
Microstructure and phase composition of Ti-based biocomposites with different contents of nano-hydroxyapatite
LI Wei(李 卫)1, PANG Peng-sha(庞鹏沙)1, 2, LIU Ying(刘 英)1
1. Department of Materials Science and Engineering, Jinan University, Guangzhou 510632, China;
2. GRIPM Advanced Materials Co., Ltd., General Research Institutes for Nonferrous Metals, Beijing 101407, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Nano-hydroxyapatite (nHA) and titanium (Ti) powders with different ratios were prepared by mechanical ball milling, and then sintered in vacuum environment. The microstructure and phase composition of Ti-based biocomposites with different contents of nHA(5% and 10%, in volume fraction) were investigated. Meanwhile, the phase composition of pure Ti was studied for contrast. The results show that Ti phase forms a finer continuous network microstructure with few porous after milling and sintering. The higher amount of nHA powders are added, the higher amount of porous are achieved, while the fracture morphology becomes coarser. The specimen with contents of 10% nHA has serious interface reaction after sintering at 1 100 ℃, it varies with the pure Ti specimen. Combined with the XRD and EDS analysis, it can be founded that elements Ca, P, O and Ti diffuse on the interface, and the phases of Ti, Ti2O, Ti5P3, CaTiO3 and TiOx can be ascertained in nHA/Ti composites.
Key words:
Ti-based biocomposites; nano-hydroxyapatite; interface reaction; powder metallurgy;
1 Introduction
Metals such as titanium and its alloys have been found wide use in orthopedic applications involving hard tissue replacement, owing to their combination of excellent mechanical properties, corrosion resistance and proven biocompatibility[1-3]. However, the elastic modulus (100 GPa) of titanium is much higher than the elastic modulus (7-25 GPa) of natural bone. When embedded in the human body, a fibrous tissue encapsulates the implant isolating it from the surrounding bone forms, known as the “stress shielding effect”[4]. Also these materials are not bioactive, i.e., the healing process is slower compared with other implant materials with bioactive properties such as bioceramic of hydroxyapatite[5-8]. Therefore, the attention has been focused on Ti-based biocomposites by powder metallurgy (PM), for improving bioactivity and decreasing elastic modulus. An important aspect of PM technology is the size prepared powders. Different approaches on the HA powders have been published using micro grades. In order to make biocomposites acquire high performance, it necessary to explore nHA for the sintering with Ti, this may decrease the sintering temperature and improve the bioactivity.
However, the biocomposite with different ratios of nHA sintering in vacuum is rarely reported up to now. Based our previous works[9-10] of charactering the nHA/Ti composite powders, the present study aims to investigate the microstructure and phase composition of Ti-based biocomposites with different contents of nHA via powder metallurgy (PM) technology. Meanwhile, their interface reaction after sintering at 1 100 ℃ were concluded by X-ray diffractometry (XRD), optical microscopy (OM), scanning electron microscopy (SEM) and energy disperse spectra (EDS).
2 ExperimentalCommercially pure titanium with average diameters of 45 μm and different contents of nHA (0, 5% and 10%, volume fraction) were milled by ball milling in alcohol media. The composite powders were cold isostatic pressed (CIP) under a pressure of 200 MPa and sintered at 1 100 ℃ into a vacuum atmosphere. Phase compositions were examined by X-ray diffractometry (XRD, RIGAKU) using Cu Kα radiation between 2θvalues of 20? -80? with step size of 0.02?. Microstructure and fracture morphology of sintered materials were investigated by optical microscopy (OM, LEICA DMIRM) and scanning electron microscopy (SEM, JSM-5910). Energy disperse spectra (EDS, THERMAL NORAN) of selected areas was simultaneously carried out duding SEM observations.
3 Results and discussion3.1 Phase composition
Fig.1 shows the XRD patterns of the phase compositions of 90%Ti-nHA composite pre and after sintering at 1 100 ℃ in vacuum environment. As a contrast, the phase composition of pure Ti pre and after sintering at 1 100 ℃ is also studied (Fig.2). It can be seen that the phase compositions of 90%Ti-nHA composite sintered at 1 100 ℃ were consisted of Ti, Ti2O, Ti5P3, CaTiO3 and TiOx.
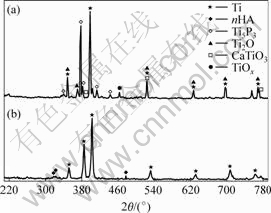
Fig.1 XRD patterns of 90%Ti-nHA: (a) Sintered in-vacuum at 1 100℃; (b) Starting composite powers
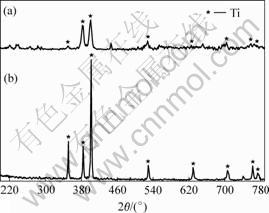
Fig.2 XRD patterns of Ti: (a) Sintered in-vacuum at 1 100 ℃; (b) Starting Ti powers
3.2 Microstructure analysis
Figs.3 and 4 show the optical micrographs of 90%Ti-nHA composite and 95%Ti-nHA composite after etching with acid solutions (n(HF)?n(HNO3) = 1?3). It can be seen that white phases distribute the whole region as a dominated phase with low magnification. The net like microstructure is observed with high magnification. Combining with the previous phase analysis and examination of these microstructures, it can conclude that white areas are Ti phase, black areas are porous and gray areas are nHA/Ti reaction layer.
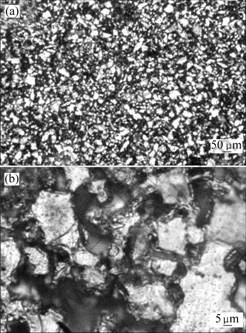
Fig.3 Optical micrographs of 90%Ti-nHA composite (after etching): (a) Low magnification; (b) High magnification
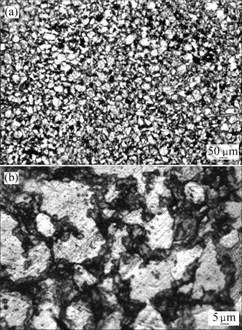
Fig.4 Optical micrographs of 95%Ti-nHA composite (after etching): (a) Low magnification; (b) High magnification
3.3 Fracture surface
SEM image and EDS analysis of in-vacuum heat-treated 90%Ti-nHA specimens are shown in Fig.5. It is observed that the in-vacuum heat-treated 90%Ti-nHA composite specimen is composed of granular aggregation and porous morphology. It is suggested that the grey area exhibits granular aggregation with domination of Ti elements, however the white edge area consists of mixture of Ca, P and O.
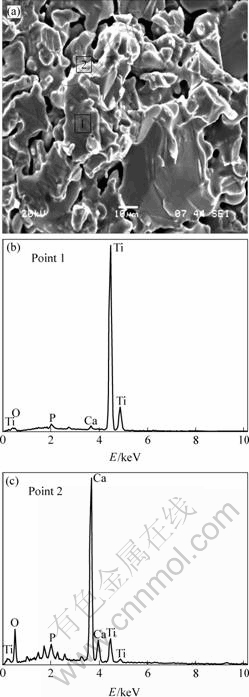
Fig.5 Fracture morphology and EDS analysis of 90%Ti-nHA composite
3.4 Discussion
The phase composition of 90%Ti-nHA and pure Ti specimens sintered at 1 100 ℃ in-vacuum are shown in Figs.1 and 2, respectively, suggesting some reactions of 90%c system. It is different from our previous work about pure nHA system, where pure nHA specimen sintered at 1 100 ℃ has merely decreased on diffraction intensity and doesn’t form any new phase. However, the 90%Ti-nHA specimen sintered at 1 100 ℃ has changed previous sintering mechanism. It suggests that 90%Ti-nHA system is a process with formation of Ti2O, Ti5P3, CaTiO3 and TiOx compounds, substituting the process of the growth and decomposition of pure nHA system. There is no decomposition phase of nHA, which is different from other reports about the phase of Ca4P2O9[11] and Ca3(PO4)2[12]. It can be concluded that the lower fraction of nHA results in un-obvious decomposition phases. Meanwhile, it is found that interface reaction productions consist of Ti5P3, CaTiO3, Ti2O and TiOx because of the elemental diffusion of Ti with Ca, P and O after sintering at 1 100 ℃ in-vacuum. Since the low oxygen in-vacuum, it is easy to form the lower oxygen content compound such as Ti2O due to weaker diffusion capacity of O element. However it may come into higher oxygen content compounds at local area with higher concentration of O element originated from nHA, and it can be testified with existence of TiOx. The reaction of Ti and nHA is supporting by the following equation:
![]()
![]()
As a contrast, it concludes that the pure Ti sintered at 1 100 ℃ has the same microstructure with pre-sintering powders as well as broadening of diffraction peak. It may be the existence of amorphous microstructure coating at specimen surface. NAD et al[13] had explained the amorphous microstructure formed by micro-melt Ti at heat treating. It also can be seen that purity of Ti can be guaranteed at this experimental process, it is facilitate to realize the essential of interface reaction of Ti-nHA system. Meanwhile, the micro-melt Ti at heat treatment with wet function promotes the sintering process.
Both the 90%Ti-nHA and 95%Ti-nHA composites have net-like microstructure by OM observation, except a few of micro-porous is dark area as shown in Fig.3 and 4. As the previous work[9], the ideal structure is derived from prepared composite powders after ball milling treatment. Considering the biological principle, the net-like microstructure with Ti as a framework may bear a majority of loads. When it implants in human body, it may promote new bone tissue growth into porous region with the biodegradable of bioactivity components such as calcium phosphate.
The bonding strength nHA and Ti seriously influences mechanical properties of composites. The fracture morphology of SEM micrographs is shown in Fig.5, revealing the interfacial region in Ti-nHA system. EDS analysis of the principal elements of the 90%Ti-nHA is presented for the reactive interfacial system. These elemental scans reveal that Ti, Ca, P and O had co-diffuse at the interface. With the XRD analysis, it can be concluded that the Ti and nHA present chemical reaction at elevated temperatures. Obviously, a chemical and metallurgical bonding forms at the interface. It is facilitate to implant in human body with longer time and avoid the weak mechanical link at the interface by coating technology [14-15].
4 Conclusions1) The 90%Ti-nHA specimen composite sintered at 1 100 ℃ has serious interface reaction in-vacuum, the reaction productions consist of Ti2O, Ti5P3, CaTiO3 and TiOx compounds.
2) The net-like microstructure is formed after ball milling and heat treating of 90%Ti-nHA specimen composite, no micro crack can be found besides a few of micro porous that is introduced by PM technology.
3) The detailed detection by EDS finds that the co-diffusion of Ca, P, O and Ti elements exists at Ti and nHA system, and 90%Ti-nHA composite specimen after sintering at 1 100 ℃ in-vacuum has a chemical reaction at interface of Ti and nHA. A chemical and metallurgical bonding also forms at the interface increases the stability of implant.
References
[1] WEI M, RUYS A J, SWAIN M V, MILTHORPE B K, SORRELL C C. Hydroxyapatite-coated metals interfacial reactions during sintering[J]. Journal of Materials Science: Materials in Medicine, 2005, 16: 101-106.
[2] YANG Y Z, KIM K H, AGRAWAL C M, ONG J L. Interaction of hydroxyapatite-titanium at elevated temperature in vacuum environment[J]. Biomaterials, 2004, 25(15): 2927-2932.
[3] CHU C L, ZHU J C, YIN Z D, LI M W, WANG S D, XING S Z. Hot pressing of hydroxyapatite(HA)-Ti material and stability of HA[J]. Trans Nonferrous Met Soc China, 2000, 10(4): 505-510.
[4] TAKAMASA O, KAZUYUKI H, TOHIYUKI H. New technique for bonding hydroxyapatite ceramics and titanium by the hydrothermal hot-press method[J]. Scripta Materialia, 2005, 52: 767-770.
[5] GROSS K A, BHADANG K A. Sintered hydroxyfluorapatites. Part Ⅲ: Sintering and resultant mechanical properties of sintered blends of hydroxyapatite and fluorapatite[J]. Biomaterials, 2004, 25: 1395-1405.
[6] WANG C Y, DUAN Y R, MARKOVIC B, BARBARA J, HOWLETT C R, ZHANG X D, ZREIQAT H. Proliferation and bone-related gene expression of osteoblasts grown on hydroxyapatite ceramics sintered at different temperature[J]. Biomaterials, 2004, 25: 2949-2956.
[7] HUANG J, BEST S M, BONFIELD W, BROOKS R A, RUSHTON N, JAYASINGHE S N, EDIRISINGHE M J. In vitro assessment of the biological response to nano-sized hydroxyapatite[J]. Journal of Materials Science: Materials in Medicine, 2004, 15: 441-445.
[8] CHU C L, ZHU J C, YIN Z D, WANG S D. Hydroxyapatite-Ti functionally graded biomaterial fabricated by powder metallurgy[J]. Mater Sci Eng A, 1999, 271: 95-100.
[9] PANG P S, LI W, LIU Y. Effect of ball milling process on the microstructure of titanium-nanohydroxyapatite composite powder[J]. Rare Metals, 2007, 26(2): 118-123.
[10] PANG P S, LI W, LIU Y, XIA D S. Preparation of hydroxyapatite-titanium nano-composites powder by mechanical milling ante it microstructure[J]. Special Casting and Nonferrous Alloys, 2007, 27(1): 068-070. (in Chinese)
[11] FILIAGGI M J, PILLIAR R M, COOMBS N A. Post-plasma-spraying heat treatment of the HA coating/Ti-6Al-4V implant system[J]. Journal of Biomedical Materials Research, 1993, 27(2): 191-198.
[12] ERGUN C, DOREMUS R, LANFORD W. Hydroxyapatite and titanium: interfacial reactions[J]. J Biomed Mater Res A, 2003, 65(3): 336-343.
[13] NAD S, SHARMA P, ROY I, MAITRA A. Anomalous nanostructured titanium dioxide[J]. J Colloid Interface Sci, 2003, 264(1): 89-94.
[14] INAGAKI M, YOKOGAWA Y, KAMEYAMA T. Bond strength improvement of hydroxyapatite/titanium composite coating by partial nitriding during RF-thermal plasma spraying[J]. Surface and Coatings Technology, 2003, 173(1): 1-8.
[15] ZHENG X B, HUANG M H, DING C X. Bond strength of plasma-sprayed hydroxyapatite/Ti composite coatings[J]. Biomaterials, 2000, 21(8): 841-849.
(Edited by HE Xue-feng)
Foundation item: Project (2006B35801001) supported by the Science and Technology Key Project of Guangdong Province, China
Corresponding author: LI Wei; Tel:+86-20-85222167; E-mail: liweijn@yahoo.com.cn


