Trans. Nonferrous Met. Soc. China 23(2013) 3788-3792
Electrical conductivity of NaF-AlF3-CaF2-Al2O3-ZrO2 molten salts
Morigengaowa BAO1,2, Zhao-wen WANG1, Bing-liang GAO1, Zhong-ning SHI1, Xian-wei HU1, Jiang-yu YU1
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China;
2. Instrumental Analysis Center, Inner Mongolia University for the Nationalities, Tongliao 028000, China
Received 27 March 2013; accepted 5 September 2013
Abstract:
The electrical conductivity of NaF-AlF3-CaF2-Al2O3-ZrO2 system was studied by a tube-type cell with fixed cell constant. The results show that the electrical conductivity of NaF-AlF3-3%Al2O3-3%CaF2-ZrO2 molten salt system decreases with increase of ZrO2 content in an interval of 0-5%. The increase of 1% ZrO2 results in a corresponding electrical conductivity decrease of 0.02 S/cm, and the equivalent conductivity increases with the increase of molar ratio of NaF to AlF3. When the temperature increases by 1 °C, the electrical conductivity increases by 0.004 S/cm. At last, the regression equations of electrical conductivity relative to temperature and ZrO2 are obtained by quadratic regression analysis.
Key words:
ZrO2; molten salt electrolysis; electrical conductivity; NaF; AlF3; Al-Zr alloy;
1 Introduction
Al-Zr alloys as one of the new structural materials are used in various fields, such as aerospace, military, nuclear reactions and atomic energy [1-4]. Cryolite- zirconia can be used as electrolyte for producing Al-Zr alloy by molten salt electrolysis. Compared with the common methods of preparing Al-Zr master alloys, such as ingot metallurgy, mechanical alloying method and aluminum thermal reduction method [5-7], molten salt electrolysis exhibits many excellent advantages, such as no contamination, homogeneous dispersion in the high productivity of aluminum matrix and low fabrication costs.
It is important to study the physicochemical properties of cryolite-zirconia molten system from theoretical and technological aspects [8,9]. STERTEN and SKAR [10] investigated the Na3AlF6-ZrO2 phase diagram. But data on conductivities of the cryolite-zirconia melt are quite restricted. Conductivity directly affects the energy consumption of the molten salt electrolysis.
In this study, electrical conductivities of NaF-AlF3- CaF2-Al2O3-ZrO2 molten salts are measured by improved fixed conductivity cell. The aim of the present work is to investigate the effects of ZrO2 addition on electrical conductivity of nNaF·AlF3-Al2O3 molten salt system, so as to quest for suitable electrolyte composition to produce Al-Zr alloy by molten salt electrolysis technique.
2 Experimental
In this work, sodium fluoride, calcium fluoride, alumina and zirconia were of analytical grade. All reagents were dried at 400 °C for several hours and kept in a dry box before using. Aluminum fluoride was sublimed under low pressure (about 100 Pa) at 1050 °C and kept in a dry box before using. Boron nitride was heat treated as reference [11].
A schematic drawing of the cell is shown in Fig. 1. The cell consisted of a pyrolytic boron nitride tube of 4 mm in inner diameter and 100 mm in length. One electrode consisted of a tungsten rod (1 mm in outer diameter) placed in a fixed position inside the boron nitride (BN) tube, while the graphite crucible served as the other electrode. The crucible containing 10-15 g sample of the salt mixture was placed in a vertical laboratory furnace with argon atmosphere and heated up to the required temperature. The boron nitride tube with the tungsten electrode was immersed in the melt and repositioned several times to make sure that the composition of the electrolyte was homogeneous and any gas bubbles were removed. The temperature was measured with a Pt-Pt 10% Rh thermocouple, and it was stable within 0.5 K. Within a run, the measurements were carried out at different temperatures (typically, at four or five temperatures) for every composition.
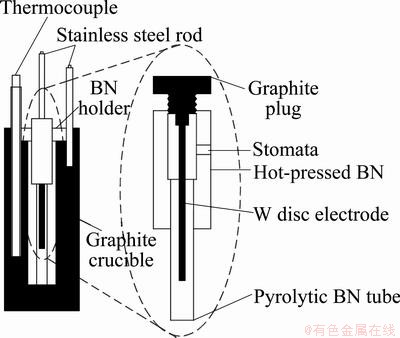
Fig. 1 Sketch of conductivity cell
Agilent impedance instrument was used to measure the cell impedance, and the electrical conductivity can be calculated by
σ=G/R (1)
where σ is the electrical conductivity; G is the electrical conductivity cell constant; R is the resistance.
3 Results and discussion
3.1 Calibration of electrical conductivity cell
Electrical conductivity cell was firstly used to measure the conductivity of molten KCl and then molten cryolite to determine the cell constant. The data for electrical conductivity of molten KCl were recommended by GRJOTHEIM et al [12]. The experimental results are listed in Table 1.
Table 1 Measurement results of KCl molten salt
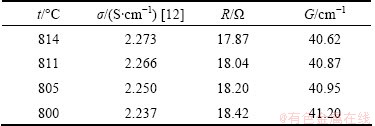
The cell constant did not vary with the temperature. The average value of electrical conductivity cell constant at different temperatures is 40.91 cm-1.
3.2 Feasibility analysis of method
Table 2 shows the comparison of our measurements with literature values for the electrical conductivity of 2.2NaF3·AlF3-5%CaF2-3%Al2O3 at 959 °C.
Table 2 Relative error of system analysis
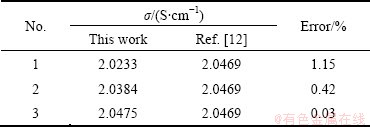
The result shows that the error is less than 1.2%, therefore the method is feasible and shows good reproducibility.
3.3 Effect of ZrO2 on electrical conductivity
Figure 2 shows experimenatal data as a function of temperature for different compositions in the system nNaF·AlF3-3%Al2O3-3%CaF2-ZrO2. The results show that the addition of ZrO2 to the melt lowers the conductivity. 1% ZrO2 addition results in a corresponding conductivity decrease of 0.02 S/cm. Some large ion species will generate according to reactions (2) [13] and (3) [14] if ZrO2 is added to the cryolite melt.
 +2ZrO2=
+2ZrO2=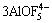 +
+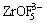 +ZrF4+2AlF3 (2)
+ZrF4+2AlF3 (2)
nZr4++nxF-=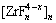 (3)
(3)
The radiuses (volumes) of these ions are larger than those of 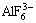 complex [15,16], which already exists in the molten salt system. Correspondingly, it will be more difficult for small ions, such as sodium ion, to move in the melt and then reduces the electrical conductivity of molten electrolyte. Therefore, ZrO2 reduces the electrical conductivity of molten electrolyte.
complex [15,16], which already exists in the molten salt system. Correspondingly, it will be more difficult for small ions, such as sodium ion, to move in the melt and then reduces the electrical conductivity of molten electrolyte. Therefore, ZrO2 reduces the electrical conductivity of molten electrolyte.
3.4 Effect of molar ratio of NaF to AlF3 on electrical conductivity
Figure 3 shows the effect of molar ratio of NaF to AlF3 on the electrical conductivity of NaF·AlF3- 3%Al2O3-3%CaF2-mZrO2 molten salt system at 985 °C. The results show that the conductivity increases with the increase of molar ratio of NaF to AlF3. When the molar ratio of NaF to AlF3 increases from 2.2 to 2.6, the electrical conductivity increases by 0.189 S/cm.
Ions in Na3AlF6-Al2O3 system include 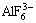 ,
, 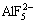 ,
, 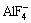 , F- and Al-O-F ion complex [16,17]. The formation of Al-O-F ion complex is shown as follows:
, F- and Al-O-F ion complex [16,17]. The formation of Al-O-F ion complex is shown as follows:
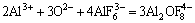 (4)
(4)
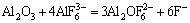 (5)
(5)
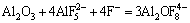 (6)
(6)
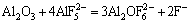 (7)
(7)
With the increase of molar ratio of NaF to AlF3, the concentration of AlF3 is decreased, and then ion complex will decrease according to the reactions. Therefore, the number of mobility ion is increased, which increases the electrical conductivity of molten electrolyte.
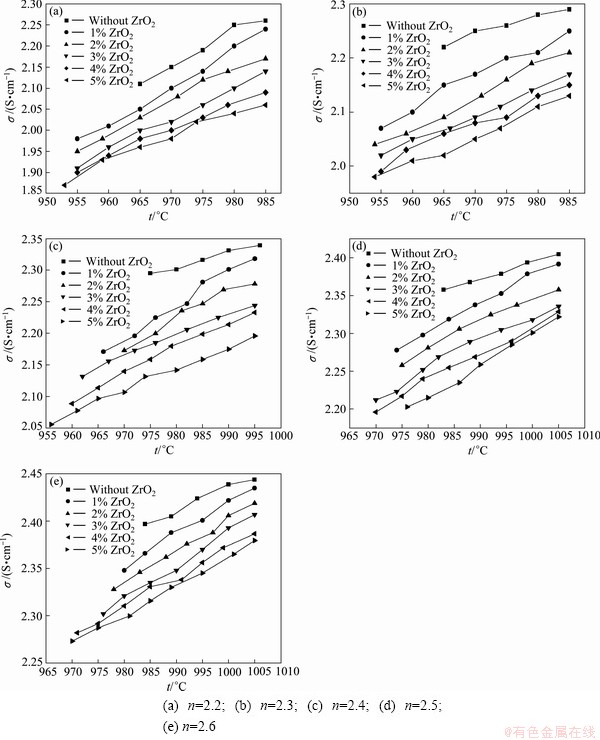
Fig. 2 Electrical conductivity for various ZrO2 additions of nNaF·AlF3-3%Al2O3-3%CaF2
3.5 Effect of ZrO2 on electrical conductivity at different superheats
Figure 4 shows the electrical conductivity of molten salts as a function of ZrO2 content at superheats of 15, 20 and 25 °C for 2.4NaF·AlF3- 3%Al2O3-3%CaF2-ZrO2 molten salt system. Increasing superheat results in increase of the electrical conductivity. The electrical conductivity increases by about 0.020 S/cm for increase of 5 °C superheat. For 1% ZrO2, the electrical conductivity increases from 2.215 to 2.271 S/cm when the superheat increases from 15 to 25 °C. For 5% ZrO2, the electrical conductivity increases from 2.078 to 2.118 S/cm when the superheat increases from 15 to 25 °C.
Because of high price of ZrO2 and significant effects of superheat on electrolytic conductance, it is reasonable to make the proper concentration of ZrO2 for industrial practice according to the above study.
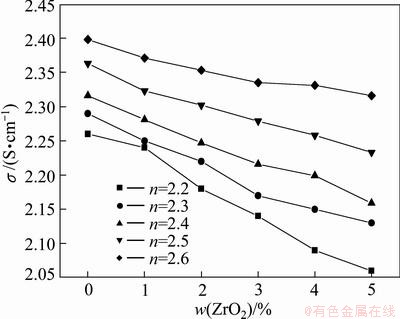
Fig. 3 Electrical conductivity for various molar ratios of NaF to AlF3 at 985 °C

Fig. 4 Electrical conductivity as function of ZrO2 content at different superheats with molar ratio of NaF to AlF3 of 2.4
3.6 Effect of temperature on electrical conductivity
The conductivity of the molten salt is mainly determined by ion movement, and the temperature is one of the most important parameters. Therefore, temperature has a great influence on the electrical conductivity. The temperature dependence of the electrical conductivity of molten salts of predominant ionic characteristic is given in the form of a basic Arrhenius equation:
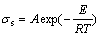 (8)
(8)
where 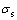 is the specific conductivity; A is the coefficient related to chemical composition of melt; E is the activation energy of conductance; T is the temperature of the melt; R is the gas molar constant.
is the specific conductivity; A is the coefficient related to chemical composition of melt; E is the activation energy of conductance; T is the temperature of the melt; R is the gas molar constant.
The A and E are constants, and in the molten salt, the logarithm form of Eq. (8) is
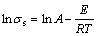 (9)
(9)
According to the formula (9), lnσs and T-1 have a linear relationship. Figure 5 shows the relationship between lnσs and T-1 of 2.2NaF·AlF3-3%Al2O3- 3%CaF2-ZrO2 molten salt system with different additions of ZrO2. The results show that lnσs and T-1 have a good linear relationship, and the correlation coefficients are higher than 0.98.
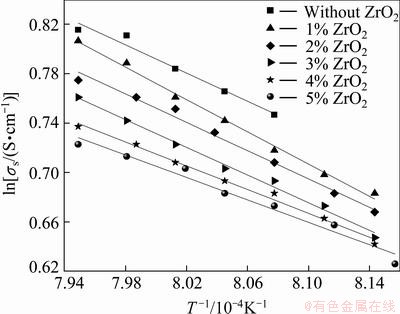
Fig. 5 Relationship between ln σs and T-1 of molten salts
Figure 5 shows that the electrical conductivity of molten salts increases with increasing temperature. When the temperature increases by 1 °C, the electrical conductivity increases by 0.004 S/cm. The heat movement of particles speeds up when the temperature increases. Bonding energy between ions with contrary charge is weakened because of acceleration of heat movement. Directional movement of ion is easier. The number of free ions increases because of decomposition of more ion complexes with increasing temperature. Decomposition of the ion complexes increases the ion mobility and thereby increases the electrical conductivity.
3.7 Regression equation of electrical conductivity
According to the measured results, the regression equations of electrical conductivity related to temperature and ZrO2 content are obtained by quadratic regression as
For 2.2 NaF/AlF3:
σ=-71.2293+0.1455t+0.448w(ZrO2)-5×10-4tw (ZrO2)-7.2×10-5t2+2.2×10-3w(ZrO2)2 (10)
Scope of application is t=965-985 °C, w(ZrO2)=0-5%.
For 2.3 NaF/AlF3:
σ=-32.1554+0.0662t-0.4223w(ZrO2)+4×10-4tw(ZrO2)-3.18×10-5t2+4.8×10-3w(ZrO2)2 (11)
Scope of application is t=965-985 °C, w(ZrO2)=0-5%.
For 2.4 NaF/AlF3:
σ=2.3516-0.0030t-0.2177w(ZrO2)+1.92×10-4tw(ZrO2)+3×10-6t2+2×10-3w(ZrO2)2 (12)
Scope of application is t=975-995 °C, w(ZrO2)=0-5%.
For 2.5 NaF/AlF3:
σ=-12.455+0.0268t-0.4939w(ZrO2)+4.8×10-4tw(ZrO2)-1.2×10-5t2+1.8×10-3w(ZrO2)2 (13)
Scope of application is t=985-1005 °C, w(ZrO2)=0-5%.
For 2.6 NaF/AlF3:
σ=-12.1895+0.0266t-0.2016w(ZrO2)+2×10-4tw(ZrO2)-1.2×10-5t2+1.25×10-3w(ZrO2)2 (14)
Scope of application is t=985-1005 °C, w(ZrO2)=0-5%.
4 Conclusions
1) Electrical conductivities of (2.2-2.6) NaF·AlF3- 3%Al2O3-3%CaF2 molten salt system decrease with increase of ZrO2 content in an interval of 0-5%. 1% ZrO2 results in a corresponding decrease of 0.02 S/cm, and the equivalent conductivity increases with the increase of molar ratio of NaF to AlF3. When the temperature increases by 1 °C, the electrical conductivity increases by 0.004 S/cm.
2) The suitable electrolyte composition for producing Al-Zr alloy by molten salt electrolysis technique is 2.4NaF·AlF3-3%CaF2-3%Al2O3-2%ZrO2 system and the electrolysis temperature is ranging from 970 to 985 °C.
References
[1] GUO J Q, OHTERA K. An intermediate phase appearing in Ll2-Al3Zr to D023-A13Zr phase transformation of rapidly solidified Al-Zr alloys [J]. Materials Letters, 1996, 27(7): 343-347.
[2] SRINIVASARAO B, SURYANARAYANA C, OH-ISHI K, HONO K. Microstructure and mechanical properties of Al-Zr nanocomposite materials [J]. Materials Science and Engineering A, 2009, 518: 100-107.
[3]  C, GOYOS L. Nanostructured Al-ZrAl3 materials consolidated via spark plasma sintering: Evaluation of their mechanical properties [J]. Journal of Alloys and Compounds, 2013, 550: 402-405.
C, GOYOS L. Nanostructured Al-ZrAl3 materials consolidated via spark plasma sintering: Evaluation of their mechanical properties [J]. Journal of Alloys and Compounds, 2013, 550: 402-405.
[4] JIA Zhi-hong, COUZINIE J P, CHERDOUDI N, GUILLOT I, ARNBERG L,  P, BR S. Precipitation behavior of Al3Zr precipitate in Al-Cu-Zr and Al-Cu-Zr-Ti-V alloys [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(8): 1860-1865.
P, BR S. Precipitation behavior of Al3Zr precipitate in Al-Cu-Zr and Al-Cu-Zr-Ti-V alloys [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(8): 1860-1865.
[5] KNIPLING K E, DUNAND D C, SEIDMAN D N. Precipitation evolution in Al-Zr and Al-Zr-Ti alloys during aging at 450-600 °C [J]. Acta Materialia, 2008, 56: 1182-1195.
[6] RAJAGOPALAN P K, SHARMA I G, KRISHNAN T S. Production of Al-Zr master alloy starting from ZrO2 [J]. Journal of Alloys and Compounds, 1999, 285: 212-215.
[7] CAO Da-li, SHI Zhong-ning, WANG Ji-kun, QIU Zhu-xian. Thermodynamics of electrolytic Al-Zr master alloys [J]. Journal of Northeastern University: Natural Science, 2006, 27: 513-516. (in Chinese)
[8] WARREN H. The liquidus enigma [J]. Light Metals, 1992, 192: 477-480.
[9] HU Xian-wei, WANG Zhao-wen, LU Gui-min, SHI Zhong-ning, CAO Xiao-zhou, CUI Jian-zhong, ZHAO Xing-liang. Equivalent circuit analysis and application for electrical conductivity measurement by continuously varying cell constant technique [J]. Transactions of Nonferrous Metals Society of China, 2008, 8(3): 551-556.
[10] STERTEN, SKAR O. Some binary Na3AlF6-MxOy phase diagrams [J]. Aluminium, 1988, 64(10): 1051-1054.
[11] SCHIEFELBEIN S L, SADOWAY D R. A high-accuracy, calibration-free technique for measuring the electrical conductivity of molten oxides [J]. Metallurgical and Materials Transactions B, 1997, 28: 1141-1149.
[12] GRJOTHEIM K, NIKOLIC R,  H A. Electrical conductivities of binary and ternary melts between MgCl2, CaCl2, NaCl, and KCl [J]. Acta Chemica Scandinavica, 1970, 24: 489-509.
H A. Electrical conductivities of binary and ternary melts between MgCl2, CaCl2, NaCl, and KCl [J]. Acta Chemica Scandinavica, 1970, 24: 489-509.
[13] WANG Xiang-wen, PETERSON R D, TABEREAUX A T. Electrical conductivity of cryolitic metals [C]//Light Metals, 1992: 481-488.
[14] BAO Morigengaowa, WANG Zao-wen, GAO Bing-liang, SHI Zhong-ning, HU Xian-wei. Effect of the addition of ZrO2 on the liquidus temperature of nNaF·AlF3-Al2O3 molten salt system [C]//World Non-Grid- Connected Wind Power and Energy Conference (WNWEC). Nanjing, China: World Wind Energy Institute, 2010: 211-213.
[15] ROLLET A. L, SALANNE M, GROULT H. Structural effects on the electrical conductivity of molten fluorides: Comparison between LiF–YF3 and LiF–NaF–ZrF4 [J]. Journal of Fluorine Chemistry, 2012, 134: 44-48.
[16] HU Xian-wei, QU Jun-yue, GAO Bing-liang, SHI Zhong-ning, LIU Feng-guo, WANG Zhao-wen. Raman spectroscopy and ionic structure of Na3AlF6-Al2O3 melts [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(2): 402-406.
[17] HU Xian-wei, WANG Zhao-wen, GAO Bing-liang, SHI Zhong-ning, LIU Feng-guo, BAO Morigengaowa. Identification of structural entities in NdF3-LiF melts with cryoscopic method [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(12): 2387-2391.
NaF·AlF3-Al2O3-CaF2-ZrO2熔盐体系的电导率
包莫日根高娃1,2,王兆文1,高炳亮1,石忠宁1,胡宪伟1,于江玉1
1. 东北大学 材料与冶金学院,沈阳 110819;
2. 内蒙古民族大学 分析测试中心,通辽 028000
摘 要:采用改进的固定电导池常数法研究NaF-AlF3-CaF2-Al2O3-ZrO2熔盐体系的电导率。结果表明,NaF-AlF3-3%Al2O3-3%CaF2-ZrO2熔盐体系的电导率随着ZrO2含量(0~5%)的增加呈降低趋势。每增加1%氧化锆,电导率相应地降低约0.02 S/cm。温度每增加1 °C时电导率相应降低约0.004 S/cm。随着NaF与AlF3摩尔比的增加,体系的电导率随之增加。最后采用二次回归正交设计方法,建立分子比相同时电解质的电导率与温度和氧化锆浓度关系的回归方程。
关键词:ZrO2;熔盐电解法;电导率;NaF;AlF3;Al-Zr合金
(Edited by Xiang-qun LI)
Foundation item: Project (2007CB210305) supported by the National Basic Research Program of China; Project (51074045) supported by the National Natural Science Foundation of China
Corresponding author: Morigengaowa BAO; Tel: +86-24-83688273; E-mail: gaowa2009@126.com
DOI: 10.1016/S1003-6326(13)62930-1
Abstract: The electrical conductivity of NaF-AlF3-CaF2-Al2O3-ZrO2 system was studied by a tube-type cell with fixed cell constant. The results show that the electrical conductivity of NaF-AlF3-3%Al2O3-3%CaF2-ZrO2 molten salt system decreases with increase of ZrO2 content in an interval of 0-5%. The increase of 1% ZrO2 results in a corresponding electrical conductivity decrease of 0.02 S/cm, and the equivalent conductivity increases with the increase of molar ratio of NaF to AlF3. When the temperature increases by 1 °C, the electrical conductivity increases by 0.004 S/cm. At last, the regression equations of electrical conductivity relative to temperature and ZrO2 are obtained by quadratic regression analysis.


