗粒的重排,通过融解-沉淀机理促进烧结,使烧结温度降低、烧结速度加快、烧结体致密性提高[31]。可以在Al2O3中单独或复合加入这类烧结助剂。典型的液相型烧结助剂还有CaO+Al2O3+ SiO2(CAS)、CaO+MgO+SiO2(CMS)、 MgO+Al2O3+SiO2 (MAS)和CaO+MgO+Al2O3+SiO2(CMAS)等玻璃相。AKIRA和MESSING[15]以纯度大于99.99%、粒径为0.2 μm的Al2O3粉末为原料,添加MgO-Al2O3-SiO2系烧结助剂,在1 460 ℃保温100 min得到的烧结体致密度达97%,而不添加烧结助剂的Al2O3粉末在相同的时间内要达到同样的致密度时烧结温度需达 1 500 ℃以上。薄占满和贺宏胜[32]选用高纯超细Al2O3为原料,添加CaO-MgO-SiO2系烧结助剂,在1 400~1 430 ℃低温下烧结得到晶粒大小约为0.6 μm的细晶Al2O3陶瓷。史国普等[33]同时掺杂质量分数为6%的CMS和1%的TiO2,在1 500 ℃温度下烧结出的Al2O3陶瓷相对密度达98.71%,且晶粒均匀,没有异常长大和二次再结晶。液相烧结助剂不仅对烧结具有不同程度的促进作用,还对A12O3陶瓷的显微结构产生很大影响,使基体中生成具有较大长径比的片状或者棒状晶粒,提高了Al2O3陶瓷基片的性能[34]。吴义权等[35]在α-Al2O3中添加CaO-MgO-SiO2系烧结助剂,在无压条件下于1 550 ℃保温3 h烧结制备出原位生长大小均匀的棒晶自增韧Al2O3陶瓷,相对密度大于97%。而不添加CAS烧结助剂的样品在1 300~1 600 ℃的烧结温度下均不能生成Al2O3棒晶。另外,液相烧结助剂的添加量要适当。液相过多会诱使晶粒异常长大,使Al2O3晶粒出现二次再结晶现象,同时液相包围Al2O3粒子会导致晶界气孔排出困难,冷却时气孔残留在Al2O3陶瓷内部,使Al2O3陶瓷相对密度降低。AHN等[36]研究在1 650 ℃的烧结温度下,添加不同质量的CaO-SiO2系烧结助剂对Al2O3显微结构尤其是晶粒异常长大的影响,发现在晶粒异常长大前有一段孵育期,孵育期时间的长短和异常长大晶粒的多少决定于液相的数量:液相含量较少时,晶粒较大,长径比也较大;液相含量较多时,晶粒较小,长径比也较小。烧结助剂的加入方式也在很大程度上影响烧结制品的性能。张静和沈卓身[37]分别以CaO、MgO和SiO2的简单氧化物和三者预烧结体的形式作为烧结助剂加入到95-Al2O3中,并在1 600 ℃烧结,发现以三者预烧结体的形式作为烧结助剂加入到Al2O3中烧结可以明显缩短烧成时间,并且烧结出的Al2O3陶瓷晶粒更加细小(5 μm左右)、致密化程度更高(98%以上)、强度更大(抗弯强度大于300 MPa)。
可与Al2O3晶体生成固溶体的烧结助剂有TiO2、MnO2、Fe2O3、Cr2O3等,这些物质晶格常数与Al2O3相近,通常能够与Al2O3形成固溶体[38]。同时,这些烧结助剂大多含有变价元素,变价作用能够使Al2O3的晶格产生缺陷,活化晶格,促进烧结。不同学者通过掺入不同烧结助剂,对促进烧结的机理有过多种分析和解释,但是至今没有一个统一明确的结论。
金红石晶体(TiO2)属四方晶系,氧离子形成有变形的六方密堆积,钛离子处在二分之一八面体空隙中。Al2O3和TiO2结构中都有大量的空隙,阳离子扩散空间较大[38]。Al2O3和TiO2可以形成有限置换固溶体,由于配位数、电价、离子半径等的差别,当Ti4+置换Al3+后,产生晶格畸变和阳离子空位,TiO2活化Al2O3晶格,对烧结的促进作用很明显[33]。KIM 等[39]研究TiO2对Al2O3烧结和显微结构的影响,发现随着烧结温度的升高和添加量的增加,Al2O3烧结体存在晶体正常生长、各向异性生长和异常长大3种情况。TiO2的加入有利于Al2O3片晶的生长。当TiO2与某些烧结助剂复合加入时,例如MnO2+TiO2、CaO+TiO2,还可以通过形成液相来促进烧结[40-41]。TiO2烧结助剂的引入可以大幅度降低Al2O3陶瓷的烧成温度,加快其烧结速度,且适当加入量对Al2O3性能的影响不大,在生产上具有明显的实际意义。
MgO可大大减小Al2O3晶界能的差异,削弱晶粒生长的各向异性,抑制Al2O3晶粒的异常长大,使微观结构细化。MgO是Al2O3烧结过程中的显微结构稳定剂[42]。MgO还能够消除晶粒的二次长大和晶粒内部包裹的气孔,使Al2O3陶瓷达到理论密度[43]。但是人们对MgO抑制Al2O3晶粒生长的机理至今仍存在争议。PARK和YOON等[44]认为,MgO单独添加或者和生成液相的烧结助剂复合添加都会使Al2O3晶粒生长为粗糙型界面,这样的界面能量较高、生长速度较慢,因此晶粒不易生长。AHN等[45]认为掺杂MgO的作用是间接的:MgO能够减小烧结过程中液相物质的粘度,促使烧结早期液相物质的流动,使液相更好更均匀地向孔隙处填充,从而消除烧结后期晶粒的异常长大现象。学术界更流行的看法是MgO与Al2O3在高温下可发生固相反应,在晶界上形成铝镁尖晶石(MgO·Al2O3)薄层,包裹在Al2O3晶粒的表面,使Al2O3晶粒之间的质点扩散受到抑制,阻碍Al2O3晶粒的长大,从而获得大小均匀的等轴状晶粒[46]。
MnO2和TiO2具有相同的结构,二者晶格常数相差不大,它们都能促进Al2O3的烧结,不同的是TiO2可以促进Al2O3的各向异性生长,而MnO2却没有这种作用。ERKALFA等[47]研究认为,在Al2O3中加入MnO2可形成固溶体,促进Al2O3晶粒的生长,当加入量在0.1%~0.5%(质量发数)之间时,烧结通过体积扩散进行;当加入量高于0.5%(质量分数)时,烧结通过晶界扩散进行;当加入量达到3%(质量分数)时会出现晶粒的异常长大现象。TOY等[48]也认为,在固溶极限范围内,加入MnO2时无第二相和液相出现,与Fe、Ti一样,Mn也可以以多种离子价态存在,变价作用会产生离子空位,从而加快体积扩散。但加入MnO2烧结形成的Al2O3晶粒内部会形成闭合的气孔,材料不可能完全致密。
TZING和TUAN[49]的研究表明,Al2O3陶瓷中加入Fe2O3可提高Al2O3陶瓷的原子扩散系数和晶粒生长速率,使试样致密度提高,晶粒尺寸增大。TARTAJ和MESSING [50]研究发现,掺有Fe2O3的Al2O3在烧结过程中会生成片晶,他们认为烧结过程中会生成Fe2+和O2-离子空位,晶界处Fe2+聚集,晶界能降低,从而使烧结速率提高。
稀土元素原子具有特殊的电子层结构,同时具有原子半径大、熔点高、化学性质活泼等优良特性,是良好的表面活性元素,可以改善材料的润湿性,降低熔点[51]。稀土元素以氧化物的形式作为烧结助剂,可以明显降低Al2O3陶瓷的烧结温度,改善其显微结构和组织,提高其使用性能[52]。稀土元素能够改善Al2O3陶瓷烧结性能的机理目前还没有统一的结论。大多数研究者认为由于稀土氧化物离子半径相对铝离子半径大很多,难以与Al2O3形成固溶体,因此主要存在于Al2O3的晶界上[53],且稀土氧化物体积较大,不仅自身难以迁移,而且还会阻碍其他离子的迁移,从而降低晶界移动速率,抑止晶粒生长,有利于形成致密结构[54-55]。也有研究者认为,稀土氧化物可以促进Al2O3与CaO、SiO2等杂质或添加剂的反应,形成低熔点液相,从而以液相传质来促进烧结。另外,添加的稀土氧化物还可以进入晶界玻璃相,使玻璃相强度得到提高,从而改善Al2O3陶瓷的力学性能[55]。
黄良钊[56]发现,加入Y2O3可以在基本不改变99-Al2O3的组成主晶相结构的条件下,极大地改善Al2O3陶瓷的显微结构,加快陶瓷的致密化程度,促进烧结,并且还可以减少晶内气孔数量、缩小孔径、细化晶粒、提高力学性能,从而降低99-Al2O3的烧成温度和烧结成本。穆柏春和孙旭东[57]研究加入Y2O3和La2O3等稀土氧化物对Al2O3陶瓷烧结温度、显微组织和力学性能的影响,发现Y2O3和La2O3主要偏聚在晶界处,可以起到细化晶粒、改善显微组织、净化界面、提高致密度和力学性能的作用。Y2O3和La2O3倾向于分布在基体颗粒的表面,并且易于形成低熔点液相,加上颗粒之间的毛细作用,促使颗粒间的物质向孔隙处填充,从而降低孔隙率、提高致密度、降低烧结温度。姚义俊等[58]对Y2O3、La2O3和Sm2O3这3种稀土氧化物对Al2O3陶瓷烧结性能和力学性能的影响进行研究,发现这几种烧结助剂的加入都可以降低95-Al2O3的烧结温度、缩短保温时间、改善烧结性能、提高Al2O3瓷的力学性能。微观结构分析表明:Y2O3、La2O3和Sm2O3可以抑止Al2O3晶粒的生长,细化晶粒,使晶粒尺寸均匀统一,形成致密化结构。他们还发现Sm2O3对改善Al2O3瓷的烧结性能及力学性能的作用比Y2O3和La2O3更显著。霍振武等[59]对MgO、Y2O3和La2O3这3种不同氧化物对高纯Al2O3致密化速率的影响进行研究,在1 250 ℃和1 400 ℃下烧结时,MgO、Y2O3和La2O3的掺杂都可以明显降低Al2O3的致密化速率。掺杂相同添加剂时,随着掺杂量的增加,致密化速率下降;在掺杂量相同的情况下,具有较大离子半径的Y2O3和La2O3比MgO具有更强的抑制Al2O3致密的作用。实验结果表明:加入烧结助剂的Al2O3晶粒尺寸明显小于纯Al2O3的晶粒尺寸,掺杂可以明显抑制晶粒长大。LI等[55]和WANG等[60]在1 200 ℃下掺杂0.01% Nd2O3时对Al2O3进行烧结,他们发现Nd2O3可以降低Al2O3晶界的扩散速率,且抑制作用可达MgO的两倍。他们认为即使在Y2O3存在的情况下,Nd2O3对Al2O3的晶界移动也可以起到主要的阻碍作用。WANG等[61]也对Nd2O3在高纯Al2O3陶瓷中的作用规律进行研究,他们发现适当控制Nd2O3含量可以在某些温度点获得较高的致密度和较小的晶粒尺寸。
4 Al2O3陶瓷的烧结方法
目前Al2O3较常用的烧结方法有6种,即常压烧结法、热压烧结法、热等静压烧结法、微波加热烧结法、微波等离子烧结法和放电等离子烧结法(SPS)。常压烧结法一般采用常规加热方式,在传统电炉中进行。常压烧结法简单、方便、经济,是Al2O3陶瓷烧结中应用最普遍的一种烧结方法[62]。吴镇江等[63]采用凝胶注模成型工艺,加入5%的TiO2在1 300 ℃温度下常压烧结出致密度达97%的Al2O3陶瓷。但是常压烧结法烧结温度较高,最终烧结体密度较低,致密化速率较慢,烧结时间较长,难以实现快速低温烧结,能源浪费大。孙致平等[64]以高纯超细Al2O3粉为原料,不掺杂烧结助剂,在4.5 GPa、1 550 ℃保温30 min高压烧结出相对密度高达98.71%的Al2O3陶瓷,而添加0.5%的MgO烧结助剂、在1 550 ℃保温30 min常压烧结出的Al2O3陶瓷相对密度仅为95.25%,致密度降低3.4%。
热压烧结是一种强化烧结,其基本原理是对胚体加热的同时施加单向轴应力,将压力的作用和表面能的减小一起作为驱动力来促进胚体的烧结。外加压力能够促进颗粒内空位(或原子)的流动,加强扩散作用,因此热压烧结可大幅度降低Al2O3的烧结温度。Al2O3粉末在常压下普通烧结必须烧至1 800 ℃,而热压(20 MPa)烧结只需烧至1 500 ℃左右[65]。热压烧结有利于坯件的致密化,可烧制出几乎无孔隙的产品,广泛用于普通无压条件下难致密化材料的制备,热压烧结也因此常被称为“全致密工艺”[66]。 彭晓峰等[67]采用热压烧结法制备出高性能的Al2O3陶瓷,在1 450 ℃保温30 min制备的Al2O3晶粒尺寸为500 nm、抗弯强度为(500±45) MPa;在1 550 ℃保温30 min制备的Al2O3陶瓷断裂韧性达(5.7±0.5) MPa·m1/2。但是热压烧结法加热和冷却所需时间都较长,只能制备形状比较简单的制品,且必须对制品进行后加工,生产效率较低。
热等静压(HIP)烧结是一种成形和烧结同时进行的新技术,其基本原理是以氮气、氩气等气体作为压力介质,在粉末加热过程中同时施加各向均衡的压力,借助高温和高压的共同作用来促进材料的致密化[68]。热等静压集热压和等静压的优点于一身,可在较低的温度、较短的时间内烧结得到各向完全同性、几乎完全致密、显微结构均匀、性能良好、形状复杂的陶瓷制品。ECHEBERRIA 等[69]在1 250 ℃的温度和150 MPa的压力下热等静压烧结出几乎完全致密的Al2O3陶瓷,晶粒大小为0.45,维氏硬度为23~24 GPa,断裂韧性达3.5 MPa·m1/2。BERNAL等[70]采用热等静压烧结法制备高强高韧Al2O3陶瓷,在1 450 ℃、150 MPa条件下烧结出的样品致密度大于98%,维氏硬度达19 GPa,断裂韧性达5.2 MPa·m1/2,各项性能均优于在相同条件下用无压烧结法制备的陶瓷样品。但是热等静压烧结设备昂贵,压力条件苛刻,需要对素坯进行包封或者预烧结,产品成本高,生产效率低,很难实现Al2O3陶瓷的工业化生产[66]。
微波加热烧结是利用微波电磁场中材料的介电损耗使材料表面和内部同时加热至烧结温度而实现烧结的技术[71]。微波烧结法与普通烧结方法的区别较大,其气热流方向是由里向外的,这更加有利于坯体内气体由内部向表面扩散溢出。微波烧结可以使材料将吸收的微波能转化为内部分子的动能和势能,从而增加粒子的动能,降低烧结活化能,提高扩散系数,促进材料的致密化过程,抑制晶粒的长大[72]。微波烧结升温快,烧结时间短,能效高,无污染,烧结体内部晶粒细小且均匀性好。微波烧结以其独特的机理和传统加热模式无法实现的许多优点,在Al2O3陶瓷烧结上具有广阔的应用前景,是最有效、最具竞争力的新一代烧结技术[73]。CHENG等[74]通过加入质量分数为0.5%的MgO烧结助剂,采用微波烧结得到性能优异的Al2O3陶瓷,平均粒径仅为40 μm,而测量密度高达3.97 g/cm3(趋近于理论密度)。但是微波烧结也存在着烧结温度分布不均匀、试样局部区域易发生热断裂等缺点。
微波等离子烧结是通过微波电离气体形成等离子体,然后用等离子体加热生坯从而得到致密陶瓷烧结体的烧结方法。微波等离子烧结法加热快速,可以减小由于表面扩散(主要发生在传统烧结的低温阶段)而引起的晶粒粗化,为晶界扩散和体积扩散提供较强的驱动力和较短的扩散途径,从而使陶瓷显微结构细 化[75]。PENG等[76]以氩气和氧气的混合气体作为等离子气体进行Al2O3陶瓷的微波等离子烧结,15 min后,坯体的相对密度达到理论密度的99%;而用传统烧结方法在相同的温度下烧结15 min,坯体的相对密度只有理论密度的63%。KIM和JOHNSON[77]利用微波等离子烧结颗粒直径为0.3 μm的α-Al2O3,当添加0.25%的MgO、升温速度为100 ℃/min时,获得的烧结体相对密度达99.5%。但是微波等离子烧结容易造成热失控效应,烧结试样性能均匀性差、局部易开裂。
放电等离子烧结(Spark plasma sintering,SPS) 是利用脉冲能、放电脉冲压力和焦耳热产生的瞬时高温场来实现烧结的新方法[78]。ZHAN等[79]以粒径为50 nm的α-Al2O3粉体为原料,在1 100 ℃下采用SPS烧结制备得到相对密度达99.8%、晶粒尺寸为0.35 μm的Al2O3陶瓷。GAO等 [80]采用放电等离子方法在 1 400~1 550 ℃的温度范围内无需保温就烧结出抗弯强度为800 MPa左右的Al2O3陶瓷,与传统烧结方法相比,陶瓷的抗弯强度提高约1倍。在1 450 ℃温度下烧制的Al2O3陶瓷的维氏硬度达18.5 GPa,抗弯强度高达860 MPa。与传统的烧结方法相比,放电等离子法烧结温度低、升温和降温速率快、保温时间短、热效率高、烧结样品晶粒均匀、致密度高、力学性能好、使用价值和应用前景广阔[81]。
5 结束语
为了满足电子系统小型化、低成本以及可靠性和电性能不断提高的市场化要求,开发可靠性好、散热性优良、价格适宜的多层陶瓷基片材料是今后相当长一段时间内的发展重点。Al2O3陶瓷基片因热导率不足等问题,应用范围受到一定限制,但Al2O3陶瓷基片工艺成熟、价格低廉,在一段时期内还将占据陶瓷基片的主要地位。电子工业的不断发展对Al2O3陶瓷基片的性能提出更新更高的要求,有必要在生产和实践中对Al2O3粉体制备和烧结技术等不断进行改进和更新。对于降低Al2O3陶瓷烧结温度和提高其致密度的研究,国内外学者已经进行了很多工作,今后可以从以下几个方面进行努力:1) 严格控制粉体颗粒的尺寸,选择颗粒尺寸相近或相对集中且分散均匀的粉体进行烧结;也可以对Al2O3粉体进行一些预处理,如煅烧、球磨、表面改性、冲击破坏等,以此增加粉体的缺陷、加大烧结推动力,以求能够实现低温下的快速烧结。2) 尝试新型种类的烧结助剂。比如有报道称加入超细金属粉作为烧结助剂可以降低Al2O3陶瓷的烧结温度,并大大改善烧结体的致密度、抗裂性、硬度、强度和耐磨性等。3) 采用更新、更高效的加热方式,应用更新型的烧结方法,如电场烧结、磁场烧结、激光烧结、辐射烧结和超高压烧结等。
参考文献:
[1] 汤 涛, 张 旭, 许仲梓. 电子封装材料的研究现状及趋势[J]. 南京工业大学学报: 自然科学版, 2010, 32(4): 105-110.
TANG Tao, ZHANG Xu, XU Zhong-zi. Research progress and trends of electronic packaging materials[J]. Journal of Nanjing University of Technology: Natural Science Edition, 2010, 32(4): 105-110.
[2] 郝洪顺, 付 鹏, 巩 丽, 王树海. 电子封装陶瓷基片材料研究现状[J]. 陶瓷, 2007(5): 24-27.
HAO Hong-shun, FU Peng, GONG Li, WANG Shu-hai. Recent achievement in research for electronic packaging ceramic substrate materials[J]. Ceramics, 2007(5): 24-27.
[3] MARKSTEIN H W. A wide choice of materials for MEMs[J]. Electronic Packaging and Production, 1997, 37(3): 34-38.
[4] 李婷婷, 彭超群, 王日初, 王小锋, 刘 兵. 电子封装陶瓷基片材料的研究进展[J]. 中国有色金属学报, 2010, 20(7): 1365-1374.
LI Ting-ting, PENG Chao-qun, WANG Ri-chu, WANG Xiao-feng, LIU Bing. Research progress in ceramic substrate material for electronic packaging[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(7): 1365-1374.
[5] 张海坡, 阮建明. 电子封装材料及其技术发展状况[J]. 粉末冶金材料科学与工程, 2003, 8(3): 216-223.
ZHANG Hai-po, RUAN Jian-ming. The development of materials and technology of electronic packaging[J]. Materials Science and Engineering of Powder Metallurgy, 2003, 8(3): 216-223.
[6] 杨会娟, 王志法, 王海山, 莫文剑, 郭 磊. 电子封装材料的研究现状及进展[J]. 材料导报, 2004, 18(6): 86-90.
YANG Hui-juan, WANG Zhi-fa, WANG Hai-shan, MO Wen-jian, GUO Lei. Present status and progress in study of electronic packaging materials[J]. Materials Review, 2004, 18(6): 86-90.
[7] ELECTRON P P. MCM process combines beryllia substrate[J]. Electronic Packaging and Production, 1996, 36(9): 81.
[8] 尹衍升, 张景德. 氧化铝陶瓷及其复合材料[M]. 北京: 化学工业出版社, 2001.
YING Yan-sheng, ZHANG Jing-de. Alumina ceramics and its composite materials[M]. Beijing: Chemical Industry Press, 2001.
[9] 张玉军, 张伟儒. 结构陶瓷材料及其应用[M]. 北京: 化学工业出版社, 2005.
ZHANG Yu-jun, ZHANG Wei-ru. Structure ceramic material and its application[M]. Beijing: Chemical Industry Press, 2005.
[10] 刘大成. 氧化铝陶瓷及其烧结[J]. 中国陶瓷, 1998, 34(5): 13-15.
LIU Da-cheng. Alumina ceramics and its sintering [J]. China Ceramics, 1998, 34(5): 13-15.
[11] SWAIN M V. 材料科学与技术丛书(第11卷)—陶瓷的结构与功能[M]. 郭景坤, 译. 北京: 科学出版社, 1998.
SWAIN M V. Materials science and technology(Vol.11)—Structure and properties of ceramics[M]. GUO Jing-kun, transl. Beijing: Science Press, 1998.
[12] 曲远方. 功能陶瓷及应用[M]. 北京: 化学工业出版社, 2003.
QU Yuan-fang. Functional ceramics and its application[M]. Beijing: Chemical Industry Press, 2003.
[13] 张立德. 超微粉体制备与应用技术[M]. 北京: 中国石化出版社, 2001.
ZHANG Li-de. Superfine powder preparation and application technology[M]. Beijing: China Petrochemical Press, 2001.
[14] 曹南萍, 王仲军, 南小英, 欧阳芳, 王静海. 降低高铝瓷烧成温度的研究[J]. 硅酸盐通报, 2006, 25(4): 150-155.
CAO Nan-ping, WANG Zhong-jun, NAN Xiao-ying, OUYANG Fang, WANG Jing-hai. Discuss on lower sintering temperature of high alumina ceramic[J]. Journal of the Chinese Ceramic Society, 2006, 25(4): 150-155.
[15] AKIRA N, MESSING G L. Liquid-Phase sintering of alumina coated with magnesium aluminosilicate glass[J]. Journal of the American Ceramic Society, 1996, 79(12): 3199-3210.
[16] GLEITER H, MARQUARDT P. Nanocrystalline structures an approach to new materials[J]. Zeitschrift Fur Metallkunde, 1984, 75(4): 263-267.
[17] BIRRINGER R, GLEITER H, KLEIN H P, MARQUARDT P. Nanocrystalline materials an approach to a novel solid structure with gas-like disorder[J]. Physics Letters, 1984, 102(8): 365-369.
[18] HERR U, BIRRINGER R, GLEITER H. Nanocrystalline materials[J]. Ceramic Forum International, 1990, 67(3): 70-74.
[19] WU Yi-quan, ZHANG Yu-feng, HUANG Xiao-xian, GUO Jing-kun. Preparation of platelike nano alpha alumina particles[J]. Ceramics International, 2001, 27(3): 265-268.
[20] POPP U, HERBIG R, MICHEL G, MULLER E, OESTREICH C H. Properties of nanocrystalline ceramic powders prepared by laser evaporation and recondensation[J]. Journal of the European Ceramic Society, 1998, 75(12): 1153-1160.
[21] 许坷敬, 杨新春, 田贵山. 采用引入晶种的水热合成法制备α-Al2O3纳米粉[J]. 硅酸盐学报, 2001, 29(6): 576-579.
XU Ke-jing, YANG Xin-chun, TIAN Gui-shan. Preparation of nano-size α-Al2O3 powders by seeding hydrothermal synthesizing method[J]. Journal of the Chinese Ceramic Society, 2001, 29(6): 576-579.
[22] WU Jie-hua, LI Bao-shun, GUO Jing-kun. A novel wet chemistry/two-step calcining process for the synthesis of ultrafine α-Al2O3 particles[J]. Materials Letters, 2001, 47(12): 40-43.
[23] 宋晓岚. 高纯超细氧化铝粉体制备技术进展[J]. 陶瓷工程, 2001, 12: 43-46.
SONG Xiao-lan. The progress of high-purity ultrafine aluminum oxide powder preparation technology[J]. Ceramic Engineering, 2001, 12: 43-46.
[24] 黄传真, 张树生, 王宝友. 溶胶-凝胶法制备纳米氧化铝粉末的研究[J]. 金刚石与磨料磨具工程, 2002, 127(1): 22-25.
HUANG Chuan-zhen, ZHANG Shu-sheng, WANG Bao-you. The research of nanoscale alumina powders prepared with the sol-gel technology[J]. Diamond and Abrasives Engineering, 2002, 127(1): 22-25.
[25] 洪 梅, 陈念贻. 溶胶-凝胶方法制备α-Al2O3超细粉的研究[J]. 功能材料, 1995, 26(2): 180-181.
HONG Mei, CHEN Nian-yi. The α-Al2O3 ultrafine powder synthesis by sol-gel proeess[J]. Functional Materials, 1995, 26(2): 180-181.
[26] 王 歆, 庄志强, 齐雪君. 金属氧化物溶胶-凝胶法制备技术及其应用[J]. 材料导报, 2000, 14(11): 42-44.
WANG Xin, ZHUANG Zhi-qiang, QI Xue-jun. Sol-gel routes and their applications for preparation of metal oxide compounds[J]. Materials Review, 2000, 14(11): 42-44.
[27] SHIAU F S, FANG T T, LEU T H. Effect of particle-size distribution on the microstructural evolution in the intermediate stage of sintering[J]. Journal of the American Ceramic Society, 1997, 80(2): 286-290.
[28] MA J, LIM L C. Effect of particle size distribution on sintering of agglomerate-free submicron alumina powder compacts[J]. Journal of the European Ceramic Society, 2002, 22(13): 2197-2208.
[29] 黄晓巍. 液相烧结氧化铝陶瓷的致密化机理[J]. 材料导报, 2005, 21, 19(Special 4): 393-395.
HUANG Xiao-wei. Mechanism of densification for alumina liquid phase sintering[J]. Materials Review, 2005, 21, 19(Special 4): 393-395.
[30] 吴振东, 叶建东. 烧结助剂对氧化铝陶瓷的烧结和显微结构的影响[J]. 兵器材料科学与上程, 2002, 25(2): 68-71.
WU Zhen-dong, YE Jian-dong. Effects of additives on sintering behavior and microstructure of Al2O3[J]. Ordnance Material Science and Engineering, 2002, 25(2): 68-71.
[31] 刘于昌, 黄晓巍. 液相烧结氧化铝陶瓷及其烧结动力学分析[J]. 硅酸盐学报, 2006, 34(6): 647-651.
LlU Yu-chang, HUANG Xiao-wei. Liquid-phase-sintering of alumina ceramics and sintering kinetic analysis[J]. Journal of the Chinese Ceramic Society, 2006, 34(6): 647-651.
[32] 薄占满, 贺宏胜. 低温烧结细晶氧化铝瓷的研究[J]. 硅酸盐学报, 1995, 23(3): 272-278.
BO Zhan-man, HE Hong-sheng. Study on fine grain alumina ceramics fired at low temperature[J]. Journal of the Chinese Ceramic Society, 1995, 23(3): 272-278.
[33] 史国普, 王 志, 侯宪钦, 孙 翔, 俎全高, 徐秋红. 低温烧结氧化铝陶瓷[J]. 济南大学学报: 自然科学版, 2007, 21(1): 17-19.
SHI Guo-pu, WANG Zhi, HOU Xian-qin, SUN Xiang, ZU Quan-gao, XU Qiu-hong. Low temperature sintered alumina ceramics[J]. Journal of University of Jinan: Science and Technology, 2007, 21(1): 17-19.
[34] WU Yi-Quan, ZHANG Yu-feng, HUANG Xiao-xian, GUO Jing-kun. Microstructural development and mechanical properties of self-reinforced alumina with CAS addition[J]. Journal of the Europaea Ceramic Society, 2001, 21(5): 581-587.
[35] 吴义权, 张玉峰, 黄校先, 郭景坤. 原位生长棒晶氧化铝陶瓷的制备[J]. 硅酸盐学报, 2002, 28(6): 585-588.
WU Yi-quan, ZHANG Yu-feng, HUANG Xiao-xian, GUO Jing-kun. Fabrication of platelike grain alumina ceramics by in situ growth[J]. Journal of the Chinese Ceramic Society, 2002, 28(6): 585-588.
[36] AHN J H, LEE J H, HONG S H, HWANG N M, KIM D Y. Effect of the liquid forming additive content on the kinetics of abnormal grain growth in alumina[J]. Journal of the American Ceramic Society, 2003, 86(8): 1421-1423.
[37] 张 静, 沈卓身. 预烧结添加剂对95%氧化铝瓷致密化的影响[J]. 电子元件与材料, 2008, 27(2): 57-59.
ZHANG Jing, SHEN Zhuo-shen. The effect of pre-sintered additives on the densification for 95% alumina ceramics[J]. Electronic Components and Materials, 2008, 27(2): 57-59.
[38] RAO W R, CUTLER I B. Effect of iron oxide on the sintering kinetics of Al2O3[J]. Journal of the American Ceramic Society, 1973, 56(11): 588-593.
[39] KIM Y M, HONG S H, KIM D Y. Anisotropic abnormal grain growth in SiO2/TiO2-doped alumina[J]. Journal of the American Ceramic Society, 2000, 83(11): 2809-2812.
[40] ERKALFA H, MISIRLI Z, BAYKARA T. Densification of alumina at 1 250 ℃ with MnO2 and TiO2 additives[J]. Ceramics International, 1995, 21(5): 345-348.
[41] HONG S H, KIM D Y. Effect of liquid content on the abnormal grain growth of alumina[J]. Journal of the American Ceramic Society, 2001, 84(7): 1597-1600.
[42] 王 珍, 党新安, 张昌松, 高 扬. 影响氧化铝陶瓷低温烧结的主要因素[J]. 中国陶瓷, 2009, 45(6): 24-27.
WANG Zhen, DANG Xin-an, ZHANG Chang-song, GAO Yang. The main influence factor on low temperature sintering for ceramics[J]. China Ceramics, 2009, 45(6): 24-27.
[43] IKEGAMI T, KOTANI K, EGUCHI K. Some roles of MgO and TiO2 in densification of a sinterable alumina[J]. Journal of the American Ceramic Society, 1987, 70(12): 885-890.
[44] PARK C W, YOON D Y. Effects of SiO2, CaO2 and MgO additions on the grain growth of alumina[J]. Journal of the American Ceramic Society, 2000, 83(10): 2605-2609.
[45] AHN J H, HONG S H, HWANG N M, KIM D Y. Characteristics of liquid penetration into undoped and magnesia-doped alumina. Journal of the American Ceramic Society, 2003, 86(12): 2206-2208.
[46] BERRY K A, HARMER M P. Effect of MgO solute on microstructure development in Al2O3[J]. Journal of the American Ceramic Society, 1986, 69(2): 143-149.
[47] ERKALFA H, MLSLRH Z, DEMIRCI M, TOY C, BAYKARA T. The densification and microstructual development of Al2O3 with manganese oxide addition[J]. Journal of the Europaea Ceramic Society, 1995, 15(2): 165-171.
[48] TOY C, DEMIRCI M, ONURLU S. A Colloidal method for manganese oxide addition to alumina powder and investigation of properties[J]. Journal of Materials Science, 1995, 30(16): 4183-4187.
[49] TZING W H, TUAN W H. Exaggerated grain growth in Fe-doped Al2O3[J]. Journal of Materials Science Letters, 1999, 18(14): 1115-1117.
[50] TARTAJ J, MESSING G L. Effect of the addition of α-Fe2O3 on the microstructural development of boehmite-derived alumina[J]. Journal of Materials Science Letters, 1997, 16(2): 168-170.
[51] 汪德宁, 徐 颖, 徐 东, 吴建生, 王家敏, 张澜庭, 毛大立. 金属间化合物FeAl与α-Al2O3的界面润湿行为及合金元素Y和Nb的作用[J]. 材料科学与工艺, 1996, 4(1): 5-9.
WANG De-ning, XU Ying, XU Dong, WU Jian-sheng, WANG Jia-min, ZHANG Lan-ting, MAO Da-li. Wettability of intermetallic FeAl/α-Al2O3 and the effect of microalloys Y and Nb[J]. Material Science and Technology, 1996, 4(1): 5-9.
[52] 许崇海, 艾 兴, 邓建新, 黄传真. 稀土元素在氧、碳、硼化物陶瓷材料中的应用研究[J]. 硅酸盐通报, 1998, 3: 64-68.
XU Chong-hai, AI Xing, DENG Jian-xin, HUANG Chuan-zhen. Applications of rare-earth elements in oxide, carbide and boride ceramic materials[J]. Journal of the Chinese Ceramic Society, 1998, 3: 64-68.
[53] FANG Jin-xin, THOMPSON A M, MARTIN P H, HELEN M C. Effect of yttrium and lanthanum on the final-stage sintering behavior of ultrahigh-purity alumin[J]. Journal of the American Ceramic Society, 1998, 80(8): 2005-2012.
[54] 刘维跃, 辛玉军, 李红岩. La2O3对ZTM陶瓷晶界性能的影响[J]. 材料研究学报, 1996, 10(1): 68-71.
LIU Wei-yue, XIN Yu-jun, LI Hong-yan. Effect of La2O3 addition on grain-boundary characterization of ZTM ceramics[J]. Chinese Journal of Materials Research, 1996, 10(1): 68-71.
[55] LI Yan-zun, WANG Chong-min, HELEN M C, RICKMAN J M, MARTIN P H. Codoping of alumina enhance creep resistance[J]. Journal of the American Ceramic Society, 1999, 82(6): 1497-1504.
[56] 黄良钊. 含钇氧化铝陶瓷的制备及性能研究[J]. 长春光学精密机械学院学报, 1999, 22(1): 6-7.
HUANG Liang-zhao. A study of manufacture and properties on alumina ceramics of yttrium addition[J]. Journal of Changchun Institute of Optics and Fine Mechanics, 1999, 22(1): 6-7.
[57] 穆柏春, 孙旭东. 稀土对Al2O3陶瓷烧结温度、显微组织和力学性能的影响[J]. 中国稀土学报, 2002, 20(增刊): 104-107.
MU Bai-chun, SUN Xu-dong. Effect of rare earths on sintering temperature, microstructure and mechanical properties of Al2O3 ceramics[J]. Journal of the Chinese Rare Earth Society, 2002, 20(supplement): 104-107.
[58] 姚义俊, 丘 泰, 焦宝祥, 沈春英. 稀土氧化物对氧化铝瓷性能的影响[J]. 真空电子技术, 2004(4): 28-30.
YAO Yi-jun, QIU Tai, JIAO Bao-xiang, SHEN Chun-ying. The effects of rare earth oxide on the properties of alumina ceramics[J]. Vacuum Electronic, 2004(4): 28-30.
[59] 霍振武, 司文捷, 刘大鹏, 苗赫濯. 掺杂对高纯氧化铝致密化速率的影响[J]. 硅酸盐通报, 2002(2): 8-11.
HUO Zhen-wu, SI Wen-jie, LIU Da-peng, MIAO He-zhuo. Influence of doping on the densification rate of high-purity alumina ceramics[J]. Journal of the Chinese Ceramic Society, 2002(2): 8-11.
[60] WANG Chong-min, JUNGHYUN C, HELEN M C, HARMER M P, RICKMAN J M. Influence of dopant concentration on creep properties of Nd2O3-doped Al2O3[J]. Journal of the American Ceramic Society, 2001, 84(5): 1010-1016.
[61] WANG Chong-Min, HELEN M C, HARMER M P. Effect of Nd2O3 doping on the densification and abnormal grain growth behavior of high-purity alumina[J]. Journal of the American Ceramic Society, 2004, 87(3): 378-383.
[62] 江 昕, 戴红莲, 陈晓明. 陶瓷烧结新工艺[J]. 陶瓷研究, 2001, 16(4): 20-23.
JIANG Xin, DAI Hong-lian, CHEN Xiao-ming. The new technology of ceramic sinting[J]. Ceramic Studies Journal, 2001, 16(4): 20-23.
[63] 吴镇江, 臧丽坤, 陈运法, 谢裕生. Al2O3凝胶注模成型及添加TiO2烧结助剂的影响[J]. 过程工程学报, 2001, 1(4): 398-401.
WU Zhen-jiang, ZANG Li-kun, CHEN Yun-fa, XIE Yu-sheng. Gelcasting of Al2O3 with TiO2 added: the effects of sintering aid and dispersant[J]. The Chinese Journal of Process Engineering, 2001, 1(4): 398-401.
[64] 孙致平, 滕元成, 齐晓敏, 陈 堃, 鲁伟员. 高纯超细Al2O3粉的常压烧结与高压烧结[J]. 化工学报, 2007, 58(11): 2932-2936.
SUN Zhi-ping, TENG Yuan-cheng, QI Xiao-min, CHEN Kun, LU Wei-yuan. Normal pressure sintering and high pressure sinterin of superfine Al2O3 powder with high purity[J]. Journal of Chemical Industry and Engineering, 2007, 58(11): 2932-2936.
[65] 李世普. 特种陶瓷工艺学[M]. 武汉: 武汉工业大学出版社, 1997.
LI Shi-pu. The technology of special ceramics[M]. Wuhan: Wuhan Industrial University Press, 1997.
[66] 李县辉, 孙永安, 张永乾. 陶瓷材料的烧结方法[J]. 陶瓷学报, 2003, 24(2): 120-124.
LI Xian-hui, SUN Yong-an, ZHANG Yong-qia. The sintering methods of ceramic materials[J]. Journal of Ceramic, 2003, 24(2): 120-124.
[67] 彭晓峰, 黄校先, 张玉峰. 高性能细晶粒Al2O3陶瓷材料的制备与研究[J]. 无机材料学报, 1998, 13(3): 327-332.
PENG Xiao-feng, HUANG Xiao-xian, ZHANG Yu-feng. Preparation and research of fine-grained alumina with high mechanical properties[J]. Journal of Inorganic Materials, 1998, 13(3): 327-332.
[68] 邬荫芳. 热等静压技术的新进展[J]. 硬质合金, 2000, 17(2): 115-119.
WU Yin-fang. The new progress of hot isostatic pressing[J]. Cemented Carbide, 2000, 17(2): 115-119.
[69] ECHEBERRIA J, TARAZONA J, HE J Y, BUTLER T, CASTRO F. Sinter-HIP of a-alumina powders with sub-micron grain sizes[J]. Journal of the European Ceramic Society, 2002, 22(11): 1801-1809.
[70] BERNAL M H B, RIOS C D, REYES A G, ELGUEZABAL A A, ECHEBERRIA J, RASCON A N. Fracture toughness of an α-Al2O3 ceramic for joint prostheses under sinter and sinter-HIP conditions[J]. Journal of Refractory Metals and Hard Materials, 2009, 27: 722-728.
[71] 王 念, 周 健. 陶瓷材料的微波烧结特性及应用[J]. 武汉理工大学学报, 2002, 24(5): 43-46.
WANG Nian, ZHOU Jian. The characteristics and application of microwave sintering in ceramics[J]. Journal of Wuhan University of Technology, 2002, 24(5): 43-46.
[72] 雷 鸣, 张礼杰, 王英伟, 曾繁明, 邹志伟, 刘景和. 透明陶瓷的研究现状与发展[J]. 中国陶瓷工业, 2005, 12(4): 45-49.
LEI Ming, ZHANG Li-jie, WANG Ying-wei, ZENG Fan-ming, ZOU Zhi-wei, LIU Jing-he. The research situation and development prospect of transparent ceramics[J]. China Ceramic Industry, 2005, 12(4): 45-49.
[73] 吴 红, 史洪刚, 冯宏伟, 范爱国. 微波烧结技术的研究进展[J]. 兵器材料科学与工程, 2004, 27(4): 59-61.
WU Hong, SI Hong-gang, FENG Hong-wei, FAN Ai-guo. Research progress in microwave sintering technology[J]. Ordnance Material Science and Engineering, 2004, 27(4): 59-61.
[74] CHENG Ji-ping, AGRAWAL D, ZHANG Yun-jin, DRAWL B, ROY R. Fabricating transparent ceramics by microwave sintering[J]. American Ceramic Society Bulletin, 2000, 79(9): 71-74.
[75] 李 江, 潘裕柏, 宁金威, 黄智勇, 郭景坤. Al2O3陶瓷低温烧结的研究现状和发展前景[J]. 中国陶瓷, 2001, 37(5): 42-45.
LI Jiang, PAN Yu-bai, NING Jin-wei, HUANG Zhi-yong, GUO Jing-kun. Low temperature sintering of alumina ceramics[J]. China Ceramic, 2001, 37(5): 42-45.
[76] PENG Jin-hui, HONG Pin-jie, DAI Shu-shan, VOLLATH D, SZABO D V. Microwave plasma sintering of nanocrystalline alumina[J]. Journal of Materials Science and Technology, 1998, 14(2): 173-175.
[77] KIM J S, JOHNSON D L. Plasma sintering of alumina[J]. American Ceramic Society Bulletin, 1983, 62(5): 620-622.
[78] TOKITA M. Trends in advanced SPS spark plasma sintering system and technology[J]. Journal of the Society of Powder Technology Japan, 1993, 30(5): 790-802.
[79] ZHAN Guo-dong, JOSHUA K, WAN Ju-lin, JAVIER G, AMIYA K M. Alumina-based nanocomposites consolidated by spark plasma sintering[J]. Scripta Materialia, 2002, 47(11): 737-741.
[80] GAO L, HONG J S, MIYAMOTO H, TORRE S D D L. Bending strength and microstructure of Al2O3 ceramics densified by spark plasma sintering[J]. Journal of the European Ceramic Society, 2000, 20(12): 2149-2152.
[81] ZHANG D M, FU Z Y, WANG Y C, ZHANG Q J, GUO J K. Heterogeneous of non-conductive materials sintering by pulse electric current[J]. Key Engineering Materials, 2002, 224(2): 729-734.
(编辑 龙怀中)
收稿日期:2010-03-23;修订日期:2010-06-25
通信作者:彭超群,教授,博士;电话:0731-88877197;E-mail: pcq2005@163.com


