DOI: 10.11817/j.ysxb.1004.0609.2021-37730
阴极改性对含钛废渣电脱氧过程的影响
王 博1, 2,陈朝轶1, 2,李军旗1, 2,王林珠1, 2,兰苑培1, 2,王仕愈1, 2,申园园1, 2
(1. 贵州大学 材料与冶金学院,贵阳 550025;
2. 贵州省冶金工程与过程节能重点实验室,贵阳 550025)
摘 要:
以CaCl2为熔盐电解质,在900 ℃、3.1 V、电解9 h条件下,对含钛废渣进行熔盐电脱氧,利用泡沫镍包裹烧结片作为阴极,石墨棒为阳极,研究碳粉掺杂量对阴极微观形貌与电解效果的影响,并分析电脱氧历程。结果表明:烧结后阴极片孔隙率随着碳粉掺杂量的增加而增大,当碳粉掺杂量为5%时,阴极片孔隙率为44.33%,具有较好的电化学活性,电解产物中Ti含量达到94.06%,颗粒尺寸均匀,呈海绵形态,与未添加碳粉相比,Ti含量增加了4.16%;当碳粉掺杂量达到10%时,阴极孔隙率增加至49.56%,颗粒间连接度降低,使得电化学活性下降,电脱氧效果明显变差。电解初期电流较高,25 min后迅速下降,并趋于稳定;掺杂适量碳粉有利于阴极电脱氧,脱氧历程为:CaTiO3→Ti2O3→TiO→TixO(x>1)→Ti。
关键词:
文章编号:1004-0609(2021)-02-0470-09 中图分类号:TF823 文献标志码:A
引文格式:王 博, 陈朝轶, 李军旗,等. 阴极改性对含钛废渣电脱氧过程的影响[J]. 中国有色金属学报, 2021, 31(2): 470-478. DOI: 10.11817/j.ysxb.1004.0609.2021-37730
WANG Bo, CHEN Chao-yi, LI Jun-qi, et al. Effect of cathode modification on electro-deoxidation process of waste slag containing titanium[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(2): 470-478. DOI: 10.11817/j.ysxb.1004.0609.2021-37730
Kroll法生产海绵钛的过程中会产生大量含钛废渣[1],直接返回熔炼炉,由于粒度较为细小,添加量超过15%会堵塞炉膛,导致利用率较低。经过近20年的研究,证实了熔盐电脱氧(FFC法[2-6]),制备金属钛[7]、铬[8]、锆[9]等具有一定的优越性,但多数以纯金属氧化物为原料,以含钛废渣为原料的研究较少,现有研究表明阴极结构对电脱氧速率产生显著影响,课题组前期的探索也发现了同样的规律[10-12]。
施瑞盟等[13]对熔盐电解动力学研究发现,钛精矿电还原速率受氧离子在产物层中的扩散控制,增加阴极孔隙率可有效缩短内扩散距离;李泽全等[14]研究表明金属氧化物脱氧缓慢是因为较低的金属/电解质接触界面,改变阴极孔隙率可提高TiO2的脱氧能力。因此,通过阴极改性获得较为适宜的孔隙率是提高阴极活性的重要因素[15]。
阴极结构改性主要有改变烧结温度、成型压力、掺杂以及包覆等方式[16-18]。LIU等[19]将阴极烧结温度从900 ℃增加至1200 ℃时发现,提高温度会降低阴极电导率,阴极的孔隙率对还原过程有直接影响,孔隙率增加有利于形成中间产物(CaTiO3)进而增加电流效率。杜继红等[20]研究表明,阴极掺杂适量的碳酸钙,可以改变烧结后阴极片孔隙率,并改变TiO2颗粒尺寸大小,对阴极电解反应起到促进作用;周忠仁等[21]通过向阴极片掺杂NH4HCO3的方式来增加孔隙率,促进电解;ZHAO等[22]在1123 K、4.0 V条件下,向TiO2前驱体中掺杂碳,电解5 h研究发现,碳粉的掺杂会导致中间产物TiCl3的生成,增加金属钛的获得效率,并指出Ti3+离子通过T3+→Ti2+→Ti两步反应机制还原为金属Ti。
目前,针对含钛废渣阴极掺杂碳的研究尚无人报道,电解过程中的影响规律和作用机制还不够明确。因此,本文以含钛废渣为原料,重点研究阴极掺杂不同碳含量对电解的影响,并分析电脱氧历程,为含钛废渣的高效利用提供理论依据。
1 实验
1.1 实验材料与表征手段
实验用含钛废渣取自贵州遵义钛厂,采用EDS能谱检测含钛废渣化学成分,分析三次取平均值,得出的原矿化学成分(质量分数)为O:40.65%;Mg:0.28%;Al:1.22%;Si:2.01%;Ca:0.65%;Ti:50.15%;Mn:1.43%;Fe:3.60%。XRD物相分析如图1所示,含钛废渣主要物相为FeTi2O5、Ti3O5、TiO2等钛的氧化物。
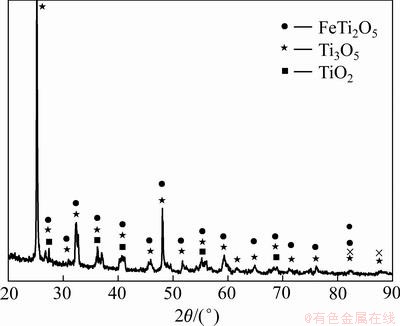
图1 含钛废渣的XRD谱
Fig. 1 XRD patterns of titanium slag
利用韩国库赛姆EM-30PLUS型扫描电镜SEM-EDS分析阴极产物形貌和成分,荷兰帕纳科公司X’Pert PRO MPD型X射线衍射仪分析阴极的物相结构。利用阿基米德原理测量阴极片的孔隙率。
1.2 实验方法
将适量碳粉掺入含钛废渣中,在研钵中充分研磨,采用8%(质量分数)的液体石蜡为黏结剂,混合均匀后,在4 MPa压力下压制成直径为15 mm、厚度为4 mm的圆片,然后于1050 ℃烧结2 h,最后随炉冷却至室温。
用泡沫镍包裹烧结片,以铁铬铝丝为引线组装成阴极。将CaCl2在120 ℃干燥去除自由水,然后升温至550 ℃去除结合水。预电解实验在氩气保护下、2.0 V、900℃,电解2 h。
预电解后,电压调至3.1 V 电解9 h;电解结束后降至室温,全程通入氩气进行气氛保护,然后取出试样,用蒸馏水及1%稀盐酸冲洗,干燥,进行后续测试。电解示意图如图2所示。
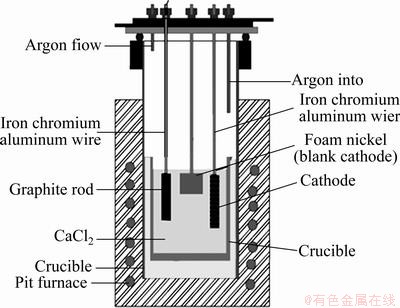
图2 实验装置示意图
Fig. 2 Schematic diagram of experiment device
2 结果与讨论
2.1 碳掺杂量对阴极形貌和组成的影响
不同碳粉掺杂量烧结后阴极的SEM像如图3所示。由图3可知,随着碳粉掺杂量增加,阴极孔隙率及孔隙尺寸明显上升且微小颗粒变大。这是因为在1050 ℃烧结时,碳粉与空气中的氧气反应生成CO或CO2,同时碳粉的加入对微细颗粒会产生一定还原作用,形成金属后发生团聚、烧结效应,之后由于金属的氧化,阴极片孔隙增多[23]。同时碳氧反应是一个放热反应,随着掺杂量的增多反应会变得更剧烈,相应孔隙越多。
图4所示为不同碳粉掺杂量所对应阴极片孔隙率。由图4可知,随着碳粉添加量的增加,阴极孔隙率几乎呈线性增加,每增加1%碳粉含量,孔隙率增加1.25%,这是因为在烧结前相同成型压力压制的阴极颗粒间的黏结程度相差不大,碳粉所占的空间与碳粉加入量呈正比关系,因此孔隙比例同碳粉加入量呈正比关系,这一结论与周忠仁等[21]研究现象类似。利用热力学软件FactSage7.2计算,以100 g钛渣为原料,在1050 ℃烧结达到热力学平衡时,碳粉掺杂量由0增加到10 g,气体生成量如图5所示,与前述结果一致。
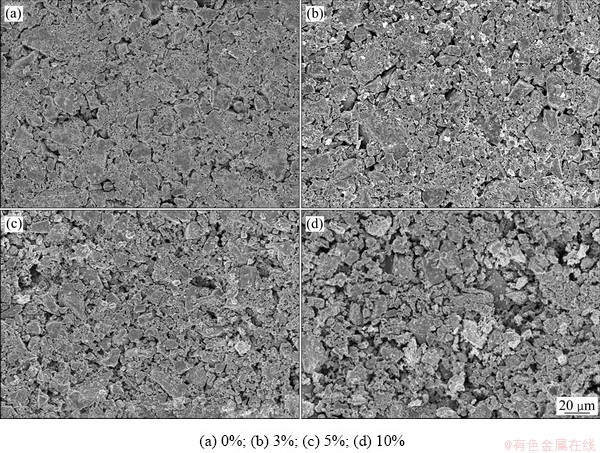
图3 不同掺碳量烧结后的阴极SEM像
Fig. 3 SEM images of cathode sintered with different carbon contents
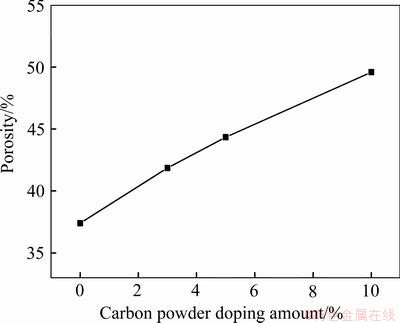
图4 孔隙率随碳粉掺杂量变化曲线
Fig. 4 Porosity change curve with carbon increasing
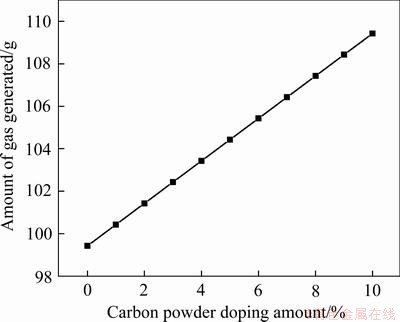
图5 气体产生量随碳粉掺杂量变化曲线
Fig. 5 Change curve of gas generation with increase of carbon doping amount
图6所示为不同碳粉掺杂量烧结后阴极的XRD谱。由图6可知,烧结后的阴极XRD衍射峰相同,物相主要为金红石型TiO2,掺杂量为10%时还有少量的Fe2TiO5,原矿中Ti3O5衍射峰消失,原因是温度低于700 ℃时,主要为Ti3O5氧化转化为锐钛型TiO2,且有少量的锐钛矿向金红石转化;钛渣在650 ℃时反应主要为Ti3O5氧化生成TiO2的过程。分析得出碳粉的添加并未改变烧结后阴极产物组成,只改变阴极孔隙率。
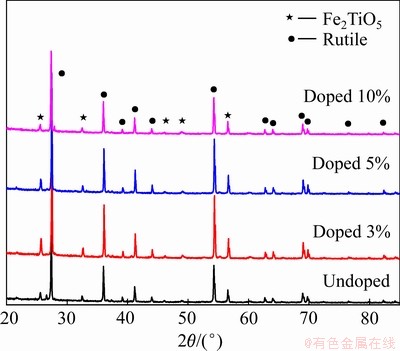
图6 不同碳粉添加量烧结后阴极XRD谱
Fig. 6 XRD patterns of cathode with different amounts of carbon powder
2.2 碳掺杂对电解产物形貌与元素分布的影响
图7所示为不同碳粉掺杂量阴极片电解9 h后的SEM像。由图7可知,随着碳粉掺杂量的增加,电解产物颗粒团聚程度降低、粒径减小,孔隙率呈增加的趋势;碳粉添加量为5%时,电解产物呈明显海绵态,颗粒较为均匀;当碳粉的添加量增加至10%时,颗粒大小变得不均匀,小颗粒数量明显增加,孔隙率在该范围内达到最大,颗粒间的黏结程度下降。
不同碳粉添加量阴极电解产物的元素分布如表1所列,孔隙率的增大会使钛/熔融CaCl2界面扩大,有利于熔盐渗入阴极溶解O2-,增加了电极的有效面积,且增加熔盐迁移至阴极内部的量,加速了电脱氧的进行,同时孔隙率的增加也能降低阴极附近氧的局部饱和度。但随着掺杂量的增加,孔隙率过大,颗粒间连接变得疏松,不利于电子传导,降低阴极电化学活性,限制电解的进行,导致产物中金属钛含量较低。电脱氧受熔盐的溶解O2-和电子导电共同决定。因此阴极掺杂碳粉的含量应在适当的范围内。
2.3 碳掺杂对电解过程的影响
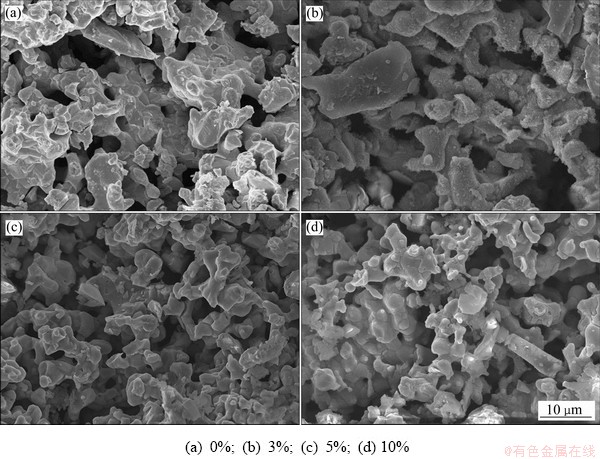
图7 不同碳粉添加量阴极电解产物SEM像
Fig. 7 SEM images of cathode electrolysis products with different carbon powder additions
表1 不同碳粉添加量阴极电解产物元素分布
Table 1 Element distribution of cathode electrolysis products with different carbon powder addition amounts
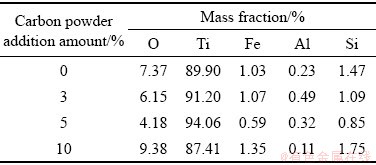
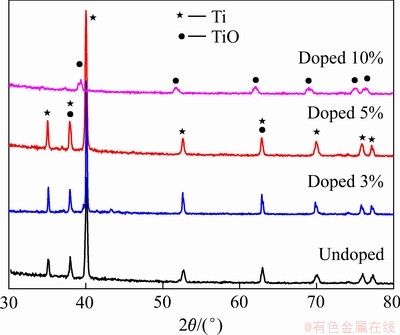
图8 不同碳粉添加量电解产物的XRD谱
Fig. 8 XRD patterns of electrolytic products with different amounts of powder addition
不同碳粉掺杂量电解9 h的XRD谱如图8所示,随着碳粉掺杂量的增加,产物的结晶度和衍射峰强度增高。当碳粉掺杂量低于5%时,电解产物的物相主要为Ti和TiO;当碳粉掺杂量为10%时,物相主要为TiO。
Ca2++2TiO2+2e=CaTiO3+TiO
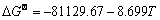 (J/mol) (1)
(J/mol) (1)
当碳粉掺杂量为5%时,900 ℃熔盐浸泡2 h及不同电解时间电解产物XRD谱如图9所示。根据式(1),在900 ℃条件下CaO与TiO2反应生成CaTiO3的 -91.33 kJ,能自发进行。随着碳粉添加量的增加,孔隙率增大,大量CaTiO3还原为钛的低价氧化物(Ti2O3, TiO),所以中间产物(CaTiO3)的形成是一个必要的过程。表2所列为不同价态的钛离子间相互转化的标准电极电位。由表2可知,Ca2+/Ca的标准电极电位为-2.866 V,(Ti4+,Ti3+)/Pt的标准电极电位为-0.04 V,远低于(Ti4+,Ti3+)/Pt的电极电位;900 ℃氩气保护下,TiO2处于低氧环境,促使TiO2晶体结构中形成氧空位[24],并产生O2-。在外加电场的情况下Ca2+定向迁移至阴极,形成置换杂质原子,Ca2+逐渐取代Ti4+形成CaTiO3和TiO;根据还原电位的大小,之后主反应为CaTiO3得电子生成钛的低价氧化物如式(3)、(4)所示;最终Ti2+被置换出来并直接从阴极得到电子形成金属钛单质[25-26]。因电解电压为3.1 V,Ca2+/Ca的标准电极电位为-2.866 V,会有少量的Ca2+被还原成金属Ca,金属Ca与钛的化合物发生钙热还原反应[27-28];基于TiO2的电脱氧历程[29-31]和实验结果,含钛废渣与TiO2的电脱氧历程一致为:CaTiO3→Ti2O3→ TiO→ TixO(x>1)→Ti。电解反应是从阴极表面到内部的渐进过程,所以TiO2阴极内部的氧空位是从电极表面传递到电极内部的过程,同时伴随着O2-从电极内部到电极表面的传质过程,孔隙率过高或者过低都不利于O2-的传质,限制电解的进行。晶体型变如图10所示。
-91.33 kJ,能自发进行。随着碳粉添加量的增加,孔隙率增大,大量CaTiO3还原为钛的低价氧化物(Ti2O3, TiO),所以中间产物(CaTiO3)的形成是一个必要的过程。表2所列为不同价态的钛离子间相互转化的标准电极电位。由表2可知,Ca2+/Ca的标准电极电位为-2.866 V,(Ti4+,Ti3+)/Pt的标准电极电位为-0.04 V,远低于(Ti4+,Ti3+)/Pt的电极电位;900 ℃氩气保护下,TiO2处于低氧环境,促使TiO2晶体结构中形成氧空位[24],并产生O2-。在外加电场的情况下Ca2+定向迁移至阴极,形成置换杂质原子,Ca2+逐渐取代Ti4+形成CaTiO3和TiO;根据还原电位的大小,之后主反应为CaTiO3得电子生成钛的低价氧化物如式(3)、(4)所示;最终Ti2+被置换出来并直接从阴极得到电子形成金属钛单质[25-26]。因电解电压为3.1 V,Ca2+/Ca的标准电极电位为-2.866 V,会有少量的Ca2+被还原成金属Ca,金属Ca与钛的化合物发生钙热还原反应[27-28];基于TiO2的电脱氧历程[29-31]和实验结果,含钛废渣与TiO2的电脱氧历程一致为:CaTiO3→Ti2O3→ TiO→ TixO(x>1)→Ti。电解反应是从阴极表面到内部的渐进过程,所以TiO2阴极内部的氧空位是从电极表面传递到电极内部的过程,同时伴随着O2-从电极内部到电极表面的传质过程,孔隙率过高或者过低都不利于O2-的传质,限制电解的进行。晶体型变如图10所示。
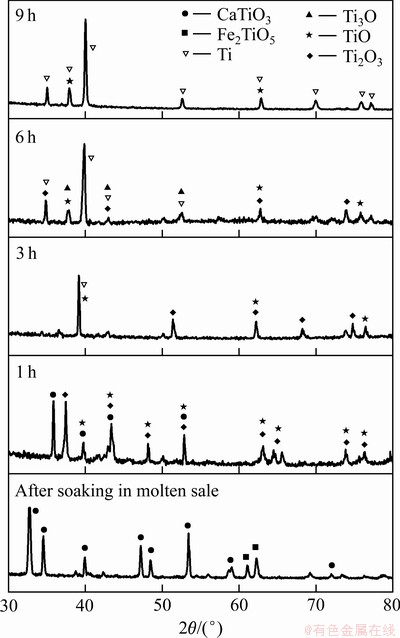
图9 不同电解时间电解产物XRD谱
Fig. 9 XRD patterns of electrolytic products at different electrolysis time
表2 标准电极电位
Table 2 Standard electrode potential

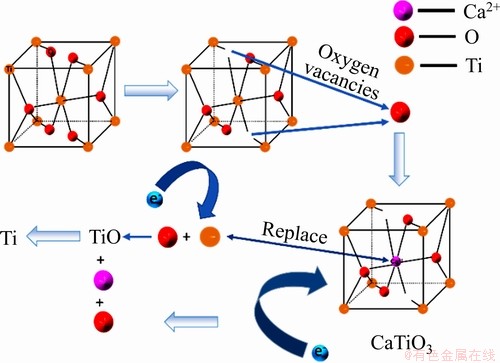
图10 晶体型变示意图
Fig. 10 Schematic diagram of crystal configuration change
Ca2++O2-+TiO2→CaTiO3 (2)
2CaTiO3+2e→Ti2O3+2Ca2++3O2- (3)
Ti2O3+2e→2TiO+Ca2++2O2- (4)
TiO+2e→Ti+O2- (5)
2.4 碳掺杂对电解电流的影响
图11所示为不同碳粉掺杂量电解9 h过程中电流-时间曲线。电解初期阴极与泡沫镍紧密接触,电脱氧主要通过电化学反应进行,发生在电极表面,使得初始电流较大。随着电解的进行,阴极中的氧逐渐被除去,一方面O2-传输距离增加,另一方面钛的低价氧化物中氧元素存在于金属钛的晶格中,需要更高的驱动力,二者均会导致电流的下降[32]。当阴极孔隙率较低时,电极内部存在闭合孔隙,三相界面较少;孔隙率过高时,颗粒间连接较疏松,且产物在高孔隙率下具有更高的电阻[19]。两者均不利于电子的传导,导致电解过程中整体电流较小。当碳粉掺杂量为3%时,当电解进行到2 h后,阴极片中产生金属化和烧结作用,孔隙率稍有增加,有利于形成更多的三相界面,电脱氧速率增加,因此,电流有所增加。当碳粉掺杂量为5%时,由于孔隙率较佳,电流始终较大,电脱氧效果较好。
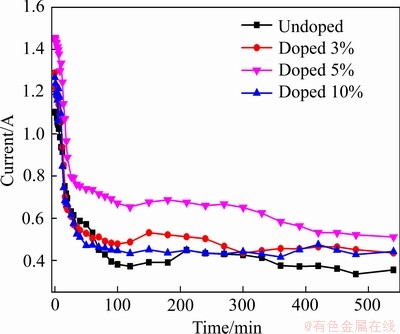
图11 不同碳粉添加量电解过程电流-时间曲线
Fig. 11 Current-time curves of electrolytic process with different amounts of carbon powder
3 结论
1) 在含钛废渣中掺杂碳粉,经烧结后孔隙率和细小颗粒尺寸变大,且随碳粉掺杂量而增加。
2) 阴极掺杂碳粉有利于改变其电化学活性,但当碳粉掺杂量为10%时,较大的孔隙率会导致烧结后的阴极片颗粒黏结程度较小,电子传导阻碍较大,降低阴极电化学活性,不利于电解,电脱氧受熔盐的溶解O2-和电子导电共同决定。当碳粉掺杂量为5%时,电解产物的颗粒尺寸最为均匀,Ti含量较高,且脱氧效果较好。
3) TiO2电解脱氧是分步进行的,合适的孔隙率有利于氧空位的形成及O2-的传质,加速电解的进行。含钛废渣电解制备金属钛的阴极电脱氧历程为:CaTiO3→Ti2O3→TiO→TixO(x>1)→Ti。
REFERENCES
[1] 王碧侠, 兰新哲, 赵西成, 等. 金属钛制备方法的研究进展[J]. 轻金属, 2005(12): 44-49.
WANG Bi-xia, LAN Xin-zhe, ZHAO Xi-chen, et al. Progress in the research of titanium preparation[J]. Light Metals, 2005(12): 44-49.
[2] ZHOU Zhon-gren, ZHANG Ying-jie, HUA Yi-xin, et al. Verification of the electro-decomposition of the CaO component in equimolar CaCl2-NaCl molten salt during the direct electrolysis of ilmenite in a two-terminal chamber[J]. Electrochimica Acta, 2018, 271: 490-497.
[3] YUAN Tie-chui, WENG Qi-gang, ZHOU Zhi-hua, et al. Preparation of high-purity titanium by molten-salt electrolysis process[J] Advanced Materials Research, 2011, 284/286: 1477-1482.
[4] 范科彪, 郑雅杰, 何汉兵. 阳极导电材料对铁钨锡合金粉电解铁的影响[J]. 中国有色金属学报, 2020, 30(4): 866-874.
FAN Ke-biao, ZHENG Ya-jie, HE Han-bing. Effects of anode conductive materials on electrolytic iron of Fe-W-Sn alloy powder[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(4): 866-874.
[5] 王碧侠, 李建新, 马兴飞, 等. 工业纯钛TA2熔盐电解法渗硼的渗层生长动力学[J]. 中国有色金属学报, 2019, 29(1): 131-137.
WANG Bi-xia, LI Jian-xin, MA Xing-fei, et al. Growth kinetics of boride layer produced by molten salt electrolytic boriding on TA2[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(1): 131-137.
[6] 范科彪, 郑雅杰, 王 钫, 等. 电解回收铁钨锡合金粉中的铁[J]. 中国有色金属学报, 2019, 29(10): 2421-2432.
FAN Ke-biao, ZHENG Ya-jie, WANG Fang, et al. Recovery iron from Fe-W-Sn alloy powders by electrolysis[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(10): 2421-2432.
[7] 邹星礼, 鲁雄刚. 攀枝花含钛高炉渣直接制备钛合金[J]. 中国有色金属学报, 2010, 20(9): 1829-1835.
ZOU Xing-li, LU Xiong-gang. Titanium alloy was directly prepared from panzhihua titanium-bearing furnace slag[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(9): 1829-1835.
[8] LIU Zheng-wei, ZHANG Hong-ling, PEI Li-li, et al. Direct electrolytic preparation of chromium metal in CaCl2-NaCl eutectic salt[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(2): 376-384.
[9] LI Qing-yu, DU Ji-hong, XI Zeng-ping. Preparation of zirconium by electro-deoxidization in molten salt[J]. Transactions of Nonferrous Metals Society of China, 2007(S1): 560-564.
[10] CHEN Chao-yi, ZHAN Chong, LI Jun-qi, et al. Direct Extraction of Ti and Ti alloy from Ti-bearing dust slag in molten CaCl2[J]. High Temperature Materials and Processes, 2015, 35(6): 591-597.
[11] 陈朝轶, 鲁雄刚, 李军旗, 等. 阴极结构对SOM法制备金属钽的影响[J]. 稀有金属材料与工程, 2012, 41(3): 522-526.
CHEN Chao-yi, LU Xiong-gang, LI Jun-qi, et al. Effect of cathode structure on reduction of Ta2O5 using SOM process[J]. Rare Metal Materials and Engineering, 2012, 41(3): 522-526
[12] 陈朝轶, 张 曼, 李军旗, 等. 含钛渣短流程直接制备钛及其合金[J]. 武汉科技大学学报, 2014, 37(4): 250-253.
CHEN Chao-yi, ZHANG Man, LI Jun-qi, et al. Direct prepara of Ti and its alloy from Ti-bearing slag[J]. Journal of Wuhan University of Science and Technology, 2014, 37(4): 250-253.
[13] 施瑞盟, 白晨光, 吕学伟. 钛精矿熔盐电解动力学研究[J]. 稀有金属材料与工程, 2016, 45(4): 907-912.
SHI Rui-meng, BAI Chen-guang, Lü Xue-wei. Kinetics of Ilmenite concentrate electrolysis process in molten salt[J]. Rare Metal Materials and Engineering, 2016, 45(4): 907-912.
[14] 李泽全, 茹黎月, 白晨光, 等. 电脱氧法电解效率主要影响因素的研究进展[J]. 稀有金属, 2011, 35(2): 301-307.
LI Ze-quan, RU Li-yue, BAI Cen-guang, et al. Progress in main influence factors for electrolysis efficiency of electro-deoxidation[J]. Chinese Journal of Rare Metals, 2011, 35(2): 301-307.
[15] WANG Feng, ZHANG Jin-qiang, DING Xing, et al. Metal coordination mediated reversible conversion between linear and cross-linked supramolecular polymers[J]. Angewandte Chemie, 2010, 49(6): 1090-1094.
[16] WANG Bo, CHEN Chao-yi, LI Jun-qi, et al. Solid oxide membrane-assisted electrolytic reduction of Cr2O3 in molten CaCl2[J]. International Journal of Minerals Metallurgy and Materials, 2020, 194(12): 60-68.
[17] ZHOU Zhong-ren, HUA Yi-xin, XU Cun-ying, et al. Preparation of ferrotitanium from ilmenite by electrolysis- assisted calciothermic reduction in CaCl2-NaCl molten salt[J]. JOM, 2016, 68(2): 532-539
[18] WANG Bo, CHEN Chao-yi, LI Jun-qi, et al. Production of Fe-Ti alloys from mixed slag containing titanium and Fe2O3 via direct electrochemical reduction in molten calcium chloride[J]. Metals—Open Access Metallurgy Journal, 2020, 10(12): 1611-1626.
[19] LIU Xu-yang, HU Mei-long, BAI Chen-guang, et al. Effect of electrical conductivity and porosity of cathode on electro-deoxidation process of ilmenite concentrate[J]. Rare Metal Materials & Engineering, 2017, 46(5): 1176-1182.
[20] 杜继红, 奚正平, 李晴宇, 等. CaCO3的掺杂对TiO2电解过程的影响[J]. 稀有金属材料与工程, 2007, 36(1): 96-99.
DU Ji-hong, XI Zheng-ping, LI Qing-yu, et al. Impact of CaCO3 doping on TiO2 performances[J]. Rare Metal Materials and Engineering, 2007, 36(1): 96-99.
[21] 周忠仁, 华一新, 徐存英, 等. 孔隙率对熔盐电解法电解还原FeTiO3的影响研究[J]. 有色金属工程, 2017, 7(1): 1-4.
ZHOU Zhong-ren, HUA Yi-xin, XU Cun-ying, et al. Study on the effect of porosity on the electrochemical electrolysis of bulk ilmenite[J]. Nonferrous Metals Engineering, 2017, 7(1): 1-4.
[22] ZHAO Kun, WANG Yao-wu, GAO Feng. Electrochemical extraction of titanium from carbon-doped titanium dioxide precursors by electrolysis in chloride molten salt[J]. Ionics, 2019, 12: 6107-6114.
[23] LI Ze-quan, RU Li-yue, BAI Chen-guang, et al. Effect of sintering temperature on the electrolysis of TiO2[J]. International Journal of Minerals Metallurgy and Materials, 2012, 19(7): 636-641.
[24] 李泽全, 张 娜, 白晨光, 等. TiO2电极中O2-的形成及迁移过程研究[J]. 稀有金属材料与工程, 2010, 39(3): 473-476.
LI Ze-quan, ZHANG Na, BAI Chen-guang, et al. Study on formation and transportation process of O2- in TiO2 electrode[J]. Rare Metal Materials and Engineering, 2010, 39(3): 473-476.
[25] 申园园, 陈朝轶, 李军旗, 等. SOM法电解过细高钛渣制备金属钛[J]. 稀有金属材料与工程, 2019, 48(5): 1671-1676.
SHEN Yuan-yuan, CHEN Chao-yi, LI Jun-qi, et al. Preparation of titanium by SOM electrolytic process from ultrafine high titanium slag[J]. Rare Metal Materials and Engineering, 2019, 48(5): 1671-1676.
[26] WENG Qi-gang, LI Rui-di, YUAN Tie-chui, et al. Valence states, impurities and electrocrystallization behaviors during molten salt electrorefining for preparation of high-purity titanium powder from sponge titanium[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2): 553-560.
[27] KATSUTOSHI O, RYOSUKE O S. A new concept for producing Ti sponge: Calciothermic reduction[J]. JOM, 2002, 54(2): 59-61.
[28] RYOSUKE O S, KOH T, KATSUTOSHI O. Calciothermic reduction of titanium oxide and in-situ electrolysis in molten CaCl2[J]. Metallurgical & Materials Transactions B, 2003, 34(3): 287-295.
[29] WANG Bin, LIU Kui-ren, CHEN Jian-she. Reaction mechanism of preparation of titanium by electro-deoxidation in molten salt[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(10): 2327-2331.
[30] ALEXANDER D T L, SCHWANDT C, FRAY D J. The electro-deoxidation of dense titanium dioxide precursors in molten calcium chloride giving a new reaction pathway[J]. Electrochimica Acta, 2011, 56(9): 3286-3295.
[31] 万贺利, 徐宝强, 戴永年, 等. 钙热还原二氧化钛的钛粉制备及其中间产物CaTiO3的成因[J]. 中国有色金属学报, 2015, 22(7): 2075-2081.
WAN He-li, XU Bao-qiang, DAI Yong-nian, et al. Preparation of titanium powders by calciothermic reduction process of titanium dioxide and formation cause of intermediate CaTiO3[J]. The Chinese Journal of Nonferrous Metals, 2015, 22(7): 2075-2081.
[32] 王 震, 李 坚, 华一新, 等. 熔盐电解法生产钛铁合金的铁-钛氧化物电极材料的制备[J]. 稀有金属, 2016, 40(3): 252-260.
WANG Zhen, LI Jian, HUA Yi-xin, et al. Preparation of Fe-Ti oxide-electrode material for production of Ti-Fe alloy by molten salt electrolysis[J]. Chinese Journal of Rare Metals, 2016, 40(3): 252-260.
Effect of cathode modification on electro-deoxidation process of waste slag containing titanium
WANG Bo1, 2, CHEN Chao-yi1, 2, LI Jun-qi1, 2, WANG Lin-zhu1, 2, LAN Yuan-pei1, 2, WANG Shi-yu1, 2, SHEN Yuan-yuan1, 2
(1. College of Materials and Metallurgy, Guizhou University, Guiyang 550025, China;
2. Guizhou Province Key Laboratory of Metallurgical Engineering and Process Energy Saving, Guiyang 550025, China)
Abstract: Titanium was prepared successfully from waste slag containing titanium by using CaCl2 molten salt as electrolyte, sintered sheet wrapped with nickel foam as cathode, and graphite as anode at 900 ℃ with cell voltages of 3.1 V under inert atmosphere. The influence of carbon doping amount on microstructures of reduced pellets, effect of electrolysis was also discussed. In addition, the process of electro-deoxidation were analyzed. The results demonstrate that the porosity of the cathode sheet after sintering increases with the increase of the amount of carbon powder. The porosity of the cathode sheet is 44.33% with 5% dopant, which has good electrochemical activity. The Ti content reached 94.06% which is higher than nodoped sample (the Ti content increased by 4.16%), and the particle size in the form of a sponge is uniform. When the carbon powder doped amount reaches 10%, the cathode porosity increases to 49.56%, and the inter-particle decreases connectivity which reduces electrochemical activity and the effect of electro-deoxidation significantly. The initial current of electrolysis was high, which decreases rapidly after 25 min and becomes stable. Doping with an appropriate amount of carbon powder is beneficial to the cathodic deoxidation. The deoxidation process is: CaTiO3→Ti2O3→TiO→TixO(x>1)→Ti.
Key words: waste slag containing titanium; molten salt electrolysis; titanium; doped
Foundation item: Projects(51664005, 51664004, 51774102) supported by the National Natural Science Foundation of China; Project(KY[2015]334) supported by Education Department of Guizhou, China; Projects(Platform Talent [2017] 5788, Talent Team Giant [2017]5626, KY(2015)334) supported by Talents of Guizhou Science and Technology Cooperation Platform, China
Received date: 2020-03-18; Accepted date: 2020-06-15
Corresponding author: CHEN Chao-yi; Tel: +86-15086015817; E-mail: Ccy197715@126.com
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51664005,51664004,51774102);贵州省教育厅项目(黔教合KY[2015]334);贵州省科技计划项目(黔科合平台人才[2017]5626,[2017]5788)
收稿日期:2020-03-18;修订日期:2020-06-15
通信作者:陈朝轶,教授,博士;电话:15086015817;E-mail:Ccy197715@126.com
摘 要:以CaCl2为熔盐电解质,在900 ℃、3.1 V、电解9 h条件下,对含钛废渣进行熔盐电脱氧,利用泡沫镍包裹烧结片作为阴极,石墨棒为阳极,研究碳粉掺杂量对阴极微观形貌与电解效果的影响,并分析电脱氧历程。结果表明:烧结后阴极片孔隙率随着碳粉掺杂量的增加而增大,当碳粉掺杂量为5%时,阴极片孔隙率为44.33%,具有较好的电化学活性,电解产物中Ti含量达到94.06%,颗粒尺寸均匀,呈海绵形态,与未添加碳粉相比,Ti含量增加了4.16%;当碳粉掺杂量达到10%时,阴极孔隙率增加至49.56%,颗粒间连接度降低,使得电化学活性下降,电脱氧效果明显变差。电解初期电流较高,25 min后迅速下降,并趋于稳定;掺杂适量碳粉有利于阴极电脱氧,脱氧历程为:CaTiO3→Ti2O3→TiO→TixO(x>1)→Ti。
[1] 王碧侠, 兰新哲, 赵西成, 等. 金属钛制备方法的研究进展[J]. 轻金属, 2005(12): 44-49.
[4] 范科彪, 郑雅杰, 何汉兵. 阳极导电材料对铁钨锡合金粉电解铁的影响[J]. 中国有色金属学报, 2020, 30(4): 866-874.
[5] 王碧侠, 李建新, 马兴飞, 等. 工业纯钛TA2熔盐电解法渗硼的渗层生长动力学[J]. 中国有色金属学报, 2019, 29(1): 131-137.
[6] 范科彪, 郑雅杰, 王 钫, 等. 电解回收铁钨锡合金粉中的铁[J]. 中国有色金属学报, 2019, 29(10): 2421-2432.
[7] 邹星礼, 鲁雄刚. 攀枝花含钛高炉渣直接制备钛合金[J]. 中国有色金属学报, 2010, 20(9): 1829-1835.
[11] 陈朝轶, 鲁雄刚, 李军旗, 等. 阴极结构对SOM法制备金属钽的影响[J]. 稀有金属材料与工程, 2012, 41(3): 522-526.
[12] 陈朝轶, 张 曼, 李军旗, 等. 含钛渣短流程直接制备钛及其合金[J]. 武汉科技大学学报, 2014, 37(4): 250-253.
[13] 施瑞盟, 白晨光, 吕学伟. 钛精矿熔盐电解动力学研究[J]. 稀有金属材料与工程, 2016, 45(4): 907-912.
[14] 李泽全, 茹黎月, 白晨光, 等. 电脱氧法电解效率主要影响因素的研究进展[J]. 稀有金属, 2011, 35(2): 301-307.
[20] 杜继红, 奚正平, 李晴宇, 等. CaCO3的掺杂对TiO2电解过程的影响[J]. 稀有金属材料与工程, 2007, 36(1): 96-99.
[21] 周忠仁, 华一新, 徐存英, 等. 孔隙率对熔盐电解法电解还原FeTiO3的影响研究[J]. 有色金属工程, 2017, 7(1): 1-4.
[24] 李泽全, 张 娜, 白晨光, 等. TiO2电极中O2-的形成及迁移过程研究[J]. 稀有金属材料与工程, 2010, 39(3): 473-476.
[25] 申园园, 陈朝轶, 李军旗, 等. SOM法电解过细高钛渣制备金属钛[J]. 稀有金属材料与工程, 2019, 48(5): 1671-1676.
[31] 万贺利, 徐宝强, 戴永年, 等. 钙热还原二氧化钛的钛粉制备及其中间产物CaTiO3的成因[J]. 中国有色金属学报, 2015, 22(7): 2075-2081.
[32] 王 震, 李 坚, 华一新, 等. 熔盐电解法生产钛铁合金的铁-钛氧化物电极材料的制备[J]. 稀有金属, 2016, 40(3): 252-260.


