
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1507-1512
Preparation of V-Ti-Fe master alloys by metallothermic reduction method
WANG Bin1, LIU Kui-ren1, CHEN Jian-she1, GAO Teng-yue1, HE Ji-lin2
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China;
2. Ningxia Orient Nonferrous Metals Group Co. Ltd., Shizuishan 753000, China
Received 16 July 2011; accepted 24 October 2011
Abstract:
V-Ti-Fe master alloys were prepared by metallothermic reduction method, and the influences of the mass ratio of V2O5 to TiO2, Al and Al-Mg alloy addition amounts on the metal recovery rates and alloy compositions were investigated. The results show that appropriate technological parameters are: the mass ratio of V2O5 to TiO2 is 0.5:1, Al addition represents 95% of the theoretical value, and the Al-Mg alloy addition amount is one third that of the Al addition. The results from energy spectrum analysis show that V and Fe distribute uniformly in the prepared alloy, while the segregation for Ti, i.e. Ti-rich phase is detected. A spray refining process was carried out to reduce the impurity contents of Al and O in the prepared alloys. The Al content drops from 4.27% to 1.86%, and the O content drops from 2.10% to 0.91% after the refining process.
Key words:
V-Ti-Fe master alloys; metallothermic reduction method; recovery rates; refining process;
1 Introduction
As a kind of clean energy, hydrogen has attracted a significant attention in recent years due to its high energy efficiency and environmental safety. To utilize hydrogen as a energy carrier in large scale, a severe challenge that must be overcome is how to store it. Many investigations have been performed to solve this problem. The storage of hydrogen in metal hydrides is considered to be one of the most attractive solutions for characteristics of large bulk density and safety. Among various kinds of hydrogen storage alloys, V-based solid solution alloys show a large hydrogen capacity of 3.8% (mass fraction), although the efficient capacity is 1.9% (mass fraction), which is higher than that of the LaNi5-based and ZrMn2-based hydrogen storage alloys [1-4].
Ternary V-based solid solutions of Ti-V-Ni [5], Ti-V-Fe [6], Ti-V-Cr [7], Ti-V-Mn [8] and quaternary alloys of Ti-V-Cr-Fe [9] and Ti-V-Cr-Mn [10] have been widely investigated. In the reseraches, the hydrogen storage alloys are generally prepared by smelting pure metals in a induction furnace or electric arc furnace. Some proper heat treatments are executed after the remlting process to improve the hydrogen storage properities of the alloy. Because V and Ti have high melting posints and V is expensive, the manufacturing cost of V-based solid solutions is very high. If the alloys can be acquired from the metal oxides directly, the manufacturing cost would be reduced markedly.
Alloys prepared by metallothermic reduction method usually contain some Al, O and Si inclusions, which is attributed to the selection of raw materials. For the V-based alloys, Al and Si impurities are harmful for the hydrogen storage [11-14]. Research shows [15] that the argon blowing process could remove inclusions from molten steel well. However, there is less study on the effects of argon blowing process on diminishing impurities in V-Ti-Fe alloys. In this work, the master V-Ti-Fe alloys were prepared by reducing metal oxides by a metallothermic reduction method, and the target composition was Ti46V44Fe10 which had a good overall property [16]. Spray refining process was carried out to reduce the impurities of the prepared alloys.
2 Experimental
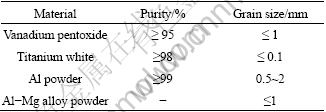
The purities and grain size of the main raw materials for alloy preparation are listed in Table 1. Scrap iron, cryolite, analytical pure CaO, CaF2 and KClO3 were also used in this experiment.
Table 1 Specification of materials
The alloy samples were prepared in a metal bucket with refractory liner, and the heat was 3100 kJ/kg. The schematic diagram of the smelting device is shown in Fig. 1. Because the reduction process of TiO2 is difficult to conduct, besides Al powder, the combined reducing agent (Al-Mg alloy) was also used as the reducing agent to increase the metal recovery rates. In addition, some CaO and CaF2 powders were added for inhibiting the formation of Ti suboxides and promoting the separation of slag-steel. The amounts of CaO and CaF2 were 90% and 35% (mass fraction) of the Al addition amount, respectively. Raw materials without KClO3 were first mixed in a mixing barrel for 1 h, and then KClO3 was introduced into the mixture. After the mixing process, partial burden was first charged into the reactor for ignition. The residual burden was continuously filled into the reactor through the feed inlet during the preparation of target alloys.
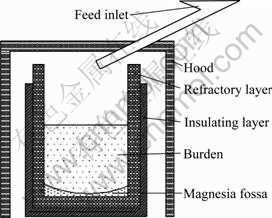
Fig. 1 Schematic diagram of smelting device used for production of V-Ti-Fe master alloys
The refining process was carried out in a YFX12/160-Y6 MoSi2 resistance furnace in argon atmosphere. The refining temperature was 1600 ℃, and the refining time was 10 min. The slag system 50%CaF2-25%CaO-25%Na3AlF6 (mass fraction) was served as the refining agent. The slag materials were injected in the alloy melt by a self-made powder-spraying device, and the argon flow was controlled at 10 L/min. The schematic diagram of the refining device is presented in Fig. 2.
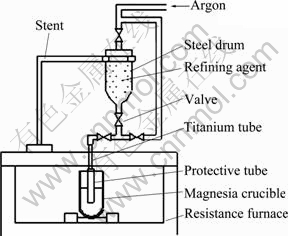
Fig. 2 Schematic diagram of refining device used for reducing impurities in alloys obtained by metallothermic reduction method
The content of oxygen was detected by a TC-436 oxygen and nitrogen analyzer, and the contents of other elements in these alloys were detected by chemical analysis. The crystalline phases of the prepared alloys were identified by a D/max-2500PC X-ray diffractometer (Japan) with Cu Kα radiation at a pip voltage of 40 kV and a current of 100 mA. The microstructure and chemical compositions were characterized by scanning electron microscope (SEM, SSX-550, Japan).
3 Results and discussion
3.1 Influences of mass ratio of V2O5 to TiO2 on metal recovery rates and compositions of prepared alloys
It is well known that the activity of V2O5 is different from that of TiO2, so the mass ratio of V2O5 to TiO2 should not be designed according to the theoretical value absolutely. The influence of the mass ratio of V2O5 to TiO2 on the metal recovery rates and the compositions of the alloys was investigated, and the results are shown in Table 2 and Fig. 3. With decreasing mass ratio of V2O5 to TiO2, the recovery rates of Ti and V decrease along with an increase in the contents of Al and O impurity. When the mass ratio is 0.3, the alloy does not separate with slag well, and most of the alloy inlays in the slag as the granular form. The recovery rates of Ti and V are very low, just 31.7% and 35.0%, respectively. Simultaneously, the contents of Al and O are also much higher than those obtained from other comparative experiments. The reasonable explanation is that with reducing mass ratio of V2O5 to TiO2, more Ti is reduced out from TiO2; however, the excessive Ti could not form alloy with V or Fe efficiently. The density of Ti (4.5 g/cm3) is close to that of the slag (3.2-3.6 g/cm3), therefore, part of Ti metal fails to separate from slag before the solidification process. When the mass ratio of V2O5 to TiO2 rises up to 0.5:1, the slag-metal separation process proceeds smoothly, the recovery rates of V and Ti reach 81% and 55.8%, respectively, and the alloy composition is close to the target composition. When the mass ratio of V2O5 to TiO2 is higher than 0.5:1, although the recovery rates are higher, the alloy composition drifts far from the target composition.
Table 2 Influence of mass ratio of V2O5 to TiO2 on composition of prepared alloys (mass fraction)

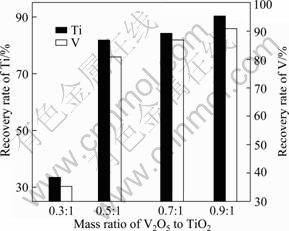
Fig. 3 Influences of mass ratio of V2O5 to TiO2 on recovery rates of V and Ti
The Si content in these alloys is about 0.5% (mass fraction) with a good reproducibility, and no obvious change tendency for Si content can be found, as seen from Table 2. It can be confirmed that a considerable proportion of Si impurity is derived from refractory materials.
3.2 Influences of Al addition amount on metal recovery rates and compositions of prepared alloys
As the main reducing agent, the amount of Al addition directly affects the recovery rate of metals and the impurity content. If the Al amount is not enough, residual Al impurity in the alloy would be less, resulting in insufficient reactions. On the other hand, Al impurity increases if the Al addition amount is high.
The influence of the ratio (n) of actual Al dosage to theoretical Al dosage on the metal recovery rates and alloying constituents was also studied. The results are shown in Table 3 and Fig. 4. It can be seen that the Al contents in the alloys are high, with the values of n higher than 0.95. When the n value is 0.85, the slag-iron separation process almost disappears for the lack of reductant, and the recovery rates of V and Ti are only 50.1% and 35.0%, respectively. The alloys are acquired close to the target alloy when the n values reach 0.95 and 0.9. But the Ti recovery rate is higher when the n value is 0.95.
Table 3 Influence of Al addition amount on composition of prepared alloys (mass fraction)
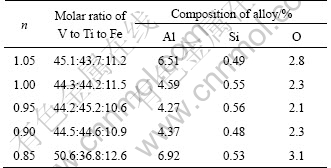
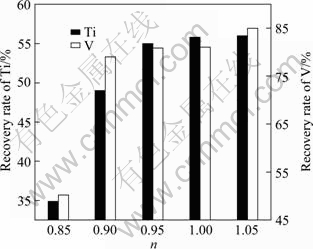
Fig. 4 Influences of Al addition amounts on recovery rates of V and Ti
3.3 Influences of Al-Mg alloy addition amount on metal recovery rates and compositions of prepared alloys
As a common reducing agent, pure Al has a limited reducibility. Moreover, the reduction process of TiO2 is difficult to conduct. So, some combined reducing agent (Al-Mg alloy) should be adopted during the preparation of the alloys. The effect of the mass ratio of Al-Mg alloy to Al was studied, and the results are shown in Table 4 and Fig. 5.
It is clearly observed from Fig. 5 that the V recovery rate increases slightly with an increase in the mass ratio of Al-Mg to Al. When the mass ratio of Al-Mg to Al is lager than 1/4, the V recovery rate basically reaches a stable value of about 80%. Therefore, it can be assumed that the V2O5 reduction process does not require too much strong reductant.
Table 4 Influence of Al-Mg addition amount on composition of prepared alloys (mass fraction)
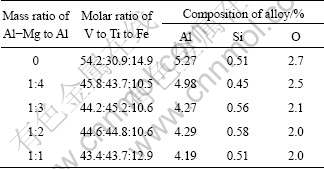
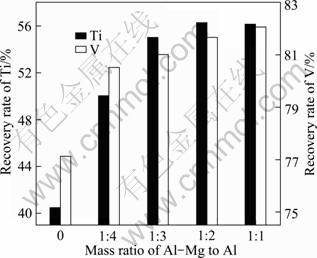
Fig. 5 Influences of Al-Mg addition amounts on recovery rates of V and Ti
Comparing with the recovery rate of V, the recovery rate of Ti varies obviously. When the reductant just consists of Al, the recovery rate of Ti is only 40.5%. With increasing mass ratio of Al-Mg to Al up to 1:3, the recovery rate of Ti is improved, and the reaction process is carried on smoothly. According to the results listed in Table 4, the composition of the alloy is also close to the target composition. When the mass ratio of Al-Mg to Al value is up to 1:2 and 1:1, the reaction process proceeds fiercely, but the recovery rate of Ti does not increase any more. The possible reason is that the Al-Mg alloy is wasted seriously in the smelting process for its high activity. In addition, considering the factor of economic cost, the mass ratio of Al-Mg to Al should not be too high.
When the mass ratio of V2O5 to TiO2 is 0.5:1, Al addition represents 95% of the theoretical values, and the Al-Mg addition amount is one third of the Al addition amount, the composition of the obtained alloy is close to the target composition. Detecting means of XRD, SEM and EDS were applied to characterize the microstructure and further identify the phases of impurities in the obtained alloys.
Figure 6 shows the XRD pattern of the alloy sample obtained by metallothermic reduction method. V-based alloys and Ti-based alloys are the primary phases. Partial Al impurity is detected also in the alloy, existing in the form of V5Al8.
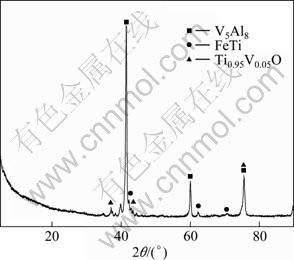
Fig. 6 XRD pattern of alloy obtained by metallothermic reduction method
Figure 7 and Table 5 show the microstructure and chemical compositions of the alloy acquired by metallothermic reduction method. The results of EDS analysis reveal that the grey phase (Area 1) and the dark grey phase (Area 2) are V-Ti-based solid solution. Fe element distributes uniformly in the solid solution. However, the segregation for Ti can be observed obviously in the form of dendrite morphologies (Area 3) in the alloys. The reason for the uneven compositions can be derived from the given analysis above. During the cooling process, the temperature of furnace decreases quickly resulting in the solidification of some Ti before diffusing homogeneously. Thus, the control of holding time seems important in the experiment. Meanwhile, further work should be carried out to improve the heat resistant property of furnace, and new heat sources should be introduced into the preparation of alloys. The deep dark phase (Area 4) is Al-O phase, according to the mass ratio of Al to O, it can be concluded that some Al2O3 inclusions also exist in the alloy.
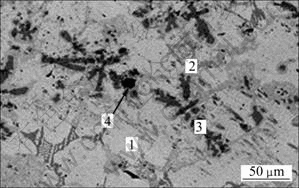
Fig. 7 SEM image of alloy obtained by metallothermic reduction method
Table 5 EDS analysis result of alloy obtained by metallothermic reduction method shown in Fig. 7 (mass fraction, %)
In order to reduce the impurities in the prepared alloy, a refining process was performed. The refining agent is CaF2–CaO–Na3AlF6 slag, because CaO and Na3AlF6 have a good dissolving ability to oxide inclusion, and CaF2 can improve the fluidity of slag. The Al, O and Si contents of the alloy reach 1.86%, 0.91% and 0.43% (mass fraction), respectively, after the refining treatment. The Al and O contents decline obviously, therefore, it can be confirmed that the preliminary refining process can remove oxide inclusions effectively. The content of Si just decreases from 0.56% to 0.43% (mass fraction), the change range is smaller. It is probably because most of the Si impurity exists in the form of V-Ti-Fe-Si alloy, and this part of Si cannot dissolve into the refining slag easily. Figure 8 shows the XRD pattern of the alloy sample after refining process. V-based alloys and Ti are the primary phases, and the oxide phase disappears in the alloy.
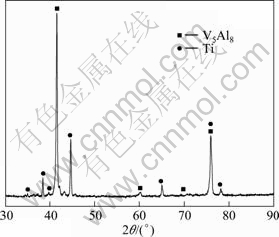
Fig. 8 XRD pattern of V-Ti-Fe master alloy after refinement
Figure 9 and Table 6 show the microstructure and chemical compositions of the refined alloy, respectively. Compared with Fig. 7, the Al-O phase cannot be detected again, and moreover, the Ti-rich phase decreases obviously. Therefore, it can be concluded that the refining process also plays a role in heat treatment.
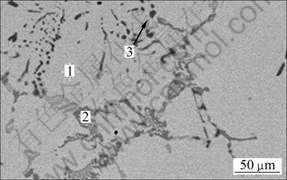
Fig. 9 SEM image of V-Ti-Fe master alloy after refinement
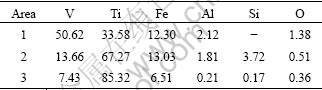
Table 6 EDS analysis result of V-Ti-Fe master alloy after refinement shown in Fig. 9 (mass fraction, %)
4 Conclusions
1) The V-Ti-Fe master alloys can be obtained by metallothermic reduction method, and the appropriate technological conditions are: the mass ratio of V2O5 to TiO2 is 0.5:1, Al addition represents 95% of the theoretical values, and the combined reducing agent amount is 1/3 that of the Al addition amount.
2) The compositions of the alloys obtained by metallothermic reduction method are inhomogeneous. V and Fe distribute uniformly, while Ti segregates in the alloys.
3) The oxide inclusions in the V-Ti-Fe master alloys can be removed effectively by the spray refining process.
References
[1] HUANG Zhuo, LIU Xiao-peng, JIANG Li-jun, DU Jun. Research and development on hydrogen storage properties of Ti-V-Fe based solid solution alloys [J]. Chinese Journal of Rare Metals, 2007, 31(6): 847-851. (in Chinese)
[2] LOTOTSKY M V, YARTYS V A, ZAVALIY I Y. Vanadium-based BCC alloys: Phase-structural characteristics and hydrogen sorption properties [J]. Journal of Alloys and Compounds, 2005, 404-406: 421-426.
[3] ZHAO Gang, LIU Shou-ping, ZHOU Shang-qi, LI Rong, REN Qin. Principle of vanadium-hydrogen reaction and development of vanadium base solid solution hydrogen alloy [J]. Iron Steel Vanadium Titanium, 2003, 24(2): 39-43. (in Chinese)
[4] ZHU Yun-feng, LI Rui, GAO Ming-xia, LIU Yong-feng, PAN Hong-ge, WANG Qi-dong. Electrochemical properties of TiV-based hydrogen storage alloys [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(1): 33-37.
[5] CHAI Yu-jun, ZHAO Min-shou. Recent development of several solid solution hydrogen storage alloys [J]. Rare Metal Materials and Engineering, 2005, 34(2): 174-177. (in Chinese)
[6] NOMURA K, AKIBA E. H2 Absorbing-desorbing characterization of the Ti-V-Fe alloy system [J]. Journal of Alloys and Compounds, 1995, 231: 513-517.
[7] ITOH H, ARASHIMA H, KUBO K, KABUTOMORI T. The influence of microstructure on hydrogen absorption properties of Ti-Cr-V alloys [J]. Journal of Alloys and Compounds, 2002, 330-332: 287-291.
[8] MATSUDA J, NAKAMURA Y, AKIBA E. Microstructure of Ti-V-Mn BCC alloys before and after hydrogen absorption- desorption [J]. Journal of Alloys and Compounds, 2011, 509: 4352-4356.
[9] YAN Y G, CHEN Y G, ZHOU X X, LIANG H, WU C L, TAO M D. Some factors influencing the hydrogen storage properties of 30V-Ti-Cr-Fe alloys [J]. Journal of Alloys and Compounds, 2008, 453: 428-432.
[10] YU X B, WU Z, XIA B J, XU N X. Enhancement of hydrogen storage capacity of Ti-V-Cr-Mn BCC phase alloys [J]. Journal of Alloys and Compounds, 2004, 372: 272-277.
[11] YAN Y G, CHEN Y G, LIANG H, WU C L, TAO M D, TU M J. Effect of Al on hydrogen storage properties of V30Ti35Cr25Fe10 alloy [J]. Journal of Alloys and Compounds, 2006, 426: 253-255.
[12] YAN Y G, CHEN Y G, LIANG H, WU C L, TAO M D. The effect of Si on V30Ti35Cr25Fe10 BCC hydrogen storage alloy [J]. Journal of Alloys and Compounds, 2007, 441: 297-300.
[13] MI Jing, LIU Xiao-peng, LI Zhi-nian, JIANG Li-jun, HUANG Zhou, WANG Shu-mao. Effect of cerium addition on microstructure and hydrogen storage property of Ti26.5Cr20(V45Fe8.5)0.98Si2 alloy [J]. Journal of the Chinese Rare Earth Society, 2009, 27(2): 241-245. (in Chinese)
[14] LIU X P, CUEVAS F, JIANG L J, LATROCHE M, LI Z N, WANG S M. Improvement of the hydrogen storage properties of Ti-Cr-V-Fe BCC alloy by Ce addition [J]. Journal of Alloys and Compounds, 2009, 476: 403-407.
[15] TANG Hai-yan, LI Jing-she, WANG Jian-bin, SUN Kai-ming, WEN De-song. Study on removal of inclusions from molten steel by blowing during LF refining [J]. Iron and Steel, 2007, 42(4): 21-23. (in Chinese)
[16] ZHENG Fang-ping, CHEN Li-xin, LIU Jian, DAI Fa-bang, CHEN Chang-pin. The phase structures and hydrogen absorption- desorption properties of Ti-V-Fe alloys [J]. Rare Metal Materials and Engineering, 2006, 35(3): 395-398. (in Chinese)
金属热还原法制备V-Ti-Fe中间合金
王 斌1,刘奎仁1,陈建设1,高腾跃1,何季麟2
1. 东北大学 材料与冶金学院,沈阳 110819;
2. 宁夏东方有色金属集团有限公司,石嘴山 753000
摘 要:采用金属热还原热法制备V-Ti-Fe中间合金,考察V2O5与TiO2的加入比例、用铝量和Al-Mg合金用量对金属回收率和合金成分的影响。结果表明:最佳工艺参数为原料中V2O5和TiO2的质量比为0.5:1,实际用铝量为理论值的95%,Al-Mg合金用量为铝量的1/3。能谱分析结果表明,合金中的V和Fe元素分布比较均匀,Ti则存在一定偏析。为降低合金中Al和O的杂质含量,进行喷吹造渣精炼。精炼后,合金中的铝含量由4.27%降为1.86%,氧含量由2.10%降为0.91%。
关键词:V-Ti-Fe中间合金;金属热还原法;回收率;精炼
(Edited by FANG Jing-hua)
Foundation item: Project (2006AA068128) supported by the High-tech Research and Development Program of China
Corresponding author: LIU Kui-ren; Tel: +86-24-83686997; Fax: +86-24-83686997; E-mail: liukr@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)61348-4
Abstract: V-Ti-Fe master alloys were prepared by metallothermic reduction method, and the influences of the mass ratio of V2O5 to TiO2, Al and Al-Mg alloy addition amounts on the metal recovery rates and alloy compositions were investigated. The results show that appropriate technological parameters are: the mass ratio of V2O5 to TiO2 is 0.5:1, Al addition represents 95% of the theoretical value, and the Al-Mg alloy addition amount is one third that of the Al addition. The results from energy spectrum analysis show that V and Fe distribute uniformly in the prepared alloy, while the segregation for Ti, i.e. Ti-rich phase is detected. A spray refining process was carried out to reduce the impurity contents of Al and O in the prepared alloys. The Al content drops from 4.27% to 1.86%, and the O content drops from 2.10% to 0.91% after the refining process.


