
Liquid-nitrogen cryopreservation of three kinds of autotrophicbioleaching bacteria
WU Xue-ling(吴学玲)1, XIN Xiao-hong(辛晓红)2, JIANG Ying(蒋 莹)1,
LIANG Ren-xing(梁任星)1, YUAN Peng(袁 鹏)1, FANG Cheng-xiang(方呈祥)2
1. Key Laboratory of Biometallurgy of Ministry of Education,
School of Minerals Processing and Bioengineering, Central South University,
Changsha 410083, China;
2. China Center for Type Culture Collection, College of Life Science, Wuhan University,
Wuhan 430072, China
Received 20 September 2008; accepted 5 November 2008
Abstract:
Three kinds of autotrophic bioleaching bacteria strains, including mesophilic and acidophilic ferrous ion-oxidizing bacteria Acidithiobacillus ferrooxidans (A. ferrooxidans), mesophilic and acidophilic sulfur-oxidizing bacteria Acidithiobacillus thiooxidans (A. thiooxidans), and moderately thermophilic sulfur-oxidizing bacteria Acidianus brierleyi, were cryopreserved in liquid nitrogen and their ferrous ion- or sulfur-oxidizing activities were investigated and compared with the original ones. The results revealed that ferrous ion/sulfur oxidation activities of the strains were almost equal before and after cryopreservation. Glycerin was used as cryoprotective agent. In conclusion, liquid-nitrogen cryopreservation is a simple and effective method for autotrophic bioleaching microorganisms.
Key words:
A. ferrooxidans; A. thiooxidans; Acidianus brierleyi; cryopreservation; liquid nitrogen; growth activities;
1 Introduction
Bioleaching is an approach of hydrometallurgy to extract metals from ores using microorganisms. This technique can offer a number of advantages in recovering metals from the low grade ore, boundary ore and tailings for its short process, low cost and little pollution. Some autotrophic bacteria play an important role in heap leaching and in-situ leaching of refractory ore[1-2]. There are diverse microbes involved in the bioleaching systems, including autotrophic or facultatively autotrophic bacteria such as A. ferrooxidans[3], A. thiooxidans, Leptospirillus ferrooxidans and Ferrobacillus ferrooxidans. And some other acidophilic thermophilic strains such as Sulfolobus acidocadarius, Sulfobacillus thermosulfidooxidans and Acidianus brierleyi[4] are also widely utilized in bioleaching[5]. Researchers have made great efforts to screen leaching microorganisms from environment. Therefore, how to preserve the bioleaching microbes becomes an important issue. The previous studies indicated that frequent subculture resulted in variation, decline of the characteristics and even death of the strains[6]. However, currently few investigations have been carried out in the terms of the preservation of bioleaching microorganisms. As far as the conservation of A. ferrooxidans is concerned, ZHOU et al[7] explored the effects of dryness preservation on the Fe2+ oxidation of A. ferrooxidans, and ZHANG et al[8-10] investigated several methods for short-term preservation of A. ferrooxidans. ZHOU et al[11] preserved A. caldus by freeze-dry preservation. Furthermore, CLELAND et al[12] reported the method of using glycine betaine as a cryoprotectant for prokaryotes including A. ferrooxidans and A. thiooxidans.
Preserving bacteria in liquid nitrogen is a routine method in strain conservation. The cells can be preserved for a long time in the liquid phase of liquid nitrogen pot at -196 ℃ or in the gas phase at -150 ℃. The technique of cryoconservation in liquid nitrogen is advantageous because of its wide range, long-term storage and little mutation on all the cells. Numerous investigations have been reported on the conservation of heterotrophic bacteria by liquid nitrogen method. However, few studies have been published for autotrophic microorganisms including bioleaching bacteria with the method. In this study, we aim at exploring the feasibility of conserving bioleaching bacteria by liquid nitrogen method.
2 Experimental
2.1 Microorganisms and culture medium
Mesophilic and acidophilic ferrous ion-oxidizing bacteria A. ferrooxidans DC[13] was grown in 9K medium[14] with initial pH of 2.0. Mesophilic and acidophilic sulfur-oxidizing bacteria A. thiooxidans DMC was grown in Starky liquid medium with initial pH of 2.0[15]. Moderately thermophilic sulfur-oxidizing bacteria Acidianus brierleyi type strain JCM8954T was purchased from JCM. The strain was grown in JCM medium 176 with initial pH of 2.0. Both A. ferrooxidans and A. thiooxidans were incubated at 30 ℃ and the Acidianus brierleyi was incubated at 65 ℃. Three strains were on a rotary shaker of 180 r/min. All the cultured media were autoclaved at 121 ℃ and 103 kPa for 30 min, respectively.
2.2 Cryoprotective agent
Two cryoprotective agents were used in this study. One of them is glycerin, the other is designated as GP provided by the China Center for Type Culture Collection(CCTCC). The glycerin was diluted to concentration of 60% (volume fraction) with deionized water and 100% GP. Two cryoprotectants were autoclaved at 121 ℃ and 103 kPa for 30 min, respectively. The different concentrations of the protectants used in the study are listed in Table 1, the suspensions with different protectant combined are listed in Table 2.
Table 1 Concentrations of cryoprotectants using for preserva- tion of bacteria
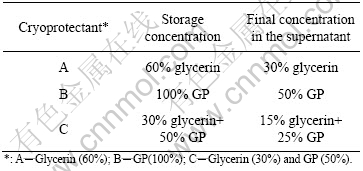
Table 2 Different combinations of bacteria with cryoprotective additives

2.3 Incubation and collection of bacteria
The cells of A. ferrooxidans, A. thiooxidans and Acidianus brierleyi were harvested during late logarithmic phase. The cultures of A. ferrooxidans and Acidianus brierleyi were divided into two test groups, respectively. The jarosite (or sulfur) were filtrated removing for one group and the other was not. For the culture of A. thiooxidans, the sulfur was removed by filtration. All the cultures were centrifuged at 10 000 r/min for 15 min and the cells were harvested, respectively. Collected cells were washed twice using H2SO4 (pH=2.0). The cell pellets were resuspended with deionized water and mixed well with equivalent volume of cryoprotective, and then transferred them into the frozen tubes, respectively.
2.4 Pre-freezing procedure and conservation
First, the frozen tubes were held at 4 ℃ for about 30-50 min and then transferred them into -20 ℃ for another 30-50 min, and then the tubes were kept at -80 ℃ for 2 h. Finally, the frozen tubes were stored in the liquid phase of liquid nitrogen.
2.5 Recovery of frozen strains and Activity measure- ment
The frozen tubes were taken out from liquid nitrogen after 7 d and immediately thawed in water bath at 37 ℃. The cryoprotective agent was removed and washed twice with culture medium without ferrous ions or sulfur. Then recovered cells were inoculated in the related fresh medium. The bacteria were harvested during late logarithmic phase and then inoculated into another corresponding medium. Cellar densities of A. ferrooxidans, A. thiooxidans and Acidianus brierleyi were 5×105, 3×106 and 5×105 mL-1, respectively. The concentrations of ferrous ion were measured using the potassium dichromate titrimetric method[16]. Change of the sulfur oxidation of A. thiooxidans and Acidianus brierleyi can be evaluated with pH variation of the medium were measured by precision acidity meter (pHS-3C). All experiments were repeated in triplicate using the original strains as controls and aseptic medium without inoculation as blank control.
3 Results
3.1 Fe2+ oxidation activity of A. ferrooxidans strain DC
During the recovery culture after cryopreservation, the complete Fe2+ oxidation time of the three filtered groups (A-Ff, B-Ff, C-Ff) was 6 d, 3 d and 4 d, respectively. Whereas the three unfiltered ones (A-UFf, B-UFf, C-UFf) took 9 d, 5 d, and 7 d to completely oxidize the Fe2+ under the same condition (Table 3). In the first activation period, the filtered group took little time to oxidize Fe2+ in the 9K medium compared with the unfiltered one.
Table 3 Time of complete Fe2+ oxidization by A. ferrooxidans strain DC

The cryoprotected strain A. ferrooxidans strain DC was inoculated in fresh 9K medium and the Fe2+ oxidation activity is shown in Fig.1. According to the Fe2+ oxidation curve of the original strain (Fig.1(a)), the bacteria turned into logarithmic phase after 18 h and Fe2+ was 100% oxidized at 75 h. Interestingly, the filtered groups A-Ff, B-Ff and C-Ff also turned into logarithmic phase after 18 h and Fe2+ was also completely oxidized at 75 h (Fig.1(b)). However, the unfiltered groups A-UFf, B-UFf and C-UFf entered into logarithmic phase after 24 h and Fe2+ was also wholly oxidized after 75 h, as shown in Fig.1(c). Moreover, the Fe2+ oxidation capability was equivalent to the original strain irrespective of the three different cryoprotective agents.
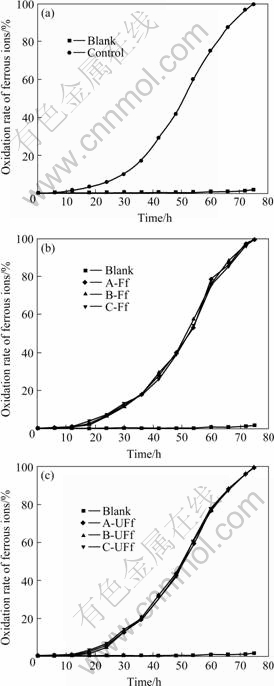
Fig.1 Ferrous ions oxidation rate in 9K medium by A. ferrooxidans strain DC: (a) Original strain; (b) Filtered groups A-Ff, B-Ff and C-Ff; (c) Unfiltered groups A-UFf, B-UFf and C-UFf
3.2 Sulfur oxidation activity of A. thiooxidans strain DMC
A. thiooxidans strain DMC was recovered using the same method in Starky liquid medium and achieved the active phase after 8 d, 10 d, and 7 d (Table 4). Obviously, A. thiooxidans strain DMC protected by 25% GP+15% glycerin took the least time to achieve the active phase in the first activation period.
Table 4 Time to achieve active phase by A. thiooxidans strain DMC

The recovered A. thiooxidans strain DMC was then transferred again to another fresh Starky liquid medium. The sulfur oxidation activity and growth curve were measured (Fig.2). According to growth curve (Fig.2(a)) and the curve of pH variation (Fig.2(b)) of the original strain, the bacteria turned into logarithmic phase for 42 h and went into active phase after 162 h. As shown in Fig.2(c) and Fig.2(d), the times of the activated strain needed to enter the logarithmic phase and active phase are 42 h and 162 h, respectively. The results revealed that the sulfur oxidation rate and growth rate of A. thiooxidans strain DMC conserved in liquid nitrogen were relatively equal to those of the original strain after recovered from frozen state.
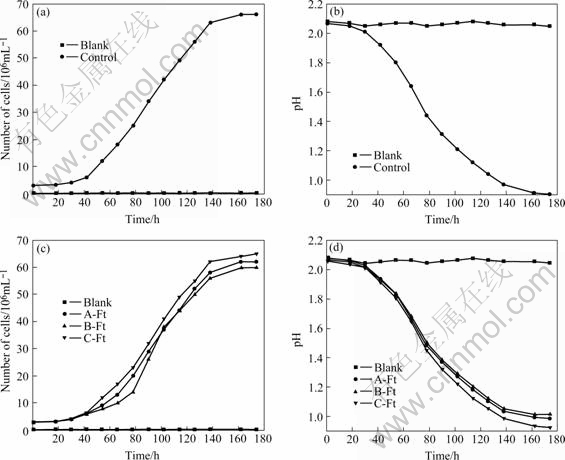
Fig.2 Curve of strain growth and pH variation of A. thiooxidans strain DMC: (a) Growth of original strain; (b) pH variation of original strain; (c) Growth of A. thiooxidans strain DMC cryopreserved; (d) pH variation of A. thiooxidans strain DMC cryopreserved
3.3 Sulfur oxidation activity of Acidianus brierleyi JCM8954T
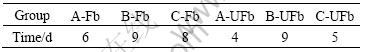
During the course of recovery cultivation, the filtered groups (A-Fb, B-Fb and C-Fb) took 6 d, 9 d, and 8 d to achieve active phase, respective; while the unfiltered groups (A-UFb, B-Ufb and C-UFb) took only 4 d, 9 d, and 5 d respectively to enter into the active phase (Table 5). It is obviously that in the first activation period, the unfiltered groups took less time to achieve active phase than the filtered groups.
Table 5 Time to achieve active phase by Acidianus brierleyi JCM8954T
The recovered cells were inoculated again into another fresh medium and the sulfur oxidation activity was measured (Fig.3). As indicated by Fig.3(a), the original strain turned into logarithmic phase after 20 h and achieved active phase after 68 h. We can also know from Fig.3(b) and Fig.3(c) that both the filtered groups (4A, 4B, 4C) and the unfiltered groups (5A, 5B, 5C) took almost the same time to turn into the logarithmic phase and active phase with the original strain. The results suggested that the sulfur oxidation capability of Acidianus brierleyi JCM8954T showed no change. It was irrespective of the kind of cryoprotective additives used.
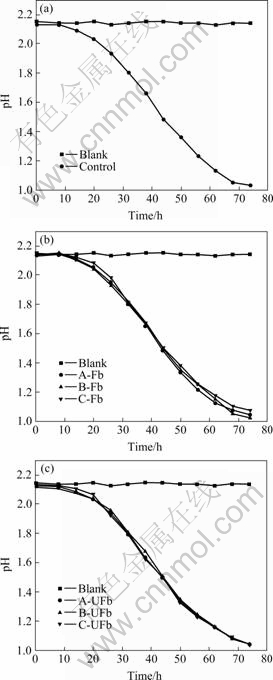
Fig.3 pH variation of Acidianus brierleyi JCM8954T in culture medium: (a) Original strain; (b) Filtered groups A-Fb, B-Fb and C-Fb; (c) Unfiltered groups A-UFb, B-UFb and C-UFb
4 Discussion
Bacterial strain is an important microbiological resource. Once a fine strain is obtained, it becomes much imperative to maintain it under feasible condition. A. ferrooxidans, A. thiooxidans and Acidianus brierleyi are all extremophiles and show unique physiological structures. They exhibit strong selectivity as well as great endurance to the living environment. In the process of cryopreservation in liquid nitrogen, cyoprotective agent is a key factor for some bacteria. Otherwise, it will reduce the survival rate of the bacterial cells[17-18].
In the present study, three strains of A. ferrooxidans, A. thiooxidans and Acidianus brierley took the least time to turn into active phase when 50% CCPA, 25% CCPA+ 15% glycerin and 30% glycerin were used as cryoprotectants, respectively. During the first recovery process, when jarosite (or sulfur) was filtered, the A. ferrooxidans strain DC took somewhat shorter time to enter into the active phase than the unfiltered one. Whereas it didn’t affect Acidianus brierleyi strain JCM8954 no matter the bacteria are filtered or not. In the second activation, three bacterial growth activities remain almost the same compared with the unfiltered one. This means that the autotrophic bioleaching strains may be unfilteredly cryopreserved in liquid nitrogen. Glycerol is a common reagent used in the laboratory. Numerous researches have proved its excellent efficiency in microbial conservation. However, the activities of autotrophic bioleaching bacteria are often inhibited by organic compounds including glycerol, agar, etc. So our results showed that 30% glycerin could be used as a cryoprotective agent to protect bioleaching strains from damage in liquid nitrogen.
5 Conclusions
1) Liquid-nitrogen cryopreservation is a simple and effective method for conservation of the unique autotrophic bioleaching bacteria including mesophilic acidophilic and moderately thermophilic bacteria, ferrous iron-oxidizing and sulfur-oxidizing bacteria.
2) 30% glycerin could be used as a cryoprotectant and insoluble sediments in the culture medium were not filtratedly removed.
3) The growth activities and ferrous iron (or sulfur) oxidation capabilities of three strains remained almost unchanged when any kind of cryoprotectant was used.
References
[1] YANG Xian-wan, SHEN Qing-feng, GUO Yu-xia. Microbial hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2003: 2.
[2] OLSON G J, BRIERLEY J A, BRIERLEY C L. Bioleaching review (part B): Applications of microbial processes by the minerals industries [J]. Applied Microbiology and Biotechnology, 2003, 63(3): 249-257.
[3] KELLY D P, WOOD A P. Reclassification of some species of Thibacillus to the newly designated genera Acidithiobacillus gen.nov., Halothibacillus gen.nov. and Thermithiobacillus gen.nov. [J]. International Journal of Systematic and Evolutionary Microbiology, 2000, 50: 511-516.
[4] SEGERER A, NEUNER A, KRISTJANSSON J K, STETTER K O. Acidianus infernus gen.nov., sp. nov., and Acidianus brierleyi comb. nov.: Facultatively aerobic, extremely acidophilic thermophilic sulfur-metabolizing archaebacteria [J]. International Journal of Systematic Bacteriology, 1986, 36(4): 559-564.
[5] ZHANG Zai-hai, WANG Dian-zuo, QIU Guan-zhou, HU Yue-hua. The bacteriological fundamental of bacterial leaching [J]. Hydrometallurgy of China, 2000, 19(3): 16-21.
[6] SHI Qiao-qing, WU Song-gang. Breeding of industrial microorganism [M]. Beijing: Science Press, 2003: 68.
[7] ZHOU Ji-kui, QIU Guan-zhou, NIU Yin-jian, QIN Wen-qing. Effect of dryness preservation on Fe2+ oxidation activity of Thiobacillus ferrooxidans [J]. Journal of Central South University: Natural Science, 2004, 35(1): 39-42.
[8] YANG Yu, ZHANG Yan-fei, HUANG Ju-fang, JIANG Dong-hai, CHEN Yong, QIU Guan-zhou. Preservation of Acidithiobacillus ferrooxidans [J]. Journal of Central South University: Natural Science, 2006, 37(3): 472-475.
[9] YANG Yu, ZHANG Yan-fei, HE Huan, WAN Min-xi, SHI Wu-yang, QIU Guan-zhou. Optimization of frozen storage cryoprotectant for Acidithiobacillus ferrooxidans by orthogonal experiment [J]. Journal of Central South University: Natural Science, 2006, 37(5): 891-895.
[10] ZHANG Yan-fei, HE Huan, WAN Min-xi, SHI Wu-yang, YANG Yu. Freeze-drying cryoprotectant optimized by orthogonal experiment for Acidithiobacillus ferrooxidans [J]. Progress in Modern Biomedicine, 2006, 6(5): 5-7.
[11] ZHOU Hong-bo, ZENG Wei-min, LI Ying, LIU Jian-she, QIU Guan-zhou. Freeze-dry cryoprotectants of moderate thermophilic bacteria for bioleaching [J]. Nonferrous Metals, 2008, 60(3): 80-83.
[12] CLELAND D, KRADER P, MCCREE C, TANG J, EMERSON D. Glycine betaine as a cryoprotectant for prokaryotes [J]. Journal of Microbiological Methods, 2004, 58(1): 31-38.
[13] WU Xue-ling, JIANG Ying, QIU Guan-zhou, LIU Xin-xing, WENG Wen-yun. Isolation of Acidithiobacillus ferrooxidans strains and effect of anions on their ferrous ions oxidation capacities [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(2): 349-355.
[14] WU Xue-ling, DING Jian-nan, GAO Jian, LIU Xin-xing, QIU Guan-zhou. Isolation and identification of metal-resistant iron-oxidizing bacteria [J]. Minerals & Metallurgical Processing, 2007, 24(1): 57-60.
[15] FU Bo, ZHOU Hong-bo, ZHANG Qian, QIU Guan-zhou. Isolation and characterization of three strains of Acidithiobacillus thiooxidans and its bioleaching of chalcopyrite [J]. Journal of Microbiology, 2008, 28(1): 12-19.
[16] HARVEY P I, CRUNDWELL F K. Growth of thiobacillus ferrooxidans: A novel experimental design for batch growth and bacterial leaching studies [J]. Applied and Environmental Microbiology, 1997, 63(7): 2586-2592.
[17] MILLS C K, GHERNA R L. Cryopreservation studies of campylobacter [J]. Cryobiology, 1988, 25(2): 148-152.
[18] FANG Cheng-xiang, ZHANG Luo-zhen, Yue Ying-yu, TAO Tian-shen, DUAN Lan-xian, XU Chun-hua, XU Wen, ZHENG Jing. Cryopreservation of glutamic acid-producing strain-brevibacterium sp. F9114 in liquid nitrogen [J]. Food and Fermentation Industries, 1996, 6: 18-21.
Foundation item: Project(50621063) supported by Chinese Science Foundation for Distinguished Group; Project(2004CB619201) supported by the National Basic Research Program of China
Corresponding author: FANG Cheng-xiang; Tel: +86-27-68752319; E-mail: cxfang@whu.edu.cn
(Edited by YANG Bing)


