J. Cent. South Univ. (2016) 23: 2199-2204
DOI: 10.1007/s11771-016-3277-8
Reagent optimization for on-line simultaneous polarographic determination of trace amounts of Cu2+Cd2+ and Co2+ in the presence of anextremely large excess of Zn2+
WANG Guo-wei(王国伟)1, YANG Chun-hua(阳春华)1, ZHU Hong-qiu(朱红求)1, 2,
LI Yong-gang(李勇刚)1, GUI Wei-hua(桂卫华) 1
1. School of Information Science and Engineering, Central South University, Changsha 410083, China;
2. School of Metallurgy and Environment, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
Reagents are optimized for the simultaneous determination of trace amounts of Cu2+, Cd2+ and Co2+ in zinc sulfate solution, which contains an extremely large excess of Zn2+. First, the reagents and their doses for the experiment are selected according to the characteristics of the zinc sulfate solution. Then, the reagent doses are optimized by analyzing the influence of reagent dose on the polarographic parameters (i.e. half-wave potential E1/2 and limiting diffusion current Ip). Finally, the optimization results are verified by simultaneously determining trace amounts of Cu2+, Cd2+ and Co2+ in the presence of an extremely large excess of Zn2+. The determination results indicate that the optimized reagents exhibit wide linearity, low detection limits, high accuracy and good precision for the simultaneous determination of trace amounts of Cu2+, Cd2+ and Co2+ in the presence of an extremely large excess of Zn2+.
Key words:
1 Introduction
Trace polymetallic ions, such as Cu2+, Cd2+ and Co2+, that coexist in zinc sulfate solution are harmful for the electrowinning process in zinc hydrometallurgy when its concentrations are substandard [1]. For example, Cd2+ and Co2+ will deteriorate the quality and morphology of cathode zinc [2], Cu2+ and Co2+ may cause “plate-burning” [3], and Co2+ will also substantially reduce the current efficiency [4]. In addition, excess ions in discharged wastewater will result in heavy metal contamination, which poses a threat to environmental protection [5]. Therefore, these ions must be controlled in the purification process [6].
The real-time and accurate determination of trace polymetallic ions is required for optimal control [7], which can provide effective feedback information. Linear sweep polarography (LSP) [8-10] is an especially suitable determination method [11] due to its superiority properties (i.e. rapid, stable, sensitive, selective, reproducible and accurate). More importantly, the working electrode of LSP doesn’t have to be replaced during the on-line determining process compared with other electrochemical methods [12]. However, the concentration ratio (CR) between the matrix (Zn2+) and the impurities (Cu2+, Cd2+ and Co2+) in the zinc sulfate solution is more than 106, which leads to the polarographic signals of impurities being easily covered by the matrix peak. In addition, overlapping peaks are inevitably generated due to the small difference in the half-wave potentials between Zn2+ and Co2+ [13]. These are the two main challenges in the simultaneous determination of trace polymetallic ions in zinc sulfate solution. Limited studies have been reported in this area. Most of the existing studies are focused on the low CR between matrix and impurities [14-15]. Nascimento et al [16] reported the one-voltammogram simultaneous determination of Cu(II), Pb(II), Cd(II), Ni(II), Co(II), and Zn(II) at ppb and sub-ppb levels in bioethanol fuel samples by adsorptive stripping voltammetry. Mixed ligands were employed providing stripping peaks for all analyses at the same voltammetric scan under optimized conditions Herrero et al [17] described a sensitive and selective method for the simultaneous determination of lead, cadmium and zinc based on the formation of their complexes with Clioquinol. Zhang et al [18] developed a new method for simultaneous determination of six trace elements (Cu(II), Pb(II), Cd(II), Ni(II), Co(II), and Zn(II)) in aqueous solutions. In conjunction with a microwave assimilation technique, the method has been used in the rapid and simultaneous determination of these trace elements in some plant medicines with satisfactory results.
In addition, high concentration cases only focus on Zn2+ and Co2+. Borovkov and MONASTYRSKAYA [19] developed a method for automated polarographic analysis for Co(II) ions in technological solutions of zinc production. The method is intended for determining the content of Co(II) in purification of a zinc sulfate solution to removed impurities and ecological monitoring of wastewater produced at nonferrous metallurgy plants. A simple and rapid on-line differential pulse polarographic (DPP) method for direct Co2+ determination in Zn plant electrolyte was developed and tested in real solutions, in which 1-nitroso-2-naphtol was applied as Co2+ to Co3+ oxidizing and chelate forming agent in ammonia buffer supporting electrolyte [20].
To improve the resolution of determining trace amounts of Cu2+, Cd2+ and Co2+ in the presence of an extremely large excess of Zn2+, a novel reagent is developed in this work. First, the reagents and their doses for the experiment are selected according to the characteristics of the zinc sulfate solution. Then, the reagent doses are optimized by analyzing the influence of reagent dose on the polarographic parameters (i.e. half-wave potential E1/2 and limiting diffusion current Ip). Finally, the optimization results are verified by simultaneously determining trace amounts of Cu2+, Cd2+ and Co2+ in the presence of an extremely large excess of Zn2+.
2 Experimental
2.1 Reagents and instruments
The zinc sulfate solution in the hydrometallurgical process contains trace amounts of polymetallic ions, and the mass concentrations are 140 g/L Zn2+, 0.6 g/L Cu2+, 0.3 g/L Cd2+ and 0.4 mg/L Co2+, which result in a high CR. The reagents should satisfy the requirements of simultaneous determination including high stability, inhibiting the sensitivity of the matrix (Zn2+), substantially increase the sensitivity of the impurities (Cu2+, Cd2+ and Co2+), extend the difference between the half-wave potentials of Co2+ and Zn2+, which will generate overlapping peaks, eliminate the interference from oxygen polarization and exhibit wide linearity and low noise.
Because Co2+ has the lowest concentration and overlapping peaks will be generated between Zn2+ and Co2+ due to the small difference in their half-wave potentials, we selected dimethylglyoxime (DMG), sodium citrate and sodium nitrite (NaNO2) as sensitizers for Co2+, which exhibit no interference with other ions. In addition, the emulsifier OP-10 was selected as an inhibitor for Zn2+. Because the pH has an important influence on the sensitivity of the impurities, an ammonia buffer solution (NH3-NH4Cl) and hydrochloric acid (HCl) were employed to adjust the pH, and NH3-NH4Cl can increase the sensitivity of Co2+. Finally, we selected anhydrous sodium sulfate (Na2SO3) as an oxygen scavenger. However, the competitive reaction and contrary effect cannot be avoided when certain dose of the reagents simultaneously act on the four ions. For example, the Ip of Co2+ will significantly decrease as the pH increases due to competitive complexation between DMG and excess NH3-NH4Cl; OP-10 can extend the half-wave potential of Co2+ and Zn2+ but inhibit the sensitivity of Cu2+, Cd2+ and Co2+, and NH3-NH4Cl is a sensitizer for Co2+ but inhibits Cu2+. In addition, competitive adsorption will occur on the electrode surface between Co(DMG)2 and the excess solvent (ethyl) of DMG. Therefore, the dose of the reagents needs to be optimized based on the experimental data. The reagent doses, instruments, experimental procedures and data handing are described below.
The polarographic measurements were performed using a JP-06B (Chengdu Instrument Factory, Chengdu, China) electrochemical system attached to a personal computer. All of the measurements were conducted using a three-electrode cell. A dropping mercury electrode was used as the working electrode, and the reference and auxiliary electrodes consisted of Ag/AgCl (0.1 mol/L KCl) and platinum, respectively. All of these components were included in the JP-06B. In addition, double-distilled Hg was used (purity was 99.99%). All of the experiments were conducted at (20±0.5) °C, and the bore diameter of the glass capillary, which was used as the working electrode, was (0.05±0.002) mm. The scanning parameters in the JP-06B system remained invariant during all of the processes with a scanning rate of 0.25 V/s and a standing time of 8.4 s.
2.2 Reagent doses
The reagents were of analytical grade and used as received without further purification. The solutions (except DMG) were prepared by dilution with double distilled water prior to use. The capacity of the volumetric flask for preparing the solutions was 50 mL. The solubility of Zn2+ in zinc sulfate solution was 23.4 g/L at 25 °C. Therefore, the concentration of Zn2+ can be reduced from 140 g/L to 23.4 g/L by controlling the solution temperature. The mass concentrations of Cu2+, Cd2+, Co2+ and Zn2+ are listed in Table 1.
Table 1 Concentrations of four ions

The solubility of oxygen in water is approximately 2.5×10-4 mol/L at ambient temperature and pressure. Therefore, the dose of Na2SO3 was set to 0.6 mL (6.0 g/L), which will completely eliminate the effect of oxygen polarization. The reagent doses and doses of the other reagents for the determination are listed in Table 2 and Table 3, respectively.
2.3 Experimental procedures
LSP at the dropping mercury electrode (DME) was performed in the solutions containing four ions. First, suitable doses of the solutions were transferred to 50 ml volumetric flasks followed by the sequential addition of HCl (used to adjust pH), the ammonia buffer solution, DMG, Na2SO3, sodium citrate, OP-10, NaNO2 and the measured sample solution. The solutions were diluted to the mark with double-distilled water and mixed well. Under the experimental conditions mentioned above, the values of the polarographic parameters (E1/2 and Ip) based on the linear sweep polarographic curves (LSPCs) were obtained.
The JP-06B ChemStation software supplied with the polarography system was used for data acquisition and initial data pre-processing. All of the calculations were performed using programs written by the authors in the MATLAB R2013a environment (The MathWorks, Natick, USA), which were run on a dual-core (Intel Pentium Dual CPU E2200 @ 2.20 GHz) computer.
3 Results and discussion
3.1 Reagent dose optimization
Based on an analysis of the statistical characteristic of the data, the least squares fitting results (influence of reagent dose on E1/2 and Ip) are shown in Figs. 1(a)-(h).
Figure 1(a) shows that OP-10 can extend the half-wave potentials (E1/2) of Co2+ and Zn2+ but inhabit the sensitivity (Ip) of Co2+, which tends to be stable while the dose of OP-10 is higher than 4.5 ml. Figures 1(b) and (c) show that pH can extend the half-wave potentials of Co2+ and Zn2+ while pH is higher than 9.6, but substantially inhibit the sensitivity of Cu2+ and Co2+ while pH is higher than 8.7. Figure 1(d) shows that the sensitivity of Co2+ reaches its maximum and the sensitivity of Cu2+ tends to be stable while the dose of DMG is 1.3 ml, and the sensitivity of Co2+ significantly decreases while the dose is higher than 2 ml. Figure 1(e) shows that the sensitivity of Co2+ substantially increases while the dose of Citrate is higher than 5 ml and tends to be stable while the dose is higher than 10 ml. Figure 1(f) shows that the sensitivity of Co2+ tends to be stable while the dose of NaNO2 is higher than 2.5 ml. Figure 1(G) and 1(H) show that NH3-NH4Cl can substantially increase the sensitivity of Zn2+ but decrease the Cu2+; the difference between the limiting diffusion currents of Co2+ and Zn2+ gradually decrease while the NH3-NH4Cl is higher than 2.0 mol/L.
Table 2 Reagent doses for determination
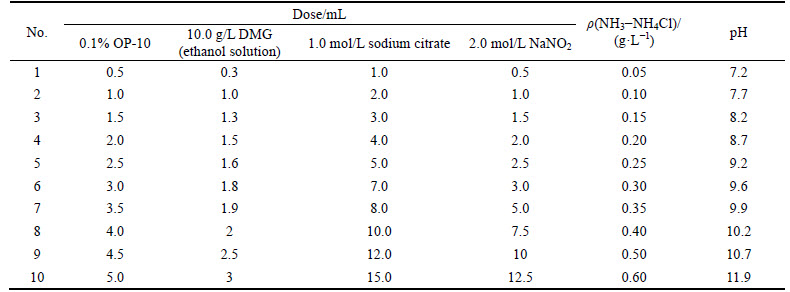
Table 3 Doses of other reagents
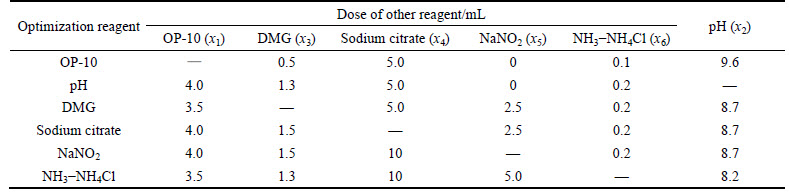
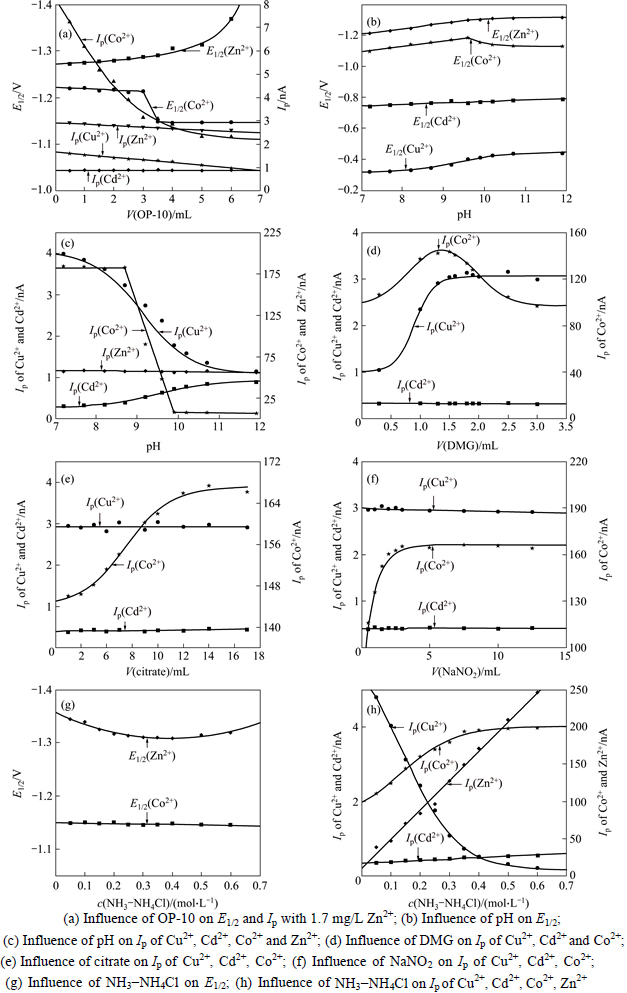
Fig. 1 Influence of reagent dose on E1/2 and Ip:
Comprehensively considering the influence of reagent dose on the polarographic parameters analyzed above, and the requirements of simultaneous determination of trace amounts of Cu2+, Cd2+ and Co2+ in the presence of an extremely large excess of Zn2+ mentioned in Section 2.1, the ranges of these reagents doses are optimized as shown in Table 4.
Table 4 Optimized ranges of reagent doses

3.2 Determination results
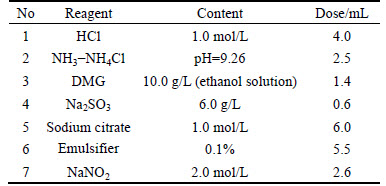
In this section, the performance of the optimized reagents in the simultaneous determination of trace amounts of Cu2+ (3.0×10-3 g/L), Cd2+ (5.0×10-3 g/L) and Co2+ (2.0×10-6 g/L) in a zinc sulfate solution (Zn2+: 0.234 g/L) is tested. The content and dose of each reagent are shown in Table 5.
Table 5 Content and dose of each reagent
The experimental procedures are listed in Section 2. To improve the resolution of LSPCs, it is necessary to require its second derivative. The determination results are shown in Fig. 2.
3.3 Performance analysis
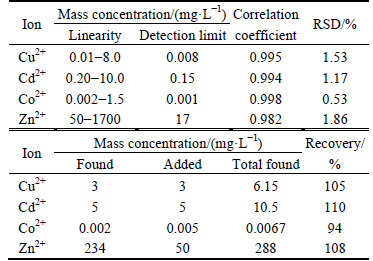
Figures 2(a) and (b) show the second- derivative determination results without and with optimized reagents, respectively. Only one obvious LSPC (Zn2+) is shown in Fig. 2(a) because the other LSPCs are covered. And four LSPCs can be observed in Fig.2(b), where the peak height of Cd2+, given by a graph with amplified coordinates, is relative lower than other ions. The peak height ratios between Zn2+ and Cu2+, Cd2+ and Co2+ are 9.8, 37.2 and 1.4 respectively, which indicates that trace amounts of polymetallic ions (Cu2+, Cd2+ and Co2+) in zinc sulfate solution (an extremely large excess of Zn2+) can be simultaneously determined by adding the optimized reagents. Performance characteristics of optimized reagents are listed in Table 6.
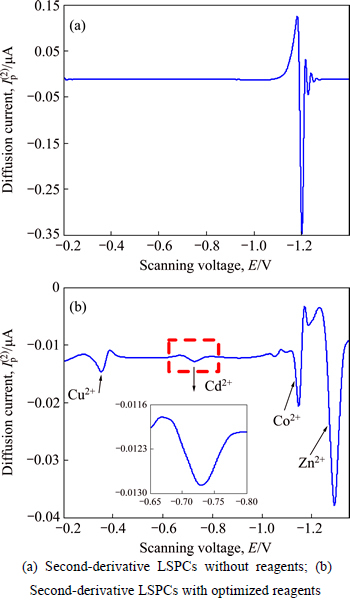
Fig. 2 Determination results without and with optimized reagents:
The determination results based on the optimized reagents exhibit wide linearity and low detection limits (ρ(Co2+) is 10-6 g/L). All of the correlation coefficients are more than 0.98, and the relative standard deviations (RSD) are less than 2%, which indicates high accuracy and good precision. The CR of the determined ions in this study is more than 105. However, in a production process, the CR is more than 106 for the failure of simultaneous determination. Therefore, the recovery of the analysis method is obtained by directly adding the sample to the solution mentioned in Section 3.2. In addition, the determined value of the recovery is 94%-110%. Therefore, the optimized reagents can be used for the simultaneous determination of trace amounts of Cu2+, Cd2+ and Co2+ in zinc sulfate solution, and satisfy the determination requirements on-line even though the concentrations of the trace ions fluctuate widely.
Table 6 Additional performance characteristics of optimized reagents
4 Conclusions
The optimized reagents (HCl: 4.0 mL, 1 mol/L; NH3-NH4Cl: 2.5 mL, pH=9.26; DMG: 1.4 mL, 10.0 g/L; Na2SO3: 0.6 mL, 6.0 g/L; sodium citrate: 6.0 mL, 1 mol/L; emulsifier OP-10: 5.5 mL, 0.1%; NaNO2: 2.6 mL, 2 mol/L) exhibit wide linearity, low detection limits, high accuracy and good precision. Therefore, the optimized reagents can be used for the on-line simultaneous determination of trace polymetallic ions (Cu2+, Cd2+ and Co2+) and are especially suitable for the high CR between Zn2+ and other ions.
References
[1] YANG Chun-hua, GUI Wei-hua, KONG Ling-shuang, WANG Ya-lin. Modeling and optimal-setting control of blending process in a metallurgical industry [J]. Computers & Chemical Engineering, 2009, 33(7): 1289-1297.
[2] GUI Wei-hua, YANG Chun-hua, CHEN Xiao-fang, WANG Ya-lin. Modeling and optimization problems and challenges arising in nonferrous metallurgical processes [J]. Acta Automatica Sinica, 2013, 39(3): 197-207.
[3] SUN Bei, GUI Wei-hua, WU Tie-bin, WANG Ya-lin, YANG Chun-hua. An integrated prediction model of cobalt ion concentration based on oxidation-reduction potential [J]. Hydrometallurgy, 2013, 140: 102-110.
[4] SARANGI C K, TRIPATHY B C, BHATTACHARYA I N, SUBBAIAH T, DAS S C, MISHRA B K. Electrowinning of zinc from sulphate solutions in the presence of perfluoroglutaric acid [J]. Minerals Engineering, 2009, 22(14): 1266-1269.
[5] HASHIM M A, MUKHOPADHYAY S, SAHUA J N, SENGUPTA B. Remediation technologies for heavy metal contaminated groundwater [J]. Journal of Environmental Management, 2011, 92(10): 2355-2388.
[6] LI Yong-gang, GUI Wei-hua, TEO K L, ZHU Hong-qiu, CHAI Qin-qin. Optimal control for zinc solution purification based on interacting CSTR models [J]. Journal of Process Control, 2012, 22(10): 1878-1889.
[7] ZHANG Bin, YANG Chun-hua, ZHU Hong-qiu, LI Yong-gang, GUI Wei-hua. Kinetic modeling and parameter estimation for competing reactions in copper removal process from zinc sulfate solution [J]. Ind Eng Chem Res, 2013, 52(48): 17074-17086.
[8] ZHANG Li-juan, ZHANG Shao-feng, WAN You-zhi. Voltammetric behavior of methaqualone and its determination by single-sweep oscillopolarography [J]. Talanta, 2003, 59(5): 1009-1013.
[9] Xu M T, Song J F, Liang Y D. Rapid determination of telmisartan in pharmaceutical preparations and serum by linear sweep polarography [J]. Journal of Pharmaceutical and Biomedical Analysis, 2004, 34(3): 681-687.
[10] PILKINGTON E S, WEEKS C, BOND A M. Determination of trace elements in zinc plant electrolyte by differential pulse polarography and anodic stripping voltammetry [J]. Analytical Chemistry, 1976, 48 (12): 1665-1669.
[11] BOND A M. 200 years of practical electroanalytical chemistry: past, present and future directions illustrated by reference to the on-line, on-stream and off-line determination of trace metals in zinc plant electrolyte by voltammetric and potentiometric techniques [J]. Analytica Chimica Acta, 1999, 400(1/2/3): 333-379.
[12] ZHANG Ming-hao, ZHOU Chun-shan, LIU Qian-hui. Catalytic wave of cobalt and its analytical application in rapid determination of trace cobalt in complex zinc electrolyte solution [J]. Journal of Central South University of Technology, 2000, 7(2): 84-87.
[13] ZHU Hong-qiu, WANG Guo-wei, YANG Chun-hua, CAO Yu, GUI Wei-hua. Overlapped peaks resolution for linear sweep polarography using Gaussian-like distribution [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(7): 2181-2186.
[14] REZAEI B, REZAEI E. Simultaneous determination of trace amounts of nickel, cobalt, and zinc in the wastewater of a galvanic workshop by using adsorptive cathodic stripping voltammetry [J]. Journal of Analytical Chemistry, 2006, 61(3): 262-265.
[15] AFKHAMI A, ABBASI-TARIGHAT M, KHANMOHAMMADI H. Simultaneous determination of Co2+, Ni2+, Cu2+ and Zn2+ ions in foodstuffs and vegetables with a new Schiff base using artificial neural networks [J]. Talanta, 2009, 77(3): 995-1001.
[16] NASCIMENTO D S, INSAUSTI M, BAND B S F, LEMOS S G. Simultaneous determination of Cu, Pb, Cd, Ni, Co and Zn in bioethanol fuel by adsorptive stripping voltammetry and multivariate linear regression [J]. Fuel, 2014, 137: 172-178.
[17] HERRERO E, ARANCIBIA V, ROJAS-ROMO C. Simultaneous determination of Pb2+, Cd2+ and Zn2+ by adsorptive stripping voltammetry using Clioquinol as a chelating-adsorbent agent [J]. Journal of Electroanalytical Chemistry, 2014, 729: 9-14.
[18] ZHANG Tai-ming, LIANG Yi-zeng, DING Feng, YANG Qing, ZHU Yu-xi. Simultaneous determination of six trace elements in plant medicines by derivative adsorption waves in conjunction with a microwave technique [J]. Instrumentation Science & Technology, 2007, 35: 95-111.
[19] BOROVKOV G A, MONASTYRSKAYA V I. Voltammetric determination of cobalt (II) in zinc sulfate solution [J]. Russian Journal of Applied Chemistry, 2001, 74(8): 1310-1317.
[20] VALERA J A, ZLATEY R K, STOYTCHEVA M S, VALDEZ B. Determination of trace concentrations of Co(II) in electrolyte for electrowinning of Zn by differential pulse voltammetry [J]. ECS Transactions, 2010, 29(1): 409-419.
(Edited by YANG Hua)
Foundation item: Projects(61533021, 61321003, 61273185) supported by the National Natural Science Foundation of China; Project(2015CX007) supported by the Innovation-driven Plan in Central South University, China; Project(13JJ8003) supported by the Joint Fund of Hunan Provincial Natural Science Foundation of China
Received date: 2015-07-13; Accepted date: 2016-05-19
Corresponding author: ZHU Hong-qiu, PhD, Associate professor; Tel: +86-13873126460; E-mail: hqcsu@csu.edu.cn
Abstract: Reagents are optimized for the simultaneous determination of trace amounts of Cu2+, Cd2+ and Co2+ in zinc sulfate solution, which contains an extremely large excess of Zn2+. First, the reagents and their doses for the experiment are selected according to the characteristics of the zinc sulfate solution. Then, the reagent doses are optimized by analyzing the influence of reagent dose on the polarographic parameters (i.e. half-wave potential E1/2 and limiting diffusion current Ip). Finally, the optimization results are verified by simultaneously determining trace amounts of Cu2+, Cd2+ and Co2+ in the presence of an extremely large excess of Zn2+. The determination results indicate that the optimized reagents exhibit wide linearity, low detection limits, high accuracy and good precision for the simultaneous determination of trace amounts of Cu2+, Cd2+ and Co2+ in the presence of an extremely large excess of Zn2+.


