J. Cent. South Univ. Technol. (2007)05-0633-05
DOI: 10.1007/s11771-007-0121-1 ![]()
Preparation of activated carbons from mesophase pitch and
their electrochemical properties
LAI Yan-qing(赖延清), LI Jing(李 晶), SONG Hai-shen(宋海申),
ZHANG Zhi-an(张治安) , LI Jie(李 劼),LIU Ye-xiang(刘业翔)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract:
The influences of molar ratio of KOH to C and activated temperature on the pore structure and electrochemical property of porous activated carbon from mesophase pitch activated by KOH were investigated. The surface areas and the pore structures of activated carbons were analyzed by nitrogen adsorption, and the electrochemical properties of the activated carbons were studied using two-electrode capacitors in organic electrolyte. The results indicate that the maximum surface area of 3 190 m2/g is obtained at molar ratio of KOH to C of 5:1, the maximum specific capacitance of 122 F/g is attained at molar ratio of KOH to C of 4?1, and 800 ℃ is the proper temperature to obtain the maximum surface area and capacitance.
Key words:
1 IntroductionSupercapacitor is a kind of new energy storage device that can fill the gap between the rechargeable battery and conventional capacitor[1-2], which has recently come to attract attention for the purpose of new energy applications[3-5]. As the electrode material for the double-layer capacitor, an active carbon powder[6-7] or fiber[8] is one of the candidates because of its high specific surface area and high conductivity. However, controlled pore size and pore size distributions are necessary for the application of activated carbons(ACs) in supercapacitors[6-7, 9]. LOZANO-CASTELL? et al[10] studied the activation of anthracite with KOH,and found that the primary pore structure is the micropore. AHMADPOUR et al[11] and MOLINA-SABIO et al[12] proposed the same result in the study of activation of seed capsule by KOH. The properties of activated carbon are determined by both the precursor and the certain activated techniques[10,13-14]. Accordingly, the understanding on the influences of activation variables on the physicochemical properties of ACs is very important in developing the porous structure of various carbons in chemical activation processes.
In this study, various ACs from mesophase pitch were prepared by means of chemical activation methods, their applicabilities to supercapacitors were evaluated, and the impacts of activated technique, including the activation temperature and molar ratio of KON to mesophase pitch, (n(KOH)/n(C)) on the pore structure and electrochemical property were investigated. In addition, the physical properties, such as BET surface area, pore size distribution, total pore volume, and micropore volume of carbons, were compared.
2 Experimental
Mesophase pitch and KOH (analytical grade, Shanghai Reagent Factory) were mixed into nickel crucible, with n(KOH)/n(C) ranging from 2?1 to 5?1; and temperature from 700 to 1 000 ℃. In the activation process, different samples were heated in argon atmosphere. The heating conditions were: argon flow of 60 mL/min, heating rate of 5 ℃/min, heating time of 2 h. After heating process, the activated carbon was washed first with 1×103 mol/L HCl, and then distilled water until neutral pH value. After rinsing process, the activated carbon was dried in an oven at 110 ℃ for 10 h. The characterization of the porous texture of the activated carbons was conducted using physical adsorption of N2 at -196 ℃ in the Autosorb-6 apparatus. Nitrogen adsorption isotherms were obtained at -196 ℃, the micropores and mesopores distributions were determined by the HK and BJH methods, respectively.
Two-electrode capacitors were built from the KOH activated carbon samples. The electrodes consist of 80% of carbon, 10% of polyvinylidene fluoride(PVDF) and 10% of acetylene black. The capacitor was constructed using a couple of electrodes face to face, with a separator inserted between these electrodes. The electrolyte was applied using 1×103 mol/L of tetraethylammonium tetrafluoroborate((C2H5)4NBF4) in an organic solvent(propylene carbonate, PC). Charging was performed at a constant current density of 5 mA/cm2 and charge-discharge cycles were performed at various current densities. The capacitance was calculated from the corresponding voltage variation of the discharge.
3 Results and discussion
In the chemical activation process many parameters exist, even keeping the precursor and the activating agent constant is important, that affect the porous texture of the activated carbon. In the present study the effect of different parameters involved in a chemical activation process on the porosity of the final activated carbon was investigated.
3.1 Influence of molar ratio of KOH to mesophase pitch on structure of activated carbons
The n(KOH)/n(C) has been found to be the most important parameter in a chemical activated process[13]. Fig.1 shows the effect of n(KOH)/n(C) on the nitrogen adsorption isotherms. It can be seen that the adsorption capacity increases with increasing n(KOH)/n(C), reaching a maximum for the n(KOH)/n(C) of 5?1. All the N2 adsorption isotherms are typical of microporous carbons according to B-D-D-T classification[15], since the knees of the isotherms are sharp and the plateaus are fairly horizontal. The adsorption amount of nitrogen at n(KOH)/n(C) of 2?1 is almost full at pressure of 0.1, and keeps constant with increasing the relative pressure. It can also be seen from Fig.1 that there is a widening of porosity with increase n(KOH)/n(C), as inferred from the opening of the knee of the isotherm and the higher slope of the plateau[15].
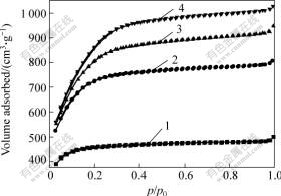
Fig.1 N2 adsorption isotherms of activated carbons produced with KOH at 700 ℃ and different n(KOH)/n(C)
n(KOH)/n(C): 1—2?1; 2—3?1; 3—4?1; 4—5?1
Fig.2 shows the pore size variation of different activated carbons prepared at different n(KOH)/n(C). It can be seen from Fig.2 that the activated carbons prepared at n(KOH)/n(C) of 2?1 are composed mainly of micropores with a peak at about 1.5 nm; with increasing of n(KOH)/n(C), the peaks at larger pore diameter appear besides the peak at 1.5, 1.8 and 2.3 nm, respectively, and the pore volume increases with increasing of n(KOH)/n(C). What’s more, with the increase of n(KOH)/n(C), the peak shifts to larger pore diameter slightly, which means that the pore diameter widens with increasing n(KOH)/n(C).
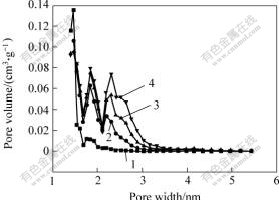
Fig.2 Effect of n(KOH)/n(C) on DFT pore diameter distribution
n(KOH)/n(C):1—2?1; 2—3?1; 3—4?1; 4—5?1
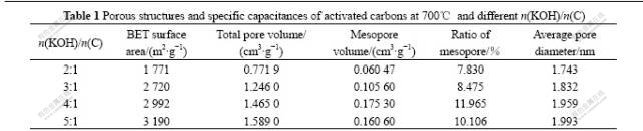
In order to discuss the influence of the variation in activation parameters explicitly, the isotherms of N2 adsorption on different carbons were employed to deduce the surface characteristics of these carbons. The effect of the n(KOH)/n(C) is listed in Table 1. It can be seen that, with increasing n(KOH)/n(C) from 2?1 to 5?1, the surface area and total pore volume both increase continuously, reaching a maximum at n(KOH)/n(C) of 5?1. When n(KOH)/n(C) exceeds 3?1, the increasing rate of surface area becomes slow, which means that n(KOH)/n(C) from 2?1 to 3?1 is important for pore generation, and at high n(KOH)/n(C) the effect of the metallic salt on the formation of microporosity is more pronounced. Meanwhile, the mesopore volume increases at n(KOH)/n(C) from 2?1 to 4?1, and almost keeps constant at n(KOH)/n(C) from 4?1 to 5?1. Thus, pore widening and the formation of microporosity may occur simultaneously with increasing n(KOH)/n(C).
It can be seen from the above result that, in chemical activation of mesophase pitch, when n(KOH)/n(C) is below 3?1, lots of micropores are formed; when n(KOH)/n(C) increases from 3?1 to 5?1, the formation of pores and pore widening occur simultaneously, and pore widening becomes dominant. But, the amount of KOH may have a proper value, or excessive addition may destroy the pore structure of activated carbons[16].
Table 1 Porous structures and specific capacitances of activated carbons at 700℃ and different n(KOH)/n(C)
3.2 Influence of temperature on structure of activated carbon
Temperature is another important parameter to affect the preparation of activated carbon in a chemical activated process[13]. Fig.3 shows the N2 adsorption isotherms of the activated carbons prepared at different activated temperatures. It can be seen from the isotherms that the N2 amount adsorbed increases when temperature increases from 700 to 800 ℃, and then decreases with further increasing the activated temperature. It can also be seen that activated carbons prepared at 700 and 800 ℃ exhibit similar adsorption isotherms, compared with those at 900 and 1 000 ℃, which means that they have similar pore structures. The N2 adsorption isotherm prepared below 800 ℃ is typical of microporous carbons, the isotherms have almost flat portions in the relative pressure range of 0.1-1.0, the adsorption amount of nitrogen is almost full at relative pressure of 0.1, and keeps constant with increase of relative pressure. But with the further increase of the activated temperature, the type of isotherm gradually changes to the type with mesopore. It can be seen that the plateaus of activated carbons prepared at 900 and 1 000 ℃ have the shape of a gradual increase along the relative pressure, which indicates that they have significant mesopore volumes. The decrease amount of N2 adsorption when temperature is higher than 800 ℃ indicates the reduction of micropore volume and increase of mesopore volume[16].
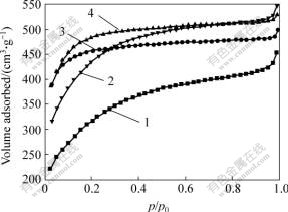
Fig.3 N2 Adsorption isotherms of activated carbons prepared at different activated temperatures
1—1 000 ℃; 2—900 ℃; 3—700 ℃; 4—800 ℃
Fig.4 shows the pore size variation of different activated carbons prepared at different temperatures. It can be seen that the pore structure of the activated carbon prepared at 700 ℃ is composed mainly of micropores with a peak at about 1.5 nm; with increasing temperature to 800 ℃, another peak at around 1.8 nm appears. With further increase of temperature to 900 ℃ and 1 000 ℃, the micropores around 1.5 nm decrease rapidly, but the larger micropores at about 1.8 nm and mesopores with pore diameters between 2 and 4 nm increase largely, and the pore volume with larger diameters increases with increasing temperature.

Fig.4 Effect of activated temperature on pore structure of activated carbons
1—1 000 ℃; 2—900 ℃; 3—800 ℃; 4—700 ℃
Thus, when the temperature is below 800℃, formation of micropores is the main process in the chemical activation, and the effect of temperature on the pore widening is small. With increasing temperature, probably due to the violent attack of mesophase pitch by chemical materials, the opening of pores is clearly damaged and partly burnt, resulting in the widening of pore diameters, i.e. the increase of mesopore volumes and decrease of micropore volumes.
Table 2 lists the porous texture characterization results of four activated carbons obtained by applying the BET equation to N2 adsorption at -196 ℃. It can be seen that, when temperature increases from 700 to 1 000 ℃, both the surface area and micropore volume increase firstly and then decrease, reaching a maximum at 800 ℃, which proves that the large surface area of activated carbon mainly comes from the contribution of micropores. When the temperature is below 800 ℃, the mesopore volume and the ratio of mesopores keep at a low value, and increase rapidly with temperature ranging from 800 to 1 000 ℃, reaching a maximum at 1 000 ℃.
Table 2 Porous texture of activated carbons prepared at n(KOH)/n(C) of 2?1 and different activation temperatures

3.3 Influence of activation techniques on electro-
chemical performance
Figs.5 and 6 show the variation of specific capacitance of porous carbon electrode prepared at different activated temperatures and n(KOH)/n(C).
It can be seen from Fig.5 that the capacitance of the carbons prepared at different n(KOH)/n(C) increases with increasing BET surface area, and decreases a little at n(KOH)/n(C) of 5?1 compared with that of 4?1, although a higher surface area. But, from Table 1, the mesopore volume of activated carbon increases with increasing n(KOH)/n(C), then decreases when n(KOH)/n(C) increases from 4?1 to 5?1. So, surface area and mesopore volume may be the most important factors to affect the capacitance of activated carbons, and the maximum capacitance of 122 F/g can be attained at n(KOH)/n(C) of 4?1, which have higher surface area and mesopore volume. This is because small diameter micropores may not be accessible to electrolyte solution, and the ions, especially those large organic ions, cannot enter those pores, thus, the surface area of those non-accessible micropores will not contribute to the total double-layer capacitance of the material[9,17-18].

Fig.5 Effect of n(KOH)/n(C) on capacitance of activated carbon
It can be seen from Fig.6 that the capacitances of the carbons have the same tendency with their BET surface areas when increasing the temperature. The surface area of activated carbon prepared at 700 ℃ is higher than those at 800 ℃ and 900 ℃, but its specific capacitance is lower than that of the laters, which confirms again the strong influence of mesopore volume on the capacitance.
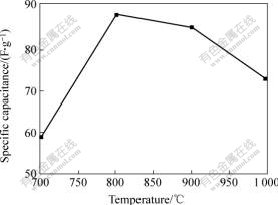
Fig.6 Effect of activated temperature on specific capacitance
Figs.7 and 8 show the cycle-voltammograms(CV) curves of the activated carbons prepared at different n(KOH)/n(C) and activated temperatures, which are measured at scan rate of 10 mV/s. It can be seen that even at high scan rate, the profiles of activated carbons show ideal rectangular-like shape, which shows the ideal capacitance property and small equivalent series resistance(ESR)[19].

Fig.7 Effect of n(KOH)/n(C) on CV curves of activated carbons
n(KOH)/n(C): 1—2?1; 2—3?1; 3—4?1; 4—5?1

Fig.8 Effect of activated temperature on CV curves of activated carbons
1—1 000 ℃; 2—700 ℃; 3—900 ℃; 4—800 ℃
Several features can be observed from Figs.7 and 8. First, all I-E curves on the positive sweeps are generally symmetric to those on their corresponding negative sweeps. In addition, the voltammeric charges integrated from the positive sweeps are very close to their corresponding charges on the negative sweeps. These results indicate the excellent reversibility of the activated carbons. Second, the voltammetric currents rapidly reach their respective plateau values for all curves when the direction of potential sweeps is changed although the scan rate of CV is as fast as 10 mV/s. This indicates the relatively low equivalent series resistance on most of electrodes. In fact, the equivalent serials resistances of the samples examined by galvanostatic measurement are less than 1 Ω/cm2 and have no obvious difference.
4 Conclusions
1) When temperature is kept at 700 ℃, with increasing n(KOH)/n(C) from 2?1 to 5?1, the surface area and micropore volume increase, and the maximum surface area is 3 190 m2/g. The mesopore volume and specific capacitance of the activated carbon increase with increasing n(KOH)/n(C) from 2?1 to 4?1, and then decrease when further increasing to 5?1, and maximum capacitance of 122 F/g can be attained at n(KOH)/n(C) 4?1.
2) When temperature increases from 700 to 800 ℃, the surface area and micropore volume of activated carbon increase, and both decrease with further increasing the temperature to 1 000 ℃. The maximum surface area of 1 836 m2/g and the maximum specific capacitance of 87 F/g are obtained at 800 ℃.
3) The capacitance of activated carbons mainly comes from the charge/discharge currents of the electric double layers. Surface area and mesopore volume are both important factors to affect the capacitance of the activated carbons, and the maximum capacitance is attained from the activated carbon that has higher surface area and mesopore volume.
References[1] KOTZ R, CARLEN M. Principle and application of electrochemical capacitors[J]. Electrochimica Acta, 2000, 45(15/16): 2483-2498.
[2] VIX-GUTERL C, SAADALLAH S. Supercapacitor electrodes from new ordered porous carbon materials obtained by a templating procedure[J]. Materials Science and Engineering B, 2004, B108(1/2): 148-155.
[3] SARANGAGAPANI S, TILAK B V, CHEN C P. Materials for electrochemical capacitors[J]. J Electrochem Soc, 1996, 143(11): 3791-3798.
[4] BURKE A. Ultracapacitors: Why, how, and where is the technology[J]. Journal of Power Sources, 2000, 91(1): 37-50.
[5] CONWAY B E, PELL W G. Power limitations of supercapacitor operation associated with resistance and capacitance distribution in porous electrode devices[J]. Journal of Power Sources, 2002, 105(2): 169-181.
[6] HANG Shi. Activated carbons and double layer capacitance[J]. Electrochimica Acta, 1996, 41(10): 1633-1639.
[7] WU F C, TSENG R L, HU C C, et al. Effects of pore structure and electrolyte on the capacitive characteristics of steam- and KOH-activated carbons for supercapacitors[J]. Journal of Power Sources, 2005, 144(1): 302-309.
[8] MERINO C, SOTO P, VILAPLANA-ORTEGO E, et al. Carbon nanofibres and activated carbon nanofibres as electrodes in supercapacitors[J]. Carbon, 2005, 43(3): 551-557.
[9] QU De-yang, SHI Hang. Studies of activated carbons used in double-layer capacitors[J]. Journal of Power Sources, 1998, 74(1): 99-107.
[10] LOZANO-CASTELL? D, LILLO-R?DENAS M A, CAZORLA-AMOR?S D, et al. Preparation of activated carbons from Spanish anthracite: I. Activation by KOH[J]. Carbon, 2001, 39(5): 741-749.
[11] AHMADPOUR A, DO D D. Preparation of activated carbon from macadamia nutshell by chemical activation[J]. Carbon, 1997, 35(12): 1723-732.
[12] MOLINA-SABIO M, RODR?GUEZ-REINOSO F. Role of chemical activation in the development of carbon porosity[J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2004, 241: 15-25.
[13] BABEL K, JUREWICZ K. KOH activated carbon fabrics as supercapacitor material[J]. Journal of Physics and Chemistry of Solids, 2004, 65(2/3): 275-280.
[14] LI Jing, LI Jie, LAI Yan-qing, et al. Influence of KOH activation techniques on pore structure and electrochemical property of carbon electrode materials[J]. Journal of Central South University of Technology, 2006, 13(4): 360-366.
[15] TENG H S, WANG S C. Preparation of porous carbons from phenol-formaldehyde resins with chemical and physical activation[J]. Carbon, 2000, 38(6): 817-824.
[16] CARVALHO A P, CARDOSO B, PIRES J, et al. Preparation of activated carbons from cork waste by chemical activation with KOH[J]. Carbon, 2003, 41(14): 2873-2876.
[17] BARBIERI O, HAHN M, HERZOG A, et al. Capacitance limits of high surface area activated carbons for double layer capacitors[J]. Carbon, 2005, 43(6): 1303-1310.
[18] KIERZEK K, FRACKOWIAK E, LOTA G, et al. Electrochemical capacitors based on highly porous carbons prepared by KOH activation[J]. Electrochimica Acta, 2004, 49(4): 515-523.
[19] WU F C, TSENG R L, HU C C, et al. The capacitive characteristics of activated carbons: Comparisons of the activation methods on the pore structure and effects of the pore structure and electrolyte on the capacitive performance[J]. Journal of Power Sources, 2006, 159(2):1532-1542.
Foundation item: Project(06FJ4059) supported by Hunan Provincial Academician Foundation
Received date: 2007-03-20; Accepted date: 2007-05-15
Corresponding author: LI Jing, Doctoral candidate; Tel: +86-731-8830474; E-mail: csu_lijing@126.com
(Edited by CHEN Wei-ping)
Abstract: The influences of molar ratio of KOH to C and activated temperature on the pore structure and electrochemical property of porous activated carbon from mesophase pitch activated by KOH were investigated. The surface areas and the pore structures of activated carbons were analyzed by nitrogen adsorption, and the electrochemical properties of the activated carbons were studied using two-electrode capacitors in organic electrolyte. The results indicate that the maximum surface area of 3 190 m2/g is obtained at molar ratio of KOH to C of 5:1, the maximum specific capacitance of 122 F/g is attained at molar ratio of KOH to C of 4?1, and 800 ℃ is the proper temperature to obtain the maximum surface area and capacitance.


