Trans. Nonferrous Met. Soc. China 24(2014) 4012-4022
Effects of substituting La with M (M=Sm, Nd, Pr) on electrochemical hydrogen storage characteristics of A2B7-type electrode alloys
Yang-huan ZHANG1,2, Peng-xin LI1, Tai YANG2, Ting-ting ZHAI2, Ze-ming YUAN2, Shi-hai GUO2
1. Key Laboratory of Integrated Exploitation of Baiyun Obo Multi-Metal Resources, Inner Mongolia University of Science and Technology, Baotou 014010, China;
2. Department of Functional Material Research, Central Iron and Steel Research Institute, Beijing 100081, China
Received 31 March 2014; accepted 16 August 2014
Abstract:
The partial substitution of M (M=Sm, Nd, Pr) for La was performed in order to ameliorate the electrochemical hydrogen storage performance of RE–Mg–Ni-based A2B7-type electrode alloys. The La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0-0.4) electrode alloys were fabricated by casting and annealing and their microstructures were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The major phases (La, Mg)2Ni7 with the hexagonal Ce2Ni7-type structure and LaNi5 with the hexagonal CaCu5-type structure make up the basic microstructure of the experimental alloys. The discharge capacities of the as-cast and annealed alloys all gain their maximum values with the M (M=Sm, Nd, Pr) content varying. The electrochemical cycle stability of the as-cast and annealed alloys clearly rises with the M (M=Sm, Nd, Pr) content growing. Furthermore, the electrochemical kinetics of the alloys, including the high rate discharge ability, charge transfer rate, limiting current density and hydrogen diffusion coefficient, all present a increase trend at first and then decrease with the rising of M (M=Sm, Nd, Pr) content.
Key words:
hydrogen storage; elements substitution; microstructure; electrochemical performance;
1 Introduction
Hydrogen storage alloys are of particular interest as a novel group in functional materials owing to their potential and practical applications in Ni-MH rechargeable batteries. Although a series of metal hydride electrode materials, including the rare earth-based AB5-type alloys [1], the AB2-type Laves phase alloys [2], the V-based solid solution alloys [3] and the Mg-based alloys [4,5], are selected as the potential electrode materials, there is no perfect choice among the above- mentioned electrode materials to meet the transport applications due to the limitations of their properties, such as the low discharge capacity of the AB5-type alloy, the poor activation capability of the AB2-type Laves phase as well as the V-based solid solution alloys and the poor cycle stability of the Mg-based electrode alloy. In such circumstance, La-Mg-Ni-system A2B7-type alloys have been considered to be the most promising candidates as the negative electrode materials of Ni/MH rechargeable battery on account of their higher discharge capacities (380-410 mA·h/g) and lower production costs since KADIR et al [6] and KOHNO et al [7] reported their research results. Although we have made some notable achievements as summarized by LIU et al [8,9], the attempt was still frustrated by the poor cycle stability of the new alloy and the production of the new type alloys as the negative electrode of Ni/MH battery has not been found in China yet. The serious challenge faced by the researchers still keeps intact, enhancing the cycle stability of the alloy without reducing its discharge capacity.
It was confirmed that the element substitution is an effective method for improving the overall properties of the hydrogen storage alloys [10]. With regard of the La-Mg-Ni series hydrogen storage alloys, the partial replacement of Ni with Co, Fe, Mn, Al, Cu [11-13] and of La with Ce, Pr, Nd [14-16] were studied systematically. Furthermore, it was convinced that the capacity deterioration of the La-Mg-Ni system alloy electrodes is mainly attributed to the pulverization of the alloy particles and the oxidation/corrosion of the elements of Mg and La [17].
In this work, it is expected that a combination of decreasing Mg content and substituting La with M (M=Sm, Nd, Pr) will improve the electrochemical performance of the La-Mg-Ni system A2B7-type alloys. To validate this, a comparative study about the effects of the substitution of M (M=Sm, Nd, Pr) for La on the structures and electrochemical hydrogen storage performance of the La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0-0.4) electrode alloys was performed.
2 Experimental
The chemical composition of the electrode alloys was La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0, 0.1, 0.2, 0.3, 0.4). For convenience, the alloys were denoted with M content as M0, M1, M2, M3 and M4, respectively. The alloy ingots were prepared in a vacuum induction furnace in helium atmosphere under the pressure of 0.04 MPa in order to prevent element Mg from volatilizing during the melting. A part of the alloy was annealed at 950 °C for 8 h in vacuum.
The phase structures and compositions of the as-cast and annealed alloys were characterized by XRD (D/max/2400). The diffraction, with the experimental parameters of 160 mA, 40 kV and 10 (°)/min, was performed with Cu Kα1 radiation filtered by graphite. The morphologies of the as-cast and as-annealed alloys were observed by SEM (QUANTA 400).
The prepared alloy ingots were crushed and mechanically ground to fine powders (passed through a sifter with the mesh sizes of 75 μm×75 μm). The round electrode pellets with 15 mm in diameter were prepared by cold pressing the mixture of alloy powder and carbonyl nickel powder with the mass ratio of 1:4 under a pressure of 35 MPa. Then the electrode pellets were immersed in a 6 mol/L KOH solution for 24 h to wet the electrodes fully before the electrochemical measurement.
The electrochemical measurements were performed at 30 °C by a tri-electrode open cell, consisting of a working electrode (the metal hydride electrode), a sintered Ni(OH)2/NiOOH counter electrode and an Hg/HgO reference electrode, which were immersed in the electrolyte of 6 mol/L KOH, and the voltage between the negative electrode and the reference one was defined as the discharge voltage. In every cycle, the alloy electrode was first charged with a constant current density. After resting for 15 min, it was discharged at the same current density to cut-off voltage of -0.500 V.
The electrochemical impedance spectra (EIS) and the Tafel polarization curves of the alloys were measured through the electrochemical workstation (PARSTAT 2273). The fresh electrodes were fully charged and then rested for 2 h until the open circuit potential was stable. For the EIS measurement, the frequency ranged from 10 kHz to 5 mHz at 50% depth of discharge (DOD), the amplitude of signal potentiostatic or galvanostatic measurements was 5 mV, and the number of points per decade of frequencies was 60. For the Tafel polarization curves, the potential range was -1.2 to 1.0 V (vs Hg/HgO) with a scan rate of 5 mV/s. For the potentiostatic discharge, the test electrodes in the fully charged state were discharged at 500 mV potential steps for 5000 s on the electrochemical workstation (PARSTAT 2273), with the electrochemistry corrosion software (CorrWare).
3 Results and discussion
3.1 Microstructure characteristics
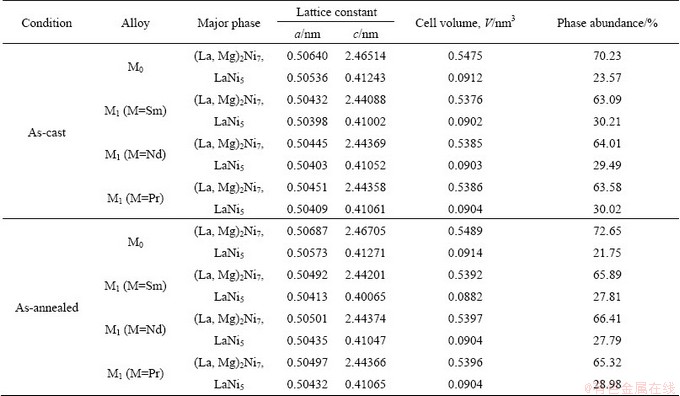
As shown in Fig. 1, the XRD profiles of the as-cast and as-annealed La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0-0.4) alloys indicate that all the as-cast and as-annealed alloys are of a multiphase structure, containing two major phases (La, Mg)2Ni7 and LaNi5 as well as a residual phase LaNi3. We note that the partial element substitution and annealing treatment obviously change the phase abundances of the alloys but have little effect on their phase composition. The lattice parameters together with the abundances of the major phases (La, Mg)2Ni7 and the LaNi5 are listed in Table 1, which were calculated by Jade 6.0 software based on the XRD data. It is found that the substitution of M (M=Sm, Nd, Pr) for La makes the lattice constants and cell volumes of the two major phases reduce visibly, due to the fact that the atom radius of M (M=Sm, Nd, Pr) is smaller than that of La. Furthermore, the reduction of the cell volume caused by M (M=Sm, Nd, Pr) substitution indicates the successfully alloying of M (M=Sm, Nd, Pr) with the two major phases. It is evident that the substitution of M (M=Sm, Nd, Pr) for La conduces to a decrease in the (La, Mg)2Ni7 phase and an increase in the LaNi5 phase. We also find that the width of the diffraction peaks of the (La, Mg)2Ni7 and LaNi5 phases become markedly narrow when annealed, suggesting that the annealing treatment reduces grain stress and lattice defects of the alloy [18]. Besides, the annealing also renders an obvious increase in the (La, Mg)2Ni7 phase and a decrease in the LaNi5 phase.
The SEM images and energy dispersive spectrometer (EDS) patterns of the as-cast and as-annealed La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloys are illustrated in Fig. 2. The EDS profiles reveal that all the experimental alloys hold a multiphase structure, being of the (La, Mg)2Ni7 and the LaNi5 as well as the LaNi3 phases, which is consistent with the XRD result. Obviously, the as-cast M0 alloy displays a dendrite structure. The substitution of M (M=Sm, Nd, Pr) for La brings on a visible refinement of the grains of the as-cast alloys. Furthermore, it can be seen that the annealing treatment turns out an obvious homogeneity of the composition segregation of the alloys.
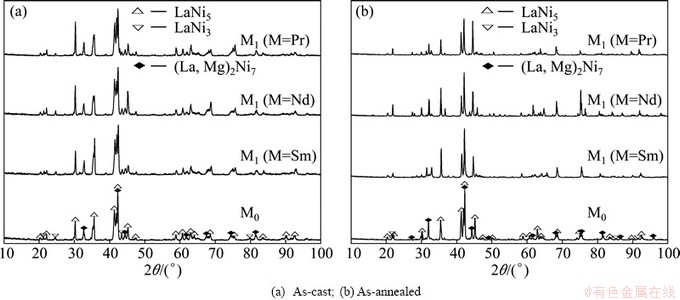
Fig. 1 XRD patterns of as-cast and annealed La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloys
Table 1 Lattice constants and abundances of (La, Mg)2Ni7 and LaNi5 major phases
3.2 Electrochemical performance
3.2.1 Activation capability and discharge capacity
Easy to be activated is very important for the alloy electrode in Ni–MH battery. Here, we use the number of charge-discharge cycles which are required for attaining the maximum discharge capacity at a constant current density of 60 mA/g to evaluate the activation capability of an alloy electrode. The fewer the number of charge-discharge cycles is, the better the activation property will be. The variations of the discharge capacity of the as-cast and as-annealed La0.8–xMxMg0.2Ni3.35Al0.1- Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloys with the cycle number are presented in Fig. 3.
Apparently, all the alloys reach their maximum discharge capacities at most two charge-discharge cycles, expressing superior activation performance. The substitution of M (M=Sm, Nd, Pr) for La has little effect on the activation capability of the alloys, whereas the annealing treatment slightly impairs the activation ability of the M0 alloy. The superior activation performance of the as-cast and as-annealed alloys comes from their multiphase structures. This is because the phase boundary not only decreases the lattice distortion and the strain energy produced in the process of hydrogen absorption but also provides good diffusion tunnels for hydrogen atoms, which facilitate to activate the alloys.
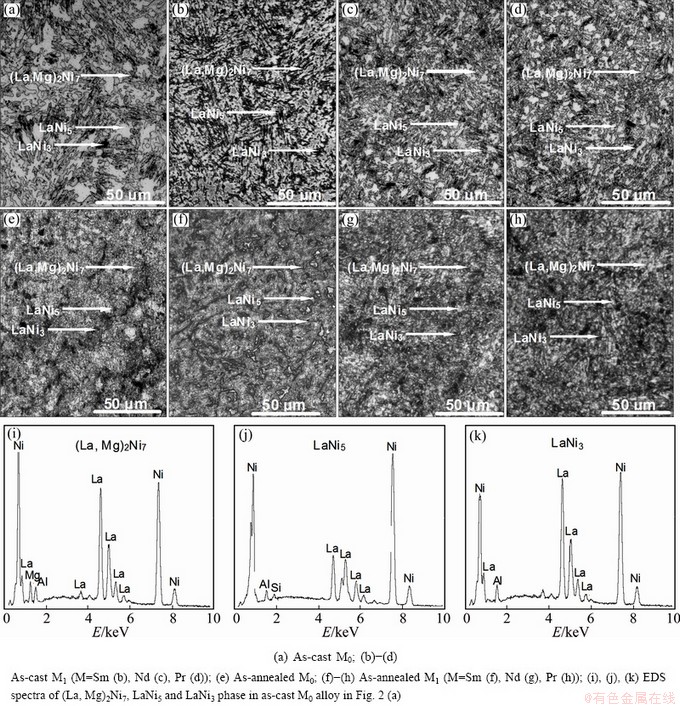
Fig. 2 SEM images and EDS spectra of La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloys

Fig. 3 Evolution of discharge capacity of as-cast (a) and as-annealed (b) La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloys with cycle number
The relationships between the maximum discharge capacity of the experimental alloys and the M (M=Sm, Nd, Pr) content are described in Fig. 4, from which it is found that the discharge capacities of all the alloys first augment and then decline with the M (M=Sm, Nd, Pr) content growing. Namely, the maximum value corresponding a special M (M=Sm, Nd, Pr) content of the discharge capacities of the as-cast and as-annealed alloys exists, which is termed as the optimal content. Evidently, the optimal content is changeable for different elements. For the as-cast alloys, the optimal content is x=0.2 for Sm and x=0.3 for Nd and Pr, and for the as-annealed alloys, it is x=0.1 for Sm and Nd and x=0.3 for Pr. The maximum discharge capacities are 346.6 mA·h/g (M=Sm), 357.9 mA·h/g (M=Nd) and 363.1 mA·h/g (M=Pr) for the as-cast alloys and 393.4 mA·h/g (M=Sm), 383.4 mA·h/g (M=Nd) and 389.7 mA·h/g (M=Pr) for the as-annealed ones, respectively. Obviously, they are in sequence (M=Pr)>(M=Nd)>(M=Sm)>(M= none) for the as-cast alloys and (M=Sm)>(M=Pr)> (M=Nd)>(M=none) for the as-annealed alloys, meaning that an amount of M (M=Sm, Nd, Pr) replacement facilitates to enhance the discharge capacities of the as-cast and as-annealed alloys. Furthermore, it is found that, the as-annealed alloy exhibits a much higher discharge capacity than the as-cast one, suggesting that the annealing treatment gives rise to a positive contribution to the discharge capacity.

Fig. 4 Evolution of maximum discharge capacity of as-cast (a) and as-annealed (b) La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0-0.4) alloys with M content x
As to the capacity, it has been ascertained to be determined by multiple factors, such as its crystal structure, phase composition and structure, grain size, composition uniformity and surface state. The observed variations of the discharge capacity of alloys generated by M (M=Sm, Nd, Pr) substitution and the annealing are definitely associated with their changed microstructures. Firstly, the substitution of M (M=Sm, Nd, Pr) for La makes the alloy grains clearly refined, creating a huge amount of grain boundary. This benefits the discharge capacity since the grain boundary possesses the maximum distribution of hydrogen concentrations [19]. Hence, it is understandable that such substitution gives rise to an increase of the discharge capacities of the as-cast and as-annealed alloys. On the other hand, this substitution enables both the cell volume and the (La, Mg)2Ni7 phase to decrease visibly, which is harmful to the discharge capacity of the alloy. Here, it must be mentioned that the LaNi5 phase works not only as a hydrogen reservoir but also a catalyst to activate the (La, Mg)2Ni7 phase to absorb/desorb hydrogen reversibly in the alkaline electrolyte [17,20]. It is just the above contrary effects that result in an optimum M (M=Sm, Nd, Pr) content for the discharge capacities of the as-cast and annealed alloys. As regards the positive impact of the as-annealing treatment on the discharge capacity, it may be attributed to the homogenization of the composition and the changes of the phase abundance and the lattice parameters originated by the annealing.
3.2.2 Cycle stability
The cyclic stability of hydrogen storage alloy is a major factor to estimate whether it can be applied as a negative electrode material or not. Generally, it is symbolized by the capacity retaining rate (Sn) which is defined as Sn=Cn/Cmax×100%, where Cmax is the maximum discharge capacity and Cn is the discharge capacity of the nth charge-discharge cycle at a current density of 300 mA/g. Figure 5 describes the variations of the Sn values of the as-cast and as-annealed La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloys with the cycle number, from which the degradation process of the discharge capacity of the alloys can be seen clearly. We can qualitatively estimate the degradation rate of the discharge capacity during the charge-discharge cycles by means of the slopes of the curves. The substitution of M (M=Sm, Nd, Ni) for La engenders an inconspicuous change for the curve’s slopes of the as-cast alloys, but it makes the curve’s slopes of the as-annealed alloys obviously decline, suggesting that such substitution brings on a positive contribution to the cycle stability of the as-annealed alloy. The relationships between the S100 (n=100) values of the as-cast and as-annealed alloys and the M (M=Sm, Nd, Pr) content are also inserted in Figs. 5(a) and (b), respectively. Clearly, the S100 (n=100) values of the as-cast and as-annealed alloys markedly grow with the M (M=Sm, Nd, Pr) content rising. To be specific, increasing the M (M=Sm, Nd, Pr) content from 0 to 0.4 enables the S100 (n=100) values to grow from 65.0% to 79.3% (M=Sm), 75.2% (M=Nd) and 77.9% (M=Pr) for the as-cast alloys, and from 72.8% to 89.4% (Sm), 89.3% (M=Nd) and 91.8% (M=Pr) for the as-annealed ones, separately. Comparing Figs. 5(a) with (b), it can be found that the annealing treatment significantly ameliorates the cycle stability of the alloy.
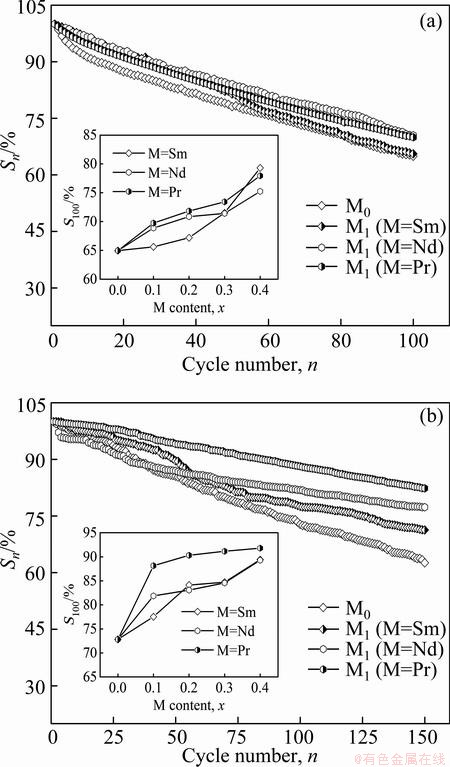
Fig. 5 Evolution of Sn of as-cast (a) and as-annealed (b) La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloys with cycle number
It is convinced that the following aspects are principally responsible for the degradation of the discharge capacity of La–Mg–Ni-based A2B7-type alloy during the charge-discharge cycling. Firstly, Mg(OH)2 or La(OH)2 surface layer forming and constantly thickening hinders the hydrogen atoms from diffusing in or out, in alkaline solution [21]. Moreover, an inevitable expansion and contraction of the cell volumes of the alloys during the charge-discharge process aggravates the alloy’s cracking and pulverizing and then makes the surface of the material apt to be oxidized, which has been confirmed by our previous work [22]. The improved cycle stability of the as-cast and as-annealed alloys by substituting La with M (M=Sm, Nd, Pr) is believed to be associated with the refinement of the grains, owing to the anti-pulverization capability of the alloy basically depending on its grain size. Furthermore, the increase of the LaNi5 phase resulting from the element substitution is beneficial for improving cycle stability of the alloy due to an undoubted fact that LaNi5 phase possesses much higher electrochemical cycle stability than (La, Mg)2Ni7 phase. As to the positive contribution of the annealing treatment to the cycle stability of the alloys, it is ascribed to the increase of the cell volumes and more homogeneous compositional distribution generated from the annealing, which facilitates to prohibit the pulverization and corrosion of the alloy [8,9].
3.2.3 Electrochemical kinetics
As is well known, increasing the discharge current density inevitably results in the discharge capacity of an electrode alloy decreasing. Currently, the HRD of an alloy electrode is used to symbolize its electrochemical kinetics, being defined as HRD=Ci/C60×100%, where Ci and C60 are the maximum discharge capacities of the alloy electrode charged-discharged at the current densities of i and 60 mA/g, respectively. The evolutions of the HRDs of the as-cast and as-annealed La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0-0.4) alloys with the discharge current density are presented in Fig. 6. From which it is found that the as-cast and as-annealed M1 (M=Sm, Nd, Pr) alloys exhibit higher HRD than the M0, meaning that such substitution makes a positive contribution on the electrochemical kinetics of the alloy. The relationships between the HRDs of the as-cast and as-annealed alloys at a fixed current density of 300 mA/g and the M (M=Sm, Pr, Nd) content are also presented in Figs. 6(a) and (b), respectively. Evidently, the HRD of all the experimental alloys first augments then declines with the growing M (M=Sm, Pr, Nd) content, which indicates that the excessive M (M=Sm, Pr, Nd) substitution will impair the electrochemical kinetics. The maximum HRDs are in sequence (M=Sm)> (M=Nd)>(M=Pr)>(M=none) for the as-cast alloys and (M=Nd)>(M=Sm)>(M=Pr)>(M=none) for the as-annealed alloys.
As a matter of fact, it is ascertained that the high rate discharge ability of an alloy electrode basically depends on the hydrogen diffusion capability in the alloy bulk and the charge-transfer rate on the surface of an alloy electrode [18]. Hence, it seems to be compulsory to investigate the effects of the M (M=Sm, Nd, Pr) on the diffusion ability of hydrogen atoms and the charge- transfer rate in order to make the mechanism of the impacted electrochemical kinetics of the alloy by substituting La with M (M=Sm, Nd, Pr) clear. The hydrogen diffusion ability which is signified by the hydrogen diffusion coefficient, can be derived by means of the semilogarithmic curves of anodic current versus working duration of an alloy electrode, as demonstrated in Fig. 7. Based on the model in Ref. [23], the diffusion coefficient of the hydrogen atoms in the bulk of the alloy could be calculated easily through the slope of the linear region of the corresponding plots according to the following formulae:
 (1)
(1)
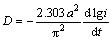 (2)
(2)
where i is the diffusion current density; D is the hydrogen diffusion coefficient; C0 is the initial hydrogen concentration in the bulk of the alloy; Cs is the hydrogen concentration on the surface of the alloy particles; a is the alloy particle radius; d is the density of the hydrogen storage alloy; t is the discharge time. The variations of the D values of the as-cast and as-annealed alloys obtained by Eq. (2) with the different M (M=Sm, Nd, Pr) contents are also inserted in Figs. 7(a) and (b), respectively. It is evident that the D values of the as-cast and as-annealed alloys first grow and then decline with the M (M=Sm, Nd, Pr) content rising, conforming well to the results shown in Fig. 6, which clarifies that the hydrogen diffusion ability is a crucial factor of the electrochemical kinetics of the alloys.
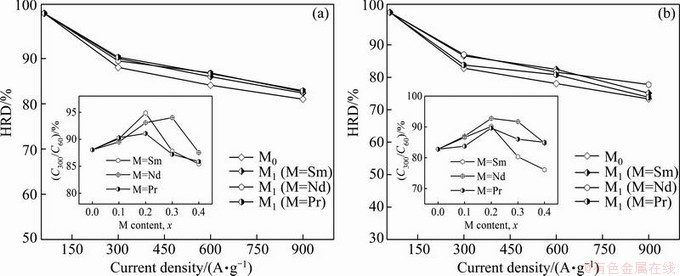
Fig. 6 Evolution of HRD of as-cast (a) and as-annealed (b) La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloys with current density
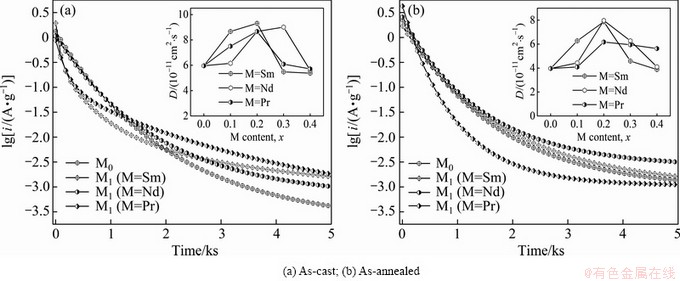
Fig. 7 Semilogarithmic curves of anodic current vs time responses of La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0-0.4) alloy electrodes
Another important electrochemical kinetic character of an alloy electrode related with the diffusion rate of hydrogen in alloy electrode is the limiting current density (iL) [24], which can be obtained by measuring the Tafel polarization curves, as depicted in Fig. 8. It can be seen that, in all cases, each anodic polarization curve has a clear inflection point, namely a critical value existing in the process, which is termed as the iL. It is viewed as the oxidation reaction takes place on the surface of the alloy electrode, the oxide layer hinders hydrogen atoms from further penetrating [25]. Here, the iL can be looked as a critical current density for passivating. Based on the obtained Tafel polarization curves of all the as-cast and as-annealed alloys, the relationships between the iL values of the as-cast and as-annealed electrode alloys and the M (M=Sm, Nd, Pr) content are founded, just as inserted in Figs. 8(a) and (b), respectively. It is found that the iL values of the alloys first mount up and then go down with the M (M=Sm, Nd, Pr) content growing. To be specific, the maximum iL values are 2.56 A/g (M=Sm), 2.30 A/g (M=Nd) and 2.29 A/g (M=Pr) for the as-cast alloys and 2.12 A/g (M=Sm), 2.14 A/g (M=Nd) and 1.94 A/g (M=Pr) for the as-annealed ones, respectively.
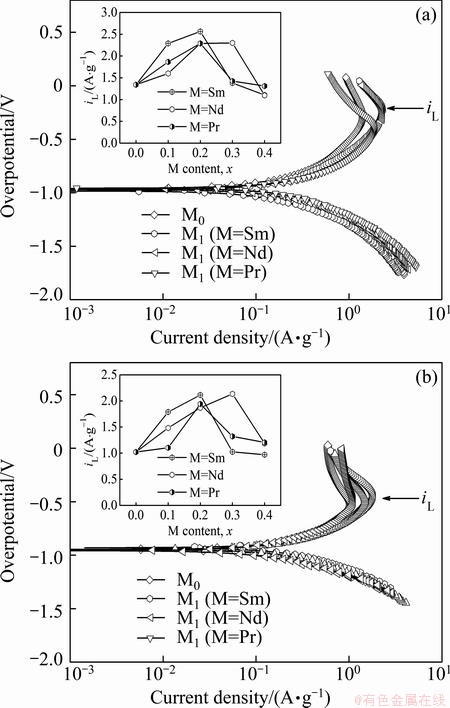
Fig. 8 Tafel polarization curves of as-cast (a) and as-annealed (b) La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloy electrodes
As for the charge-transfer ability, it can be qualitatively evaluated by its EIS based on the model of KURIYAMA et al [26]. Figure 9 shows the EIS curves of the as-cast and as-annealed La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloys. It can be seen that each EIS has two distorted capacitive loops at high and middle frequencies respectively as well as a line at low frequency, which expresses the electrochemical processes very well. The smaller semicircle in the high frequency region is regarded to reflect the contact resistance between the alloy powder and the conductive material, and the larger one in the middle frequency region represents the charge-transfer resistance on the alloy surface while the straight line in low frequency corresponds to the atomic hydrogen diffusion in the alloy. In such cases, the charge-transfer ability can be estimated easily, namely, the larger the radius of the semicircle in the middle frequency region, the higher the charge- transfer resistance of the alloy electrode. It can be seen from Figs. 9(a) and (b) that the substitution of M (M=Sm, Nd, Pr) for La enables the radii of the large semicircles of the as-cast and as-annealed alloys in the middle frequency region to shrink markedly. Besides, the effect of M (M=Sm) content on the charge-transfer ability of the as-cast alloy is described in Fig. 9(c), revealing that the radii of the large semicircles in the low frequency first shrink and then expand with the M (M=Sm) content growing. Similarly, the M (M=Nd and Pr) substitution alloys also have the same property in this aspect.
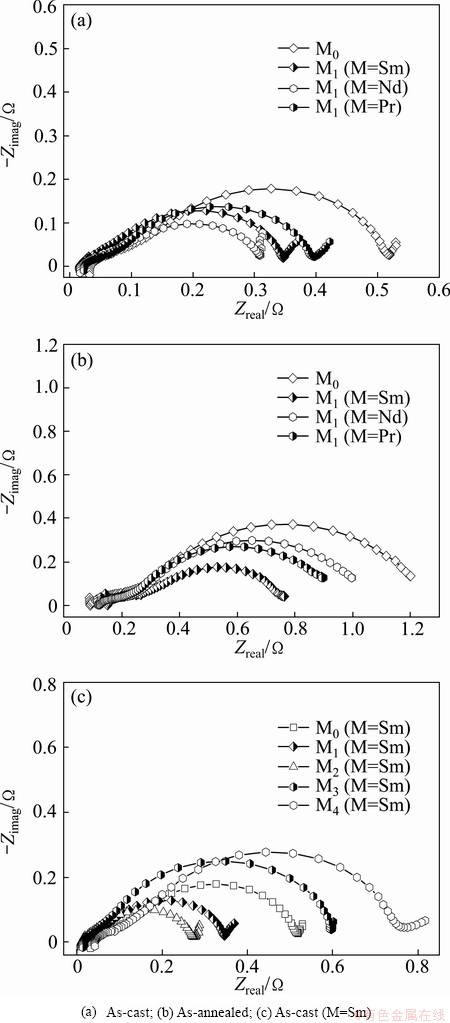
Fig. 9 EIS of as-cast and as-annealed La0.8–xMxMg0.2Ni3.35- Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4) alloy electrodes
The above-mentioned results indicate that all the HRDs and the electrochemical kinetics of the as-cast and as-annealed alloys first augment and then decline with the M (M=Sm, Nd, Pr) content rising. Maybe the next several reasons can explain why this phenomenon appears. The electrochemical hydriding/dehydriding reaction can be generalized by the following equation:
 (3)
(3)
where M represents the hydrogen storage alloy. The above equation indicates that, when the alloy electrode is charged in KOH solution, the hydrogen atoms at the alloy-electrolyte interface diffuse into bulk alloy and then store themselves in the metallic lattice in the form of hydride. In the process of discharging, the hydrogen stored in the bulk alloy diffuses toward the surface where it is oxidized. Hence, it can be concluded that electrochemical hydrogen storage kinetics of the alloy electrode is controlled by two factors, the charge-transfer rate on the surface of an alloy electrode and the diffusion capability of the hydrogen atoms in the alloy bulk. The variations of the structures and the phase abundances of the alloys resulting from substituting M (M=Sm, Nd, Pr) for La, i.e., the grain refinement and the increase of the LaNi5 phase, bring on a quite different impact on the electrochemical kinetics of the alloy. Firstly, as for the diffusion ability of the hydrogen atoms, it is convinced to be dominated by the strength of the metal-hydrogen interaction as well as the structure of the alloy [27]. The significant increase of LaNi5 phase enhances the electrocatalytic activity of the alloy electrodes. Thanks to the refined microstructure by M (M=Sm, Nd, Pr) substitution, a lot of new crystallites and grain boundaries evolve, which provide the fast diffusion paths for hydrogen absorption [28]. But it must be mentioned that the reduced cell volume incurred by M (M=Sm, Nd, Pr) substitution gives rise to a negative effect on the hydrogen diffusion because the reduction of the cell volume means the growing of the expansion and contraction degree during the process of hydrogen absorbing and desorbing. It is the above-mentioned quite opposite impact generated by M (M=Sm, Nd, Pr) substitution that makes the diffusion coefficient of the as-cast and as-annealed alloys have a maximum value with the M (M=Sm, Nd, Pr) content varying. In the case of the charge-transfer step, it is believed to be controlled by both crystallographic and electronic structures [29]. The variation of the alloy compositions on the alloy surface results in an evident impact on the valence electron configurations which dominates the charge- transfer reaction, i.e. the hydrogen dissociative reaction [30]. The increase of LaNi5 phase improves the electrocatalytic activity of the alloy, which accelerates the charge transfer on the surface of an alloy electrode. However, the refinement of the grains created by M (M=Sm, Nd, Pr) substitution impairs the charge-transfer ability on the alloy surface because it can effectively prohibit the pulverization of the alloy particles and hardly form new surface, decreasing the rate of charge transfer at the alloy-electrolyte interface. At present, it is found that similar to the hydrogen diffusion, the M (M=Sm, Nd, Pr) substitution also exerts both positive and negative effects on the charge transfer rate on the surface of an alloy electrode. Based on the aforementioned discussion, it is clear that why the HRD of the as-cast and as-annealed alloys have a maximum value with the M (M=Sm, Nd, Pr) content growing. With regard to the negative effect produced by annealing treatment, it is considered that the annealing treatment eliminates the casting internal strain and diminishes crystalline defects such as dislocations and grain boundaries, which not only increases the charge-transfer resistance of the alloy electrodes but also hinders the hydrogen diffusion from inner of the bulk to the surface, and subsequently brings on a drop in the electrochemical kinetic property.
4 Conclusions
1) The substitution of M (M=Sm, Nd, Pr) for La makes the grains of the as-cast and as-annealed alloys refined significantly. Moreover, such substitution gives rise to a decrease of the (La, Mg)2Ni7 phase and an increase of the LaNi5 phase in the alloys without altering the major phase structures of the alloys.
2) The substitution of M (M=Sm, Nd, Pr) for La nearly does not affect the activation ability of the as-cast and as-annealed alloys. The maximum values of discharge capacities of the alloy appear with the M (M=Sm, Nd, Pr) content growing, which are 346.6 mA·h/g (M=Sm), 357.9 mA·h/g (M=Nd) and 363.1 mA·h/g (M=Pr) for the as-cast alloys and 393.4 mA·h/g (M=Sm), 383.4 mA·h/g (M=Nd) and 389.7 mA·h/g (M=Pr) for the as-annealed ones, respectively. The electrochemical cycle stability of the alloys augments with the M (M=Sm, Nd, Pr) content growing. Moreover, the HRD values of the as-cast and as-annealed alloys first increase then decrease with the M (M=Sm, Nd, Pr) varying.
3) Furthermore, the annealing treatment significantly enhances the discharge capacity and cycle stability of the alloy, whereas it slightly impairs the electrochemical kinetic property of the alloy.
References
[1] WILLEMS J J G, BUSCHOW K H J. From permanent magnets to rechargeable hydride electrodes [J]. Journal of the Less-Common Metals, 1987, 129(15): 13-30.
[2] OVSHINSKY S R, FETCENKO M A, ROSS J. A nickel metal hydride battery for electric vehicles [J]. Science, 1993, 260(5105): 176-181.
[3] TSUKAHARA M, KAMIYA T, TAKAHASHI K, KAWABATA A, SAKURAI S, SHI J, TAKESHITA H T, KURIYAMA N, SAKAI T. Hydrogen storage and electrode properties of V-based solid solution type alloys prepared by a thermic process [J]. Journal of the Electrochemical Society, 2000, 147(8): 2941-2944.
[4] SONG W J, LI J S, ZHANG T B, HOU X J, KOU H C, XUE X Y, HU R. Microstructure and hydrogenation kinetics of Mg2Ni-based alloys with addition of Nd, Zn and Ti [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(12): 3677-3684.
[5] ZHANG Y H, YANG T, BU W G, CAI Y, ZHANG G F, ZHAO D L. Effect of Nd content on electrochemical performances of nanocrystalline and amorphous (Mg24Ni10Cu2)100-xNdx(x=0-20) alloys prepared by melt spinning [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(12): 3668–3676.
[6] KADIR K, SAKAI T, UEHARA I. RMg2Ni9 (R=La, Ce, Pr, Nd, Sm and Gd) built from MgNi2 Laves-type layers alternating with AB5 layers [J]. Journal of Alloys and Compounds, 1997, 257(1-2): 115-121.
[7] KOHNO T, YOSHIDA H, KAWASHMA F, INABA T, SAKAI I, YAMAMOTO M, KANDA M. Hydrogen storage properties of new ternary system alloys: La2MgNi9, La5Mg2Ni23, La3MgNi14 [J]. Journal of Alloys and Compounds, 2000, 311(2): L5-L7.
[8] LIU Y F, PAN H G, GAO M X, WANG Q D. Advanced hydrogen storage alloys for Ni/MH rechargeable batteries [J]. Journal of Materials Chemistry, 2011, 21(13): 4743-4755.
[9] LIU Y F, CAO Y H, HUANG L, GAO M X, PAN H G. Rare earth–Mg–Ni-based hydrogen storage alloys as negative electrode materials for Ni/MH batteries [J]. Journal of Alloys and Compounds, 2011, 509(3): 675-686.
[10] ZHANG Y H, REN H P, CAI Y, YANG T, ZHANG G F, ZHAO D L. Structures and electrochemical hydrogen storage performance of Si added A2B7-type alloy electrodes [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2): 406-414.
[11] LIU Y F, PAN H G, GAO M X, LI R, LEI Y Q. Effect of Co content on the structural and electrochemical properties of the La0.7Mg0.3Ni3.4-xMn0.1Coxhydride alloys: II. Electrochemical properties [J]. Journal of Alloys and Compounds, 2004, 376(1–2): 304-313.
[12] LIU B Z, LI A M, FAN Y P, HU M J, ZHANG B Q. Phase structure and electrochemical properties of La0.7Ce0.3Ni3.75Mn0.35Al0.15Cu0.75-x- Fex hydrogen storage alloys [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(11): 2730-2735.
[13] WEI F S, LI L, XIANG H F, LI H, WEI F N. Phase structure and electrochemical properties of La1.7+xMg1.3-x(NiCoMn)9.3 (x=0-0.4) hydrogen storage alloys [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(8): 1995-1999.
[14] SHEN X Q, CHEN Y G, TAO M D, WU C L, DENG G, KANG Z Z. The structure and high-temperature (333K) electrochemical performance of La0.8-xCexMg0.2Ni3.5(x=0.00–0.20) hydrogen storage alloys [J]. International Journal of Hydrogen Energy, 2009, 34(8): 3395-3403.
[15] ZHANG Y H, HOU Z H, LI B W, REN H P, CAI Y, ZHAO D L. Electrochemical hydrogen storage characteristics of as-cast and annealed La0.8-xNdxMg0.2Ni3.15Co0.2Al0.1Si0.05(x=0-0.4) alloys [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1403-1412.
[16] ZHANG Y H, HOU Z H, YANG T, ZHANG G F, LI X, ZHAO D L. Structure and electrochemical hydrogen storage characteristics of La0.8-xPrxMg0.2Ni3.15Co0.2Al0.1Si0.05 (x=0-0.4) electrode alloys [J]. Journal of Central South University of Technology, 2013, 20(5): 1142-1150.
[17] LIU Y F, PAN H G, YUE Y J, WU X F, CHEN N, LEI Y Q. Cycling durability and degradation behavior of La–Mg–Ni–Co-type metal hydride electrodes [J]. Journal of Alloys and Compounds, 2005, 395(1-2): 291-299.
[18] PAN H G, CHEN N, GAO M X, LI R, LEI Y Q, WANG Q D. Effects of annealing temperature on structure and the electrochemical properties of La0.7Mg0.3Ni2.45Co0.75Mn0.1Al0.2 hydrogen storage alloy [J]. Journal of Alloys and Compounds, 2005, 397(1-2): 306-312.
[19] ZHANG Y H, LI B W, REN H P, GUO S H, QI Y, WANG X L. Structures and electrochemical hydrogen storage characteristics of La0.75-xPrxMg0.25Ni3.2Co0.2Al0.1(x=0–0.4) alloys prepared by melt spinning [J]. Journal of Alloys and Compounds, 2009, 485(1-2): 333-339.
[20] SHEN X G, CHEN Y G, TAI M D, WU C L, DENG G, KANG Z Z. The structure and 233K electrochemical properties of La0.8-xNdxMg0.2Ni3.1Co0.25Al0.15(x=0.0–0.4) hydrogen storage alloys [J]. International Journal of Hydrogen Energy, 2009, 34(6): 2661-2669.
[21] DORNHEIM M, DOPPIU S, BARKHORDARIAN G, BOESENBERG U, KLASSEN T, GUTFLEISCH O, BORMANN R. Hydrogen storage in magnesium-based hydrides and hydride composites [J]. Scripta Materialia, 2007, 56(10): 841–846.
[22] ZHANG Y H, LI C, CAI Y, HU F, LIU Z C, GUO S H. Highly improved electrochemical hydrogen storage performances of the Nd–Cu-added Mg2Ni-type alloys by melt spinning [J]. Journal of Alloys and Compounds, 2014, 584: 81-86.
[23] ZHENG G, POPOV B N, WHITE R E. Electrochemical determination of the diffusion coefficient of hydrogen through a LaNi4.25Al0.75 electrode in alkaline aqueous solution [J]. Journal of the Electrochemical Society, 1995, 142(8): 2695–2698.
[24] RATNAKUMAR B V, WITHAM C, BOWMAN JR R C, HIGHTOWER A, FULTZ B. Electrochemical studies on LaNi5-xSnx metal hydride alloys [J]. Journal of the Electrochemical Society, 1996, 143(8): 2578–2584.
[25] ZHAO X Y, DING Y, MA L Q, WANG L Y, YANG M, SHEN X D. Electrochemical properties of MmNi3.8Co0.75Mn0.4Al0.2 hydrogen storage alloy modified with nanocrystalline nickel [J]. International Journal of Hydrogen Energy, 2008, 33(22): 6727–6733.
[26] KURIYAMA N, SAKAI T, MIYAMURA H, UEHARA I, ISHIKAWA H, IWASAKI T. Electrochemical impedance and deterioration behavior of metal hydride electrodes [J]. Journal of Alloys and Compounds, 1993, 202(1–2): 183–197.
[27] ZHAO X Y, DING Y, YANG M, MA L Q. Effect of surface treatment on electrochemical properties of MmNi3.8Co0.75Mn0.4Al0.2 hydrogen storage alloy [J]. International Journal of Hydrogen Energy, 2008, 33(1): 81–86.
[28] WU Y, HANA W, ZHOU S X, LOTOTSKY M V, SOLBERG J K, YARTYS V A. Microstructure and hydrogenation behavior of ball-milled and melt-spun Mg-10Ni-2Mm alloys [J]. Journal of Alloys and Compounds, 2008, 466(1-2): 176-181.
[29] KLEPERIS J,  G, CZERWINSKI A, SKOWRONSKI J, KOPCZYK M, BELTOWSKA-BRZEZINSKA M. Electrochemical behavior of metal hydrides [J]. Journal of Solid State Electrochemistry, 2001, 5(4): 229-249.
G, CZERWINSKI A, SKOWRONSKI J, KOPCZYK M, BELTOWSKA-BRZEZINSKA M. Electrochemical behavior of metal hydrides [J]. Journal of Solid State Electrochemistry, 2001, 5(4): 229-249.
[30] NOBUHARA K, KASAI H, DINO W A, NAKANISHI H. H2 dissociative adsorption on Mg, Ti, Ni, Pd and La surfaces [J]. Surface Science, 2004, 566-568: 703-707.
M (M=Sm, Nd, Pr)替代La对A2B7型贮氢合金电化学性能的影响
张羊换1, 2,李鹏欣1,杨 泰2,翟亭亭2,袁泽明2,郭世海2
1. 内蒙古科技大学 内蒙古自治区白云鄂博矿多金属资源综合利用重点实验室,包头 014010;
2. 钢铁研究总院 功能材料研究所,北京 100081
摘 要:采用M (M=Sm, Nd, Pr)部分替代La,用合金熔炼及退火的方法制备La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0–0.4)电极合金,以提高RE–Mg–Ni系A2B7型贮氢合金的电化学性能。用X射线衍射(XRD)及扫描电子显微镜(SEM)分析合金的相组成和显微结构。结果表明,合金由六方结构Ce2Ni7型的(La, Mg)2Ni7相与六方结构CaCu5型的LaNi5相组成。随着M替换量的增加,铸态及退火态合金的放电容量均出现最大值。铸态及退火态合金的循环稳定性均随着M替换量的增加而增加。此外,合金的电化学动力学性能(包括高倍率放电性能、电荷传递速率、极限电流密度、氢扩散系数)均随着M替换量的增加呈现先上升后下降的趋势。
关键词:贮氢;元素替换;显微结构;电化学性能
(Edited by Xiang-qun LI)
Foundation item: Projects (51161015, 51371094) supported by the National Natural Science Foundations of China
Corresponding author: Yang-huan ZHANG; Tel: +86-10-62183115; Fax: +86-10-62187102; E-mail: zhangyh59@sina.com
DOI: 10.1016/S1003-6326(14)63563-9
Abstract: The partial substitution of M (M=Sm, Nd, Pr) for La was performed in order to ameliorate the electrochemical hydrogen storage performance of RE–Mg–Ni-based A2B7-type electrode alloys. The La0.8–xMxMg0.2Ni3.35Al0.1Si0.05 (M=Sm, Nd, Pr; x=0-0.4) electrode alloys were fabricated by casting and annealing and their microstructures were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The major phases (La, Mg)2Ni7 with the hexagonal Ce2Ni7-type structure and LaNi5 with the hexagonal CaCu5-type structure make up the basic microstructure of the experimental alloys. The discharge capacities of the as-cast and annealed alloys all gain their maximum values with the M (M=Sm, Nd, Pr) content varying. The electrochemical cycle stability of the as-cast and annealed alloys clearly rises with the M (M=Sm, Nd, Pr) content growing. Furthermore, the electrochemical kinetics of the alloys, including the high rate discharge ability, charge transfer rate, limiting current density and hydrogen diffusion coefficient, all present a increase trend at first and then decrease with the rising of M (M=Sm, Nd, Pr) content.


