文章编号:1004-0609(2011)10-2448-17
锂离子电池纳米钒基正极材料的研究进展
梁叔全,潘安强,刘 军,钟 杰,陈 涛,周 江
(中南大学 材料科学与工程学院,长沙 410083)
摘 要:
锂离子电池因为其较高的能量密度、优良的循环性能及较强的荷电保持能力被广泛应用于便捷式电子器件中。同时作为混合动力汽车(HV)和电动汽车(EV)潜在的电源设备也被广泛地研究,但是,目前其电化学性能还不能完全满足高能量密度、大功率的要求。主要是因为商品化和即将进入开发性研究的正极材料大多是嵌锂过 渡金属氧化物,这些正极材料存在致命的本征制约 —— 较低的比容量。钒基正极材料,如V2O5、LiV3O8和Li3V2(PO4)3等,由于可以嵌入多个Li+离子,从而具有较高的理论比容量,但受材料微结构的影响,这类材料的实际比容量远低于理论值。材料微结构纳米化,可以形成独特形貌,获得高比表面积,缩短Li+离子的扩散距离,使这类材料的实际比容量接近理论值,从而有可能在能量的高效率储存中扮演十分重要的角色。本文作者重点综述钒基正极材料的主要晶体结构特点和相关纳米材料合成方法、结构表征及其对应电化学性能的研究进展。
关键词:
纳米材料;锂离子电池;V2O5;LiV3O8;Li3V2(PO4)3;电化学性能;
中图分类号:TM912.9;O646.54 文献标志码:A
Research developments of V-based nanomaterials as cathodes for lithium batteries
LIANG Shu-quan, PAN An-qiang, LIU Jun,ZHONG Jie, CHEN Tao, ZHOU Jiang
(School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Lithium-ion batteries (LIBs) were widely used in portable electronic devices, mainly due to their high energy density, good cycle performance and charge retention ability. Moreover, as the potential power sources of the hybrid vehicles (HV) and electric vehicles (EV), LIBs were widely studied. But at present their electrochemical properties cannot fully meet the requirements of high energy density, high power for power sources of HV and EV. This is mainly because most commercial and studied cathode materials are lithium transition metal oxides, which have an intrinsic constraint, i.e. low capacity. V-based cathode materials, such as V2O5, LiV3O8 and Li3V2(PO4)3, possess relatively high theoretical specific capacity because of their abilities to intercalate more Li+ ions per formula. However, due to the structure limitation of these materials, their actual capacity is much lower than the theoretical value. Synthesis of these materials with nanostructures can greatly enlarge their surface areas and reduce the Li+ ion diffusion distance significantly, resulting in the fact that the actual specific capacity is closer to the theoretical value. Such V-based nanomaterials may make LIBs play an important role in the high efficiency store of energy, especially for power sources of HV and EV. This review focuses on the research development of synthesis of V-based nanomaterials, characterization and their corresponding electrochemical properties.
Key words: nanomaterials; lithium ion batteries; V2O5; LiV3O8; Li3V2(PO4)3; electrochemical performance
随着石油、天然气等不可再生能源的快速消耗和生态环境的日益恶化,在可支撑经济和社会可持续发展的新清洁能源出现之前,人类只能在不断提高能量的使用效率上下功夫,因此,对能量的储存和释放将提出越来越高的要求。便携、清洁、高能量密度和大功率的能源供给系统越来越受到青睐[1-3]。锂离子电池因为其能量密度高、设计灵活、循环稳定性好、寿命长、环境友好等[4-6],在便携式电子器件中应用最为广 泛[7-9]。同时也正在被考虑作为动力电源应用在大功率的汽车等交通工具中[10]。当前,无论是在便捷式电子器件中的应用,还是在大功率、高能量密度装置中的应用,锂离子电池都受到一个关键制约性因素的影响,即电池正极材料的比容量明显偏低。
锂离子电池通过法拉第反应实现化学能和电能之间的转化,同时伴随着物质的传输和电子的迁移,其原理如图1所示[11]。在电池反应进行中还伴随着整个电极的尺寸变化。因此,电极材料的比表面积和传输距离对电极材料的电化学性能至关重要。化学成分、晶体结构和微观形貌等对反应的速率、电荷传输的过程以及循环稳定性都具有重要影响。电池可储存和释放的能量(E)正比于电池的工作电压(V)和容量(C)。由于负极材料的电位大多数在1 V以下,接近于Li金属或Li+离子的电位,所以,电池的工作电压主要决定于高电位的正极材料,同时,负极材料的容量一般远高于正极材料的。因此,开发具有较高工作电压(V)和大容量(C)的电池材料成为锂离子电池研究的两个主要方向。
由于受到目前已广泛采用的有机溶剂电解液体系
最高安全电压(约为4.6 V,vs Li/Li+)的限制,通过进一步提高电池工作电压的方式来提高能量储存和释放效率的空间十分有限,在此限制没有突破之前,锂离子电池正极材料容量的提高已成为当前和今后一个时期锂离子电池研究的重点领域和突破口。获得高容量的一种有效方式是寻找高价态的氧化物,如钒、鉻、铌和钼等,这是因为氧化还原时这些氧化物可以嵌入多个Li+离子,发生多个价态的变化[12],有助于获得较大的比容量。但是,因为W和Nb等本身的元素较重,其比放电容量并没有得到真正提高。Mo氧化物中单个Mo虽可以嵌入1.5 个Li+离子,但是其活性较差。图2所示为目前已商品化和正在研发的主要正极材料容量和工作电压分布图。由图2可见,锰基和钒基正极材料是目前重点关注的两大体系。
锰基正极材料,如掺Ni形成的LiNi0.5Mn1.5O4,其Li+离子嵌入基体的电压平台达到4.8 V,是开发高电压平台较好的例子[13],但该系材料容量明显偏低,只有140 mA?h/g (C/7)。钒基正极材料,如V2O5和LiV3O8等钒氧化物,嵌锂时,其V5+化合价分别降为+3.5(ω-Li3V2O5)和+3.67 (Li5V3O8) [9],根据理论比容量计算公式:Cm=26 800n/M(n为Li+离子的个数;M为相对分子质量),它们分别可以获得442和 372 mA?h/g的理论比容量,当3个Li+离子从Li3V2 (PO4)3中脱出时,也可以释放197 mA?h/g, 该比容量在已经报道的磷酸盐体系中是最高的。此外,V2O5?nH2O理论比容量可高达600 mA?h/g[14]。除了上述性能优势外,钒的储量丰富,价格相对低廉,这使得钒氧化物作为电池正极材料具有较好的应用前景,有可能满足便捷式电子器件对高品质电池的要求及混合动力汽车(HV)和电动汽车(EV)对高能量密度和大功率电池的要求,成为目前重点研究的新一代正极材料之一。
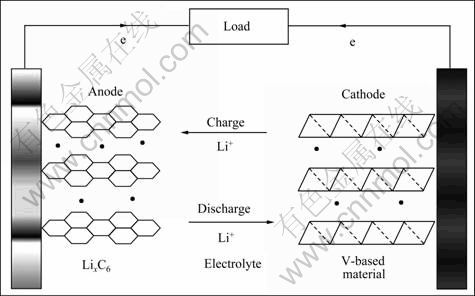
图1 Li+离子电池充放电示意图[11]
Fig.1 Illustration of charge-discharge process involved in lithium ion cell[11]
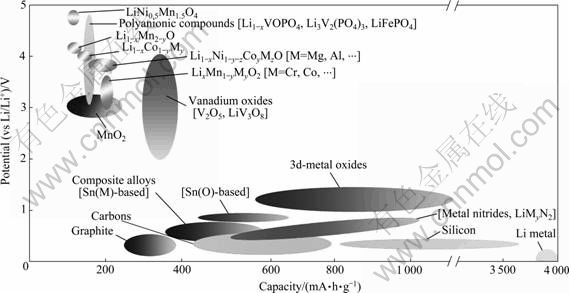
图2 正、负极材料电压和容量的分布[9]
Fig.2 Distribution of potential versus capacity for positive- and negative-electrode materials[9]
由于这些材料较低的Li+离子扩散系数和较弱电子传输能力,要获得优良电化学性能,还有许多障碍要跨越。材料结构纳米化为解决上述不足提供了一条有效的途径,这是因为钒基材料结构纳米化,极大地缩短了Li+离子的扩散距离、提高了电荷输运效率,因此,有可能通过对钒基正极材料进行纳米化或纳米复合处理,显著提高其电化学性能。本文作者综述了目前正在研究的几种主要钒基正极材料的纳米化研究进展。
1 几种含钒氧化物典型结构与特性
V2O5作为锂离子电池的正极材料已研究30多 年[14-15],其嵌锂机理如下[14]:
xLi+V2O5=LixV2O5 (1)
WHITTINGHAM[1]及CHERNOVA等[2]对锂离子电池正极材料包括锂金属氧化物和钒氧化物的类型进行了详尽的综述。钒氧化物最有代表性的是具有正交结构的V2O5,属Pmmn空间群,a=1.154 0 nm,b= 0.357 1 nm,c=0.438 3 nm,V=0.180 68 nm3,其晶体结构如图3所示。[VO5]四方棱锥通过共边和共角形 成的层堆积而成[16-17]。V=O顶角的双键比其他4个键的距离都短。该结构说明了该材料的二维层状结构特点[16]。1976年,WHITTINGHAM[15]报道了V2O5在室温下的可逆嵌锂结构转变和循环性能。图4所示为Li+离子嵌入V2O5中形成LixV2O5的相转变过程以及ω相的循环特征。随着Li+离子嵌入量的不断增加,形成不同的相:当LixV2O5中x<0.01和0.35<x<0.7时,分别得到α和ε相[18];当x=1时,对应的相为δ相[19-20];当x≤1时,LixV2O5脱锂后,可以重新转变为V2O5的初始结构,相变在整个过程中是可逆的。当嵌入的锂含量继续增加时(x>1),结构重排导致δ相不可逆地转变为γ相[18]。γ相可在0<x<2的范围内循环,结构并不改变[21]。当嵌入第3个Li+离子(x=3)后,γ相变为ω相,该转变过程不可逆。ω相可以通过电化学嵌锂合成[22],也可以用化学的方法合成[23]。
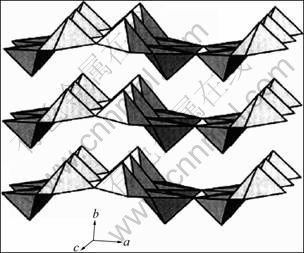
图3 V2O5的晶体结构
Fig.3 Crystal structure of V2O5
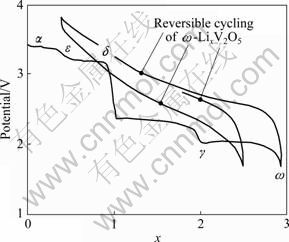
图4 随着Li嵌入V2O5 的不断深入,LixV2O5相的演变和ω相的循环曲线[1]
Fig.4 Electrochemical lithium intercalation into V2O5 showing evolution of phases with degree of lithium intercalation and cycling of ω-phase[1]
Li1+xV3O8是另一个可以嵌入多个Li+离子的层状钒基正极材料(如LiV3O8为单斜结构,空间群P21/m,a=0.668 nm,b=0.360 nm,c=1.203 nm,V=0.275 45 nm3)。单斜结构的Li1+xV3O8由(V3O8)-层沿着a轴排列,而(V3O8)-层由两个基本结构单元、[VO6]八面体和[VO5]扭曲棱锥双金字塔组成。它们通过氧原子角共用形成V—O层。Li+离子可以占据V—O层之间不同的八面体和四面体位置[24],其结构如图5所示。一般的层结构只是通过范德尔斯键连接在一起,而LiV3O8的层结构之间通过位于八面体位置的Li离子连接在一起。这种在电化学嵌入过程中不参与充、放电的Li+离子将层和层很牢固地连接在一起,以确保在充电和放电过程中Li1+xV3O8具有很好的结构可逆性和稳定性,同时也不会阻碍嵌入的Li+离子占据四面体间隙位置[25-28]。位于层与层之间四面体位置的Li+离子对应于数值x,循环时参与放电和充电过程。PANERO 等[29]研究了较低锂含量的Li1+xV3O8在电流密度为0.5~2 mA/cm2时的放电行为。3个Li+离子可以可逆地嵌入Li1+xV3O8中,循环多次后可以获得高于100 mA?h/g的稳定容量,Li1+xV3O8的开路电位与锂含量有关。当1+x≤3时,嵌锂过程为单相转变,Li4V3O8形成后出现两相转变[30]。放电曲线和循环伏安曲线显示出多个平台和电流峰值,表明在锂嵌入的过程中,发生了一些结构的重组。最近,7Li和51V MAS NMR被用来重新研究循环过程中Li+离子嵌入Li1+xV3O8形成的相[31]。
V2O5?nH2O是另一个被广泛研究的锂离子电池的钒基正极材料。ZAKHAROVA和VOLKOV[32]及DONG等[33]综述了V2O5?nH2O干凝胶和气凝胶的嵌锂行为。例如含有P2O5的V2O5?nH2O玻璃或者其他网络结构的V2O5?nH2O[34]、V2O5?nH2O气凝胶[35]和V2O5?nH2O干凝胶[36-37]等形式存在的低结晶度的V2O5?nH2O材料,其较高的比容量和能量密度显示出其应用的广阔前景,例如V2O5?nH2O干凝胶作为正极材料可以获得700 kW?h/kg的能量密度[37]。PETKOV等[38]最近用Bragg衍射数据库中的数据,根据原子双对分布函数方法确定了V2O5?nH2O的三维结构,如图6所示,该结构可以看作是[VO5]四方棱锥组成的双层结构,在双层结构中有部分水分子。
除了钒氧化物之外,Li3V2(PO4)3是近年来研究比较多的钒基正极材料,特别是Li3Fe2(PO4)3研发的部分成功,理应引起更多关注。Li3V2(PO4)3主要有两种存在形式:热力学较稳定的单斜α-Li3V2(PO4)3(空间群为P21/n,a=0.860 6 nm,b=0.859 1 nm,c=1.203 6 nm,β=90.61,V=0.899 83 nm3)和离子交换制备的菱形相(菱形结构的Li3V2(PO4)3有两个Li+离子可以脱出,但是只能可逆地嵌入1.3个Li+离子,电化学性能比单斜相的差,不宜作为正极材料)。单斜结构的Li3V2(PO4)3的原子排列更紧密,是磷酸盐中理论容量最高的(197 mA?h/g)[39],其晶体结构如图7所示。由图7可见,V(1)和V(2)位置与O结合,实际上代表稍微扭曲的[VO6]八面体,其中,V—O的键长为0.200 3和 0.200 6nm,化合价为+3价。Li的位置分别由位于四面体位置的Li(1)和两个位于伪四面体位置的Li(2)和Li(3)组成。Li(2)和Li(3)具有额外的Li—O长键,配位数为5[40]。该材料的电化学行为比较复杂,充电时可以看到一系列的两相转变平台;重新嵌入0~2个Li+离子后形成固溶相,第3个Li+离子的嵌入属于两相转变行为[41-42]。最近的结果表明,3个Li+离子可可逆脱出,获得接近理论值的比放电容量。虽然在循环过程中有一些相的转变,但中子衍射结果表明,在循环过程中,材料的结构并没有太大的变化[43]。Li+离子在材料中的传输能力对于电池材料的性能具有重要的影响。CAHILL等[44]用7Li NMR 和二维交换光谱来探测Li+离子在3个位置的移动情况。发现Li+离子在这些位置间的跳跃变换是微秒级别的。单斜结构的Li3V2(PO4)3中,Li+离子可以在三维尺寸内快速跃迁,因此,具有很好的倍率性能。菱形结构的Li3V2(PO4)3有两个Li+离子可以脱出,但是只能可逆地嵌入1.3个Li+离子。
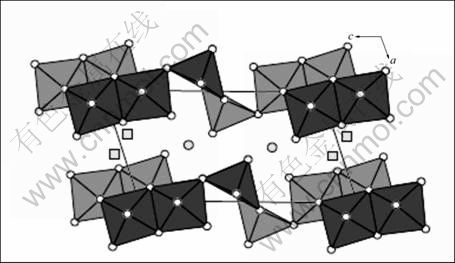
图5 Li1.1V3O8的结构以及VO6和VO5多面体[24]
Fig.5 Structure of Li1.1V3O8, VO6 and VO5 polyhedra (○—Octahedral sites; □—Partly occupied tetrahedron gaps)[24]
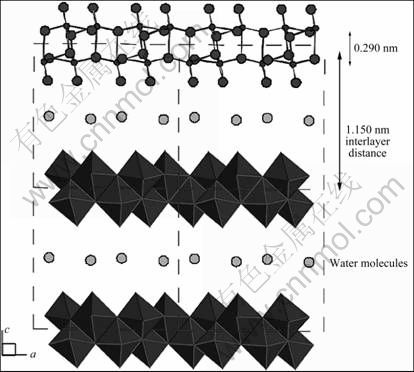
图6 V2O5?nH2O的晶体结构[38]
Fig.6 Crystal structure of V2O5?nH2O[38]
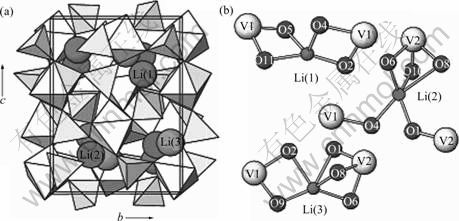
图7 单斜Li3V2(PO4)3的晶体结构及Li的环境配位[39]
Fig.7 Crystal structure of monoclinic Li3V2(PO4)3 (a) and Li coordination environments (b)[39] (V1 (V3+)—O and V2 (V3+)—O octahedra are shown in gray, and P—O tetrahedra are shown in yellow. Li(1), Li(2) and Li(3) atoms are shown in magenta, green, and orange, respectively)
事实上,很多不同的分子和离子都可以嵌入V2O5的层结构中,如有机分子[45]、Ag或Cu金属元素[46]。特别是含Ag或Cu的钒氧化物,如Ag2V4O11、Cu1.1V4O11化合物引起了人们广泛的兴趣[47-48],因为Ag和Cu可以从晶体点阵中脱出,从而增加正极材料的导电性。据报道,其可逆性比Ag2V4O11氧化物的更强。电化学氧化可以形成新化合物Cu1.1V4O11,该新化合物具有更好的循环性能,在0.1~1 mA/cm2的电流密度下,循环20周之后仍然可以获得200 mA?h/g的容量[49]。
2 纳米钒氧化合物的制备与评价
钒氧化合物材料纳米化后Li+离子所需经过的路程有可能大大缩短,比表面积显著增大,可增加电极材料与电解液的接触面积,有助于减少极化;材料纳米化后可以更好地适应脱出/嵌入过程中的体积变化,从而降低反应的能垒。因此,材料纳米化为解决钒氧化物较低的 Li+离子扩散系数以及较低的电导率问题提供了很好的途径,可以获得更高容量、更好的倍率性能和循环稳定性[50]。
2.1 纳米氧化钒的制备与评价
纳米氧化钒制备方法有很多,主要有模板法[51]、水热法[52]、热分解法[4]和静电纺丝法[53]等。不同的制备技术获得的材料微观形貌及电化学性能有明显差别,即使同一种合成技术,参数控制不同,也会得到不同的结果。
模版法因其结构可控、形貌均匀,被广泛应用于材料的合成中[54-60]。LI等[55]将三异丙脂钒氧化物首先沉积到聚碳酸脂的多孔模版中,之后通过高温分解有机物模版制备了氧化钒纳米棒阵列,并研究了它们的电化学性能。在200 C和500 C倍率下放电,可释放的容量分别是薄膜电极的3和4倍,主要结果如图8所示[55-57]。
他们还用NaOH对聚碳酸脂模版预先进行刻蚀处理,再用于V2O5的合成,用该方法增加模版的孔隙度,提高V2O5材料单位体积的能量密度。最近,SIDES和MARTIN[61]制备了不同直径的V2O5纳米棒,并比较了其在低温工作时的电化学性能。在同样的条件下,V2O5纳米棒材料(70 nm)比微米尺寸的V2O5棒状材料具有更高的比放电容量。因为纳米材料的表面积更 大,Li+离子扩散所需要经过的距离更短,可以缓解低温下材料的动力学扩散速率较慢的不足。
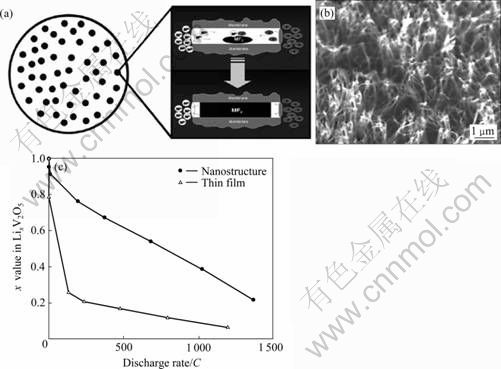
图8 模版法制备纳米线示意图以及V2O5 纳米棒阵的SEM像和电化学性能[55-57]
Fig.8 Schematic diagram of synthesis of nanowires by template method (a), SEM image of V2O5 nanorod array by this method (b) and their electro- chemical performances (c)[55-57]
TAKAHASHI等[62-64]用电化学和电泳沉积的方法将V2O5沉积到聚碳酸脂模版的圆柱形孔洞中,形成棒状V2O5纳米单晶材料。电化学和电泳沉积方法中 可用钒前驱体分别为VO2+溶液和V2O5 溶胶。这两种方法合成的纳米棒阵列的比放电容量和倍率性能比V2O5多晶薄膜的性能好。例如从VO2+溶液中得到的V2O5纳米棒阵列在0.7 A/g的电流密度下,其比放电容量是薄膜电极比容量的5倍。WANG等[65]通过调 整电极两端电压和控制沉积时间制备了水合V2O5纳米管阵列,其初始比放电容量(300 mA?h/g)是V2O5?nH2O 薄膜容量的两倍。这主要归因于电解液和电极材料之间更大的接触面积:纳米管内壁、外壁以及管端口都可以与电解液接触。但是,该纳米管的放电容量随着电流密度的增加衰减较快。该纳米管较差的导电能力是容量降低的主要原因。为进一步改性,V2O5?nH2O溶胶被电泳沉积到Ni纳米棒阵的表面,制备了核-外壳结构的Ni-V2O5?nH2O纳米复合材料[66]。因Ni骨架的导电作用,该复合材料的导电能力大大增强。其容量是单晶纳米棒阵的10倍,是溶胶-凝胶方法制备的V2O5?nH2O薄膜容量的20倍。但是,该方法的合成成本较高,实验中的影响因素较多,规模制备还比较困难。
SPAHR等[67]、KRUMEICH等[68]及MUHR等[69]首先用水热法在溶剂中存在胺的情况下,对V2O5?nH2O溶胶进行水热处理,获得卷形的钒氧化物纳米材料。在该过程中,胺起着结构引导剂的作用,最后形成纳米管或纳米卷轴,图9所示为该结构的TEM像[2, 70]。
当在水热合成过程中用氨作为结构引导剂时,可以得到钒氧化物纳米管,该管壁由一些不同间距的层组成[70]。与其他纳米管的结构相比,纳米卷与电解液之间有4个不同的接触区域,即管口处、管外壁、管内壁和层之间的空隙。该材料具有200 mA?h/g的比放电容量。循环过程中,其结构有所破坏,性能也有 降低。
该方法可以合成不同形貌的氧化钒,如沿[010]方向生长的带状纳米线[71]和沿[130]方向双向生长的纳米板[72]。另外,对V2O5、H2O2和HCl进行水热处理,可以合成介于纳米板和纳米线形貌之间的V2O5?nH2O纳米片层结构[73],但是未报道对应的嵌锂性能。
最近,LI等[74]利用V2O5和H2O2反应生成的凝胶经过水热处理,合成了宽度为100~300 nm、厚度为30~40 nm、长度为几十微米的正交结构纳米带V2O5单晶。电化学性能测试表明,在4.0~1.5 V 的电压范围内,该材料的起始容量为288 mA?h/g,第2周循环后的比放电容量降为191 mA?h/g,之后的4周循环具有较稳定的容量。SENG等[75]直接将V2O5 溶于H2O2形成透明溶液,再经过水热处理,合成了直径为30~50 nm、长度为几厘米的V2O5纳米线,然后再利用传统的造纸工艺制备了柔性V2O5纳米线纸张,如图10(a)和(b)所示。电化学测试表明,在4.0~2.5 V的电压范围内,该V2O5纳米线纸在1.7 C充放电时,具有140 mA?h/g的容量,且40周循环后,容量没有明显的下降;在6.7 C充放电时,仍然具有132 mA?h/g的容量,10周循环后,其容量保持不变[75],如图10(c)所示。
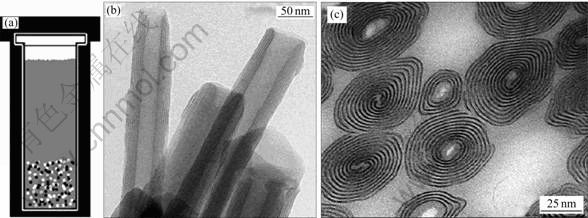
图9 水热法装置图及其制备钒氧化物纳米管和纳米卷的TEM像[2, 70]
Fig.9 Hydrothermal setup (a) and TEM images of synthesis of vanadium oxide nanotube (b) and cross section of nanoscroll (c) [2, 70]
PAN等[4]最近报道了一种高效合成V2O5纳米颗粒的方法。首先将V2O5和草酸反应生成VOC2O4前驱体,该前驱体在空气中热分解后得到V2O5纳米颗粒。V2O5和H2C2O4的比例对最后产物的形貌具有重要的影响。当n(V2O5):n(H2C2O4)=1:5时,可以获得棒状的纳米颗粒。图11所示为在该比例下获得的纳米颗粒[4]。纳米颗粒直径比较均匀,颗粒之间空隙很大。棒状结构的纳米颗粒在局部区域平行排列,直径为20~100 nm。在147 mA/g的电流密度下放电,可以获得270 mA?h/g的比放电容量,该值与嵌入两个Li+离子时的理论容量(290 mA?h/g)非常接近。即使在电流倍率为8 C(2 352 mA/g)下放电,仍然可以获得144 mA?h/g的比放电容量,且具有很好的循环稳定性。
静电纺丝是一种广泛用于聚合物、复合材料和陶瓷纳米线合成的技术[76-79](见图10)。通过控制静电合成参数获得不同形貌的纳米线[80-85]。除了可以宏观控制纳米线的排布外,静电纺丝还可以调整单根纳米线的二次结构,增加结构的复杂性,如制备具有核-外壳、空心和多孔结构的纳米线。静电纺丝合成的V2O5纳米线因为其典型的层结构和优异的电化学性能受到人们的关注[7, 86]。
OSTERMANN等[7]采用静电合成技术和后续煅烧工艺,合成了V2O5纳米棒复合材料。V2O5纳米棒形貌可以通过改变纳米线成分和煅烧温度来控制。BAN等[87]通过对静电合成的材料进行水热处理,再在500 ℃煅烧得到V2O5纳米晶粒。该纳米颗粒具有很 好的库仑效率,其比放电容量可以达到350 mA?h/g。如图12所示,MAI等[86]和ZIABARI等[88]以NH4VO3作为前驱体,采用静电纺丝技术合成了V2O5纳米 线,其直径为100~200 nm、长度为几毫米。该方法降低了合成成本,更适合于规模制备。电化学测试表明,在1.75~4.00 V的电压范围内,该材料的初始容量为390 mA?h/g,50周循环后为201 mA?h/g;在2.00~4.00 V 的电压范围内,该材料的初始容量为275 mA?h/g,50周循环后为187 mA?h/g。
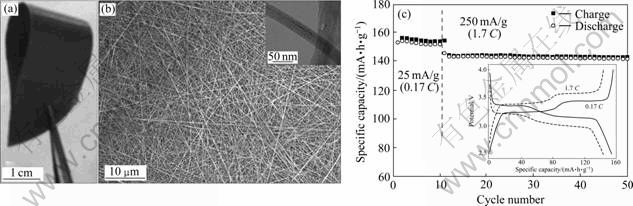
图10 水热法制备的V2O5纳米线宏观图、SEM像和TEM像以及该纳米线的电化学性能曲线[75]
Fig.10 Macro-view of hydrothermal prepared V2O5 nanowires (a), SEM and TEM images (b) and electrochemical performance (c) of as-prepared samples[75]
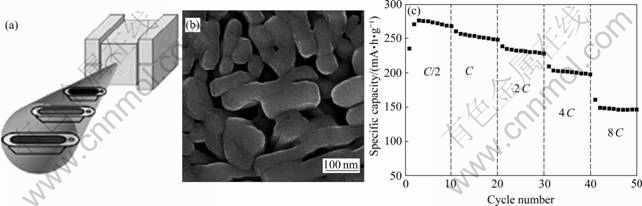
图11 纳米颗粒的热分解示意图、热分解形成的棒状纳米颗粒形貌及其在不同倍率下的循环性能[4]
Fig.11 Illustration of thermal decomposition of nano-particles (a), SEM image of thermal decomposition prepared V2O5 nanorods (b) and its cycle capability (c) at different rates[4]
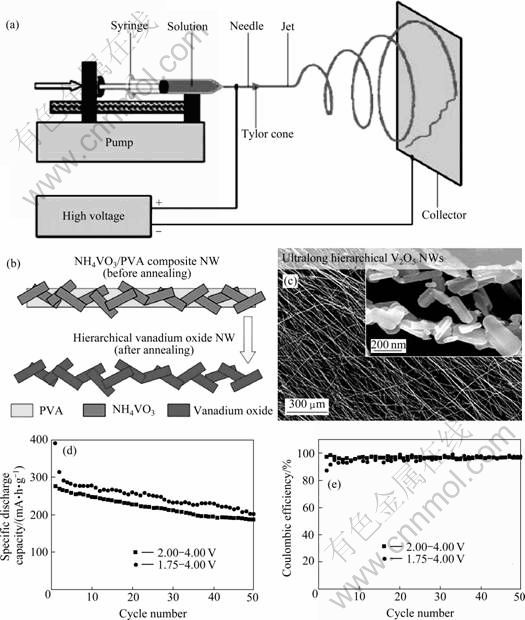
图12 静电纺丝装置示意图、电纺丝制备V2O5纳米线过程示意图以及V2O5纳米线SEM像、循环性能和库仑效率性能[86, 88]
Fig.12 Illustration of electrospinning setup (a), schematic diagram of V2O5 nanowire preparation process (b) and SEM images (c), cycling performance (d) and coulombic efficiency (e) of V2O5 nanowires[86, 88]
2.2 多孔纳米氧化钒的制备与评价
多孔结构材料的比表面积大、空隙连通,有利于电解液的渗透和离子的扩散,从而能获得优异的倍率性能,因此,纳米钒氧化物的多孔化是改善钒氧化物正极特性的有效途径之一。LIU等[89]在VOSO4和 P123 (Pluronic 123, H(—OCH2CH2—)20{—OCH—(CH3)CH2—}70—(—OCH2CH2—)xOH))的水和酒精溶液中,用电化学沉积方法合成了孔径为3~4 nm的氧化钒多孔材料。从VOSO4溶液中电沉积得到的V2O5层结构结晶度较低,层间距为1.1 nm,与V2O5干凝胶的层间距接近。P123在水溶液中作为模版,改变了氧化钒的微观结构。该多孔材料在50 C倍率下放电,其比放电容量为125 mA?h/g。
超临界干燥V2O5凝胶可以得到无定形的V2O5气凝胶 (ARG),其比表面积为450 m2/g,比孔体积为2.3 cm3/g,可以作为Li嵌入的可逆高容量基体[90]。恒电流间歇滴定(GITT)和化学嵌锂显示单位摩尔的V2O5可以储存4个Li+离子。V2O5 气凝胶的比能量可以超过1 600 W?h/kg, 这在所报道的V2O5的基体材料中是最高的。但是,其合成成本较高,体积能量密度较低。
CAO等[91]提出了一种简便自组装合成V2O5球形颗粒的方法,其形貌如图13所示。乙二醇与钒前驱体在聚乙烯吡咯烷酮(PVP)存在的情况下相互作用,得到的空心钒固体前驱物经煅烧得到晶体结构的V2O5空心球(见图13(c))。该方法制备的V2O5空心球在2.0~4.0 V电压范围内循环时具有286.4 mA?h/g的比放电容量和97.2%的库仑效率。
2.3 纳米氧化钒/C复合材料的制备与评价
氧化钒因为自身导电能力较差,需要与其他导电能力良好的材料复合,如聚合物、金属和导电炭等,复合将提高材料的综合性能,ODANI等[92]在密闭体系中,通过高温自发热分解生成炭包覆的VOx。
该核心为V2O3材料,其直径为30~100 nm,炭外壳厚度约为15 nm。所有V2O3纳米颗粒表面都实现了炭包覆,高放大倍数的TEM像显示,该包覆的炭形貌均匀、结构致密。该复合材料在2.00~4.00 V的电压范围内的比放电容量为270 mA?h/g。
HU等[93]将草酸钒前驱体水溶液渗透到双层纳米炭管(CTIT)中,然后在400 ℃空气中煅烧2 h,得到形貌均匀的V2O5/CTIT 纳米复合材料。图14清晰地显示了该复合结构[93]。在2.00~4.00 V的电压范围、58 mA/g电流密度下放电,可以获得280 mA?h/g的可逆容量和99%的库仑效率。循环20周后,其容量 为265 mA?h/g、库仑效率为100%。在0.140、0.588、1.176、2.352及5.880 A/g的电流密度下,分别可以获得 250、223、200、160和90 mA?h/g 的比放电容量。该优异的电化学性能是因为 CTIT不仅可以作为电子的导体,也可以为电解液的扩散提供通道。
SAKAMOTO和DUNN[94]用溶胶-凝胶的方法成功地将V2O5气凝胶复合到单壁炭纳米管导电基体中,实现了彼此在纳米尺度上的紧密接触。另外,电解液可以渗透炭纳米管和V2O5气凝胶到整个复合材料中。因此,该复合材料在高倍率下放电,仍然可以获得超过400 mA?h/g的比容量。
2.4 纳米Li1+xV3O8的制备与评价
Li1+xV3O8虽然在晶体结构稳定性上比V2O5晶体材料更具有优势,但是其倍率性能也受到Li+离子扩散和电子传输的制约。材料纳米化被认为是提高该材料电化学性能的一种主要方式。目前,已用溶胶-凝胶法[95-96]、水热法[97-99]、冷冻-干燥法[100]合成等合成了形貌、尺寸、结晶度和电化学性能各不相同的Li1+xV3O8 [101-102]。
PAN等[6]采用溶胶-凝胶法制备了锂钒氧化物前驱体,之后在空气中热分解,制备了形貌均匀的LiV3O8纳米棒材料,该纳米棒直径为50~150 nm,棒的长度可以达到几微米,另外,纳米棒之间具有很大的空隙(见图15(a))。该结构有利于电解液的渗透,同时因为纳米棒直径为30~150 nm,大大缩短了Li+离子扩散所需经过的路程。因此,在高倍率放电和充电条件 下,材料的利用率和比容量更高。如图15(b)所示,在100 mA/g的电流密度下放电,350 ℃合成的电极(LT350)显示了更明显的电压平台和更高的比放电容量。在第2周循环后,其比放电容量为320 mA?h/g,几乎是传统方法合成电极(HT680)比放电容量的3倍。该方法合成的LiV3O8 电极同时具有很高的比放电容量和很好的循环稳定性。在100 mA/g下放电可以获得320 mA?h/g的比放电容量,单次循环的容量损失仅为 0.23%。在更高电流密度 1 A/g下循环100次之后,其比放电容量仍可达158 mA?h/g。
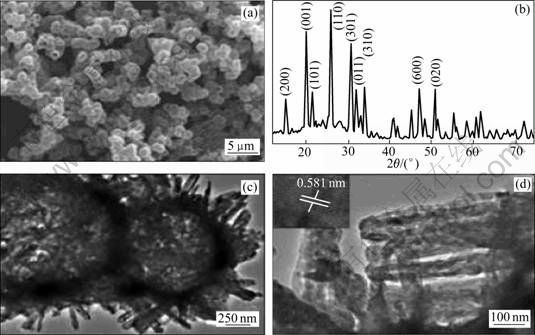
图13 煅烧得到的V2O5中空微米球的SEM像、XRD谱以及低倍和高倍TEM像[91]
Fig.13 SEM image (a), XRD pattern (b), TEM images of low (c) and high (d) maginifications of hollow V2O5 spheres after calcination[91]
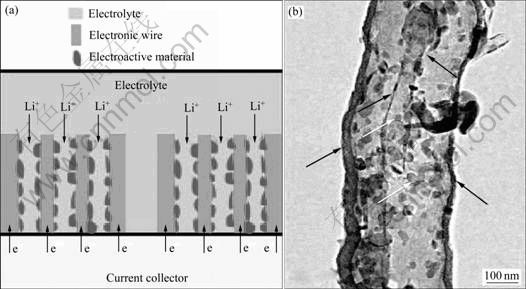
图14 复合材料的导电网络结构示意图及V2O5/CTIT 纳米复合材料的典型TEM像[93]
Fig.14 Schematic diagram of efficient mixed-conducting network (a) and typical TEM image of V2O5/CTIT nanocomposites (b) (V2O5 nanoparticles indicated by red arrows and CTIT by black arrows)[93]
为了提高循环稳定性,添加PEG聚合物到LiV3O8电极材料的前驱体溶液中,合成了具有纳米形貌的LiV3O8[5]。其电化学性能如图16所示。由图16可知,在100 mA/g充放电时,其初始容量达到260 mA?h/g,且具有100%的容量保持能力。循环100周后,仍然可以得到262 mA?h/g的比放电容量,是所有已经报道的结果中是最好的。该研究结果表明,材料的形貌对材料的性能具有重要影响。
2.5 纳米Li3V2(PO4)3的制备与评价
虽然Li+离子在单斜Li3V2(PO4)3中的传输是三维的,但是要获得理想的倍率性能,还需要进一步缩短Li+离子的扩散距离和提高材料的导电性。REN等[103] 将Li3V2(PO4)3纳米颗粒混合在蔗糖的水溶液中,经过水热处理,制备了炭包覆的Li3V2(PO4)3/C复合材料。循环伏安和电化学阻抗结果显示,炭包覆有助于提高Li+离子的扩散和导电能力,也可以减缓固体电解液界面膜的形成。该材料在 28 mA/g电流密度下放电,可以获得126 mA?h/g的容量。最近,WANG等[104]用静电喷涂沉积技术,将前驱体材料沉积到石墨炭表面,制备了Li3V2(PO4)3/C 薄膜。TEM结果显示,炭包覆的纳米颗粒直径大约为50 nm,很好地分布在炭基体中。在3.00~4.30 V的电压范围、1 C放电,该材料的比放电容量为118 mA?h/g。在24 C放电,可以仍然可以获得80 mA?h/g的容量。该结果表明,减小纳米颗粒的直径可以明显提高材料的倍率性能。最近,PAN等[105]将Li3V2(PO4)3的前驱体溶液渗入具有很多细孔的多孔炭材料中,合成了颗粒尺寸小于50 nm 的Li3V2(PO4)3/C 纳米复合材料。该复合材料具有极优的倍率性能和循环稳定性,这是因为纳米尺寸颗粒有效地降低了Li+离子扩散所需要经过的距离,与多孔导电炭基体的接触紧密提高了电子传输的能力。导电炭的多孔结构为电解液的渗透提供了通道,也增加了活性材料与电解液的接触面积。在3.00~4.30 V的电压范围内,第1周循环的比放电容量为122 mA?h/g、库仑效率为90%。该数值比文献[103]报道的没有引入多孔KB炭到前驱体溶液中合成的材料在0.2 C倍率下的比放电容量(110 mA?h/g)高。在1 C下循环22周之后,仍然可以获得120 mA?h/g的比放电容量。然后,在2 C放电、1 C充电,比放电容量没有降低。当在4 C和 8 C倍率下放电时,比放电容量分别为118、115 mA?h/g。从1 C到8 C倍率放电,复合材料的比放电容量的差别只有5 mA?h/g。即使在16 C和32 C倍率下放电,仍然可以分别获得105 和83 mA?h/g的比放电容量。该复合材料优异的倍率性能和很好的循环稳定性归因于Li3V2(PO4)3/C独特的结构。因为当纳米颗粒大小为20~50 nm时,可以缩短Li+离子的扩散时间,同时这些颗粒能较好地嵌入炭基体中,使得电极材料具有较强的导电能力。多孔炭基体也可以为电解液的渗透提供通道,更有利于电极材料的润湿。为进一步改性,在有机气氛中通过自组装,PAN等[3]合成了纳米带形貌的Li3V2(PO4)3,如图17所示,该纳米颗粒分布均匀、形状一致。纳米带的厚度大约为50 nm,宽度为200 nm, 长度为500 nm。纳米带朝不同方向生长,所以,带状颗粒之间存在较大空隙。其电化学性能如图18所示,在3.00~4.30 V放电时,可以获得131 mA?h/g的比放电容量,该数值与理论容量132 mA?h/g非常接近。在2、4和8 C充放电时,分别可以获得128、122和110 mA?h/g的比放电容量。在4 C和8 C充放电时,仍然可以分别获得122 和110 mA?h/g的比放电容量。Li3V2(PO4)3纳米带具有非常优异的循环性 能,连续循环120周,其比放电容量从131 mA?h/g降为130 mA?h/g,容量损失几乎可以忽略。
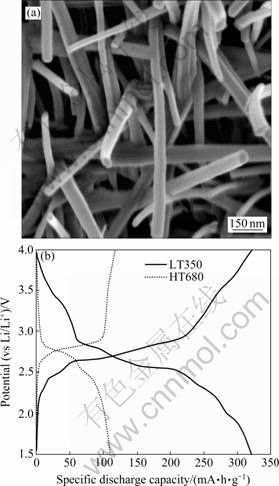
图15 低温热分解得到的LiV3O8和采用不同方法合成的电极的充放电曲线[6]
Fig.15 Morphology of LiV3O8 prepared by low temperature thermal decomposition (a) and charge-discharge curves of samples prepared by two different methods (b)[6]
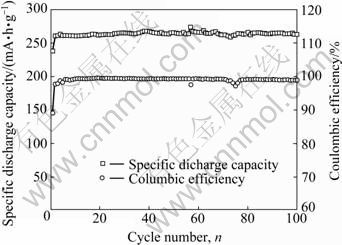
图16 LiV3O8纳米片比放电容量和库仑效率随循环次数的变化曲线[5]
Fig.16 Plots of specific discharge capacity and coulombic efficiency of LiV3O8 nanosheets versus cycle number[5]

图17 Li3V2(PO4)3 纳米带的低倍和高倍SEM像[3]
Fig.17 SEM images of Li3V2(PO4)3 nanobelts at low (a) and high (b) magnifications[3]
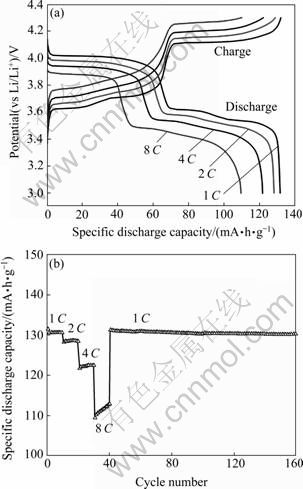
图18 Li3V2(PO4)3 纳米带在不同倍率下的充放电曲线和循环性能(1 C=132 mA/g)[3]
Fig.18 Charge-discharge curves (a) and cycling performance (b) of Li3V2(PO4)3 nanobelts at various rates (1 C=132 mA/g)[3]
3 结论与展望
通过对几种钒氧化物和Li3V2(PO4)3等钒基正极材料原子尺度结构的研究和纳米化制备技术的系统比较分析,钒基正极材料正在受到广泛关注,很有可能发展成为新一代锂离子电池的主流正电极材料,关键在于原子尺度结构的可逆性和稳定性以及可将材料结构比较经济地纳米化,并获得稳定的电化学性能。已有研究表明,不同原子尺度结构的钒基正极材料,其嵌锂能力各不相同;当材料的尺度由微米减小到纳米时,材料的能量储存和释放性能大大提高,这主要得益于材料结构纳米化产生的独特形貌和大比表面积;溶胶-凝胶法、水热法和静电纺丝法等是目前用来合成不同形貌的钒氧化物纳米材料的主要制备技术,不同的制备技术获得材料的微观形貌及电化学性能具有明显差别。同一种合成技术参数控制不同也会得到不同的结果,有必要进一步完善相关的制备技术。未来的工作可以围绕着如下的几方面展开:1) 深入研究钒基正极材料在Li+离子嵌入和脱出过程中原子尺度结构可逆转变的机制,为最终在这类材料中选出具有原子尺度结构优势的候选材料奠定物质结构基础;2) 更加深入地研究材料的形貌,合成方法对钒基纳米正极材料性能的影响,为今后的研究提供借鉴,并最终将这类材料推向主流市场提供理论支撑;3) 对现有技术作出重要改进或探寻新的、经济有效的合成技术,显著降低此类材料的制备成本,为最终将这类材料推向主流市场提供技术支持。
REFERENCES
[1] WHITTINGHAM M A. Lithium batteries and cathode materials[J]. Chem Rev, 2004, 104: 4271-4301.
[2] CHERNOVA N A, ROPPOLO M, DILLON A C, WHITTINGHAM M S. Layered vanadium and molybdenum oxides: Batteries and electrochromics[J]. J Mater Chem, 2009, 19: 2526-2552.
[3] PAN A, CHOI D, ZHANG J G, LIANG S Q, CAO G Z, NIE Z M, AREY B W, LIU J. High-rate cathodes based on Li3V2(PO4)3 nanobelts prepared via surfactant-assisted fabrication[J]. J Power Sources, 2011, 196: 3646-3649.
[4] PAN A, ZHANG J G, NIE Z M, CAO G Z, AREY B W, LI G, LIANG S Q, LIU J. Facile synthesized nanorod structured vanadium pentoxide for high-rate lithium batteries[J]. J Mater Chem, 2010, 20: 9193-9199.
[5] PAN A, ZHANG J G, CAO G Z, LIANG S Q, WANG C M, NIE Z M, AREY B W, XU W, LIU D W, XIAO J, LI G, LIU J. Nanosheet-structured LiV3O8 with high capacity and excellent stability for high energy lithium batteries[J]. J Mate Chem, 2011, 21: 10077-10084.
[6] PAN A, ZHANG J G, CAO G Z, XU W, NIE Z M, XIAO J, CHOI D, AREY B W, WANG C M, LIANG S Q. Template free synthesis of LiV3O8 nanorods as a cathode material for high-rate secondary lithium batteries[J]. J Mater Chem, 2011, 21: 1153-1161.
[7] OSTERMANN R, LI D, YIN Y, MCCANN J T, XIA Y. V2O5 nanorods on TiO2 nanofibers: A new class of hierarchical nanostructures enabled by electrospinning and calcination[J]. Nano Letters, 2006, 6: 1297-1302.
[8] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414: 359-367.
[9] 刘国强, 曾潮流, 徐 宁, 高 虹, 杨 柯. 锂蓄电池正极材料LiV3O8的合成和充放电性能[J]. 中国有色金属学报, 2002, 12(1): 70-73.
LIU Guo-qiang, ZENG Chao-liu, XU Ning, GAO Hong, YANG Ke. Synthesis and electrochemical properties of LiV3O8 as cathode material for rechargeable lithium batteries[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(1): 70-73.
[10] BURKE A F. Batteries and ultracapacitors for electric, hybrid, and fuel cell vehicles[J]. Proceedings of the IEEE, 2007, 95: 806-820.
[11] MANTHIRAM A. Materials challenges and opportunities of lithium ion batteries[J]. J Phys Chem Lett, 2011, 2: 176-184.
[12] KIM J H, MYUNG S T, YOON C S, KANG S G, SUN Y K. Comparative study of LiNi0.5Mn1.5O4-δ and LiNi0.5Mn1.5O4 cathodes having two crystallographic structures: Fd3m and P4332[J]. Chem Mater, 2004, 16: 906-914.
[13] BESENHARD J O, SCHWAKE M, MISAILIDIS N. Modified chromium oxides for high-rate lithium intercalation cathodes[J]. J Power Sources, 1989, 26: 409-414.
[14] WANG Y, CAO G Z. Developments in nanostructured cathode materials for high-performance lithium-ion batteries[J]. Adv Mater, 2008, 20: 2251-2269.
[15] WHITTINGHAM M S. The role of ternary phases in cathode reactions[J]. J Electrochem Soc, 1976, 123: 315-320.
[16] MURPHY D W, CHRISTIAN P A, DISALVO F J, WASZCZAK J V. Lithium incorporation by vanadium pentoxide[J]. Inorg Chem, 1979, 18: 2800-2803.
[17] ABELLO L, HUSSON E, REPELIN Y R, LUCAZEAU G. Vibrational spectra and valence force field of crystalline V2O5[J]. Spectrochim Acta, 1983, 39: 641-651.
[18] COCCIANTELLI J M, DOUMERC J P, POUCHARD M, BROUSSELY M, LABAT J. Crystal chemistry of electrochemically inserted LixV2O5[J]. J Power Sources, 1991, 34: 103-111.
[19] JEAN G. Vanadium pentoxide and vanadium oxide bronzes—Structural chemistry of single (S) and double (D) layer MxV2O5 phases[J]. J Solid State Chem, 1992, 100: 229-245.
[20] CAVA R J, SANTORO A, MURPHY D W, ZAHURAK S M, FLEMING R M, MARSH P, ROTH R S. The structure of the lithium-inserted metal oxide δ-LiV2O5[J]. J Solid State Chem, 1986, 65: 63-71.
[21] BROUSSELY M, LABAT J, BODET J M, COCCIANTELLI J M. Recent developments in rechargeable lithium batteries[J]. Journal of Power Sources, 1991, 13: 429-436.
[22] DELMAS C, BRETHES S, MENETRIER M. ω-LixV2O5—A new electrode material for rechargeable lithium batteries[J]. J Power Sources, 1991, 34: 113-118.
[23] DELMAS C, CONGNAC-AURADOU H, COCCIANTELLI J M, COCCIANTELLI J M, MENETRIER M, DOUMERC J P. The LixV2O5 system: An overview of the structure modifications induced by the lithium intercalation[J]. Solid State Ionics, 1994, 69: 257-264.
[24] WADSLEY A D. Crystal chemistry of non-stoichiometric pentavalent vanadium oxides: Crystal structure of Li1+xV3O8[J]. Acta Crystallographic, 1957, 10: 261-267.
[25] KAWAKITA J, MORI H, MIURA T, KISHI T. Formation process and structural characterization of layered hydrogen vanadium[J]. Solid State Ionics, 2000, 131: 229-235.
[26] ZHANG X, FRECH R. Spectroscopic investigation of Li1+xV3O8[J]. Electrochem Acta, 1996, 43: 861-868.
[27] KAWAKITA J, KATAYAMA Y, MIURA T, KISHI T. Structural properties of Li1+xV3O8 upon lithium insertion at ambient and high temperature[J]. Solid State Ionics, 1998, 107: 145-152.
[28] KAWAKITA J, MIURA T, KISHI T. Lithium insertion into Li4V3O8[J]. Solid State Ionics, 1999, 120: 109-116.
[29] PANERO S, PASQUALI M, PISTOIA G. Rechargeable Li/Li1+xV3O8 cells[J]. J Electrochem Soc, 1983, 130: 1225-1227.
[30] KAWAKITA J, KATAYAMA Y, MIURA T, KISHI T, Structural properties of Li1+xV3O8 upon lithium insertion at ambient and high temperature[J]. Solid State Ionics, 1998, 107: 145-152.
[31] DUPRE N, GAUBICHER J, GUYOMARD D, GREY C P. 7Li and 51V MAS NMR study of the electrochemical behavior of Li1+xV3O8[J]. Chem Mater, 2004, 16: 2725-2733.
[32] ZAKHAROVA G S, VOLKOV V L. Intercalation compounds based on vanadium (Ⅴ) oxide xerogel[J]. Russian Chemical Reviews, 2003, 72: 311-325.
[33] DONG W, SAKAMOTO J S, DUNN B. Electrochemical properties of vanadium oxide aerogels[J]. Sci Tech Adv Mater, 2003, 4: 3-11.
[34] SAKURAI Y, OKADA S, YAMAKI J, OKADA T. Electrochemical behaviour of amorphous V2O5(-P2O5) cathodes for lithium secondary batteries[J]. J Power Sources, 1987, 20: 173-177.
[35] SALLOUX K, CHAPUT F, WONG H P, DUNN B, BREITER M W. Lithium intercalation in vanadium pentoxide aerogels[J]. J Electrochem Soc, 1995, 142: L191-L192.
[36] WEST K, CHRISTIANSEN B Z, ?STERGARD M J L, JACOBSEN T. Vanadium oxides as electrode materials for rechargeable lithium cells[J]. J Power Sources, 1987, 20: 165-172.
[37] WEST K, CHRISTIANSEN B Z, JACOBSEN T, SKAARUP S. Vanadium oxide xerogels as electrodes for lithium batteries[J]. Electrochem Acta, 1993, 38: 1215-1220.
[38] PETKOV V, TRIKALITIS P N, BOZIN E S, BILLINGE S J L, VOGT T, KANATZIDIS M G. Structure of V2O5·nH2O xerogel solved by the atomic pair distribution function technique[J]. J Am Chem Soc, 2002, 124: 10157-10162.
[39] MORGAN D, CEDER G, SAIDI M Y, BARKER J, SWOYER J, HUANG H, ADAMSON G. Experimental and computational study of the structure and electrochemical properties of LixM2(PO4)3 compounds with the monoclinic and rhombohedral structure[J]. Chem Mater, 2002, 14: 4684-4693.
[40] YIN S C, GRONDEY H, STROBEL P, ANNE M, NAZAR L F. Electrochemical property: Structure relationships in monoclinic Li3-yV2(PO4)3[J]. J Am Chem Soc, 2003, 125: 10402-10411.
[41] SAIDI M Y, BARKER J, HUANG H, SWOYER J L, ADAMSON G. Electrochemical properties of lithium vanadium phosphate as a cathode material for lithium-ion batteries[J]. Electrochem Solid-State Letter, 2002, 5: A149-A151.
[42] HUANG H, YIN S C, KERR T, TAYLOR N, NAZAR L F. Nanostructured composites: A high capacity, fast rate Li3V2(PO4)3/carbon cathode for rechargeable lithium batteries[J]. Adv Mater, 2002, 14: 1525-1528.
[43] PATOUX S, WURM C, MORCRETTE M, ROUSE G, MASQUELIER C. A comparative structural and electrochemical study of monoclinic Li3Fe2(PO4)3 and Li3V2(PO4)3[J]. J Power Sources, 2003, 119: 278-284.
[44] CAHILL L S, CHAPMAN R P, BRITTEN J F, GOWARD G R. 7Li NMR and two-dimensional exchange study of lithium dynamics in monoclinic Li3V2(PO4)3[J]. J Phys Chem B, 2006, 110: 7171-7177.
[45] HAGRMAN P J, FINN R C, ZUBIETA J. Molecular manipulation of solid state structure: Influences of organic components on vanadium oxide architectures[J]. Solid State Science, 2001, 3: 745-774.
[46] OWENS B B, PASSERINI S, SMYRL W H. Lithium ion insertion in porous metal oxides[J]. Electrochem Acta, 1999, 45: 215-224.
[47] CRESPI A, SCHMIDT C, NORTON J, CHEN K, SKARSTAD P. Modeling and characterization of the resistance of lithium/SVO batteries for implantable cardioverter defibrillators[J]. J Electrochem Soc, 2001, 148: A30-A37.
[48] MORCRETTE M, ROZIER P, DUPONT L, MUGNIER E, SNNIER L, GALY J, TARASCON J M. A reversible copper extrusion–insertion electrode for rechargeable Li batteries[J]. Nature Materials, 2003, 2: 755-761.
[49] MORCRETTE M, MARTIN P, ROZIER P, VEZIN H, CHEVALLIER F, LAFFONT L, POIZOT P, TARASCON J M. Cu1.1V4O11: A new positive electrode material for rechargeable Li batteries[J]. Chem Mater, 2005, 17: 418-426.
[50] GUO Y G, HU J S, WAN L J. Nanostructured materials for electrochemical energy conversion and storage devices[J]. Adv Mater, 2008, 20: 2878-2887.
[51] CHE G, LAKSHMI B B, FISHER E R, MARTIN C R. Carbon nanotubule membranes for electrochemical energy storage and production[J]. Nature, 1998, 393: 346-349.
[52] CAO A M, HU J S, LIANG H P, WAN L J. Self-assembled vanadium pentoxide (V2O5) hollow microspheres from nanorods and their application in lithium-ion batteries[J]. Angew Chem Int Ed, 2005, 117: 4465-4469.
[53] BANA C M, CHERNOVAA N A, WHITTINGHAM M S. Electrospun nano-vanadium pentoxide cathode[J]. Electrochem Commun, 2009, 11: 522-525.
[54] NISHIZAWA M, MUKAI K, KUWABATA S, MARTIN C R, YONEYAMA H. Template synthesis of polypyrrole-coated spinel LiMn2O4 nanotubules and their properties as cathode active materials for lithium batteries[J]. J Electrochem Soc, 1997, 144: 1923-1927.
[55] LI N, PATRISSI C J, CHE G, MARTIN C R. Rate capabilities of nanostructured LiMn2O4 electrodes in aqueous electrolyte[J]. J Electrochem Soc, 2000, 147: 2044-2049.
[56] PATRISS C J, MARTIN C R. Sol-gel-based template synthesis and Li-insertion rate performance of nanostructured vanadium pentoxide[J]. J Electrochem Soc, 1999, 146: 3176-3180.
[57] PATRISSI C J, MARTIN C R. Improving the volumetric energy densities of nanostructured V2O5 electrodes prepared using the template method[J]. J Electrochem Soc, 2001, 148: A1247-A1253.
[58] LI N, MARTIN C R. A high-rate, high-capacity, nanostructured Sn-based anode prepared using sol-gel template synthesis[J]. J Electrochem Soc, 2001, 148: A164-A170.
[59] LAKSHMI B B, PATRISSI C J, MARTIN C R. Sol-gel template synthesis of semiconductor oxide micro- and nanostructures[J]. Chem Mater, 1997, 9: 2544-2550.
[60] CHE G, JIRAGE K B, FISHER E R, MARTIN C R. Chemical-vapor deposition-based template synthesis of microtubular TiS2 battery electrodes[J]. J Electrochem Soc, 1997, 144: 4296-4302.
[61] SIDES C R, MARTIN C R. Nanostructured electrodes and the low-temperature performance of Li-ion batteries[J]. Adv Mater, 2005, 17: 125-128.
[62] TAKAHASHI K, LIMMER S J, WANG Y, CAO G Z. Synthesis and electrochemical properties of single-crystal V2O5 nanorod arrays by template-based electrodeposition[J]. J Phys Chem B, 2004, 108: 9795-9800.
[63] TAKAHASHI K, LIMMER S J, WANG Y, CAO G Z. Growth and electrochemical properties of single crystal V2O5 nanorod arrays[J]. J Appl Phys B: Part 1, 2005, 44B: 662-668.
[64] TAKAHASHI K, LIMMER S J, WANG Y, CAO G Z. Growth and electrochromic properties of single crystal V2O5 nanorod arrays[J]. Appl Phys Lett, 2005, 86: 053102.
[65] WANG Y, TAKAHASHI K, SHANG H M, LEE K H, CAO G Z. Synthesis and electrochemical properties of vanadium oxide nanotube arrays[J]. J Phys Chem B, 2005, 109: 3085-3088.
[66] TAKAHASHI K, WANG Y, CAO G Z. Ni-V2O5.nH2O core-shell nanocable arrays for enhanced electrochemical intercalation[J]. J Phys Chem B, 2005, 109: 48-51.
[67] SPAHR M E, BITTERLI P, NESPER R, MULLER M, KRUMEICH F, NISSEN H U. Redox-active nanotubes of vanadium oxide[J]. Angew Chem Int Ed, 1998, 37: 1263-1265.
[68] KRUMEICH F, MUHR H J, NIEDERBERGER M, BIERI F, SCHNYDER B, NESPER R. Morphology and tepochemical reactions of novel vanadium oxide nanotubes[J]. J Am Chem Soc, 1999, 121: 8324-8331.
[69] MUHR H J, KRUMEICH F, SCHONHOLZER U P, BIERI F, NIEDERBERGER M, GAUCKLER L J, NESPER R. Vanadium oxide nanotubes—A new flexible vanadate nanophase[J]. Adv Mater, 2000, 12: 231-234.
[70] PILLAI K S, KRUMEICH F, MUHR H J, NIEDERBERGER M, NESPER R. The first oxide nanotubes with alternating inter-layer distances[J]. Solid State Ionics, 2001, 141/142: 185-190.
[71] PAN D, ZHANG S, CHEN Y, HOU J G. Hydrothermal preparation of long nanowires of vanadium oxide[J]. J Mater Research, 2002, 17: 1981-1984.
[72] SCHLECHT U, KNEZ M, DUPPEL V, KIENLE L, BURGHARD M. Boomerang-shaped VOx belts: Twinning within isolated nanocrystals[J]. Appl Phys A, 2004, 78: 527-529.
[73] HU X K, MA D K, LIANG J B, XIONG S L, LI J Y, QIAN Y T. V2O5.nH2O crystalline nanosheets: Hydrothermal fabrication and structure evolution[J]. Chem Lett, 2007, 26: 560-561.
[74] LI G, PANG S, JIANG L, GUO Z, ZHANG Z. Environmentally friendly chemical route to vanadium oxide single-crystalline nanobelts as a cathode material for lithium-ion batteries[J]. J Phys Chem B, 2006, 110: 9383-9386.
[75] SENG K H, LIU J, GUO Z P, CHEN Z X, LIU H K. Free standing V2O5 electrode for flexible lithium ion batteries[J]. Electrochem Communi, 2011, 13: 383-386.
[76] POL V G, KOREN E, ZABAN A. Fabrication of continuous conducting gold wires by electrospinning[J]. Chem Mater, 2008, 20: 3055-3062.
[77] NAM S H, SHIM H S, KIM Y S, DAR M A, KIM J G, KIM W B. Ag or Au nanoparticle-embedded one-dimensional composite TiO2 nanofibers prepared via electrospinning for use in lithium-ion batteries[J]. Appl Mater Interfaces, 2010, 2: 2046-2052.
[78] SHUI J L, LI J C M. Platinum nanowires produced by electrospinning[J]. Nano Lett, 2009, 9:1307-1314.
[79] KLIMOV E, RAMAN V, VENKATESH R, HECKMANN W, STARK R. Designing nanofibers via electrospinning from aqueous colloidal dispersions: Effect of cross-linking and template polymer[J]. Macromolecules, 2010, 43: 6152-6155.
[80] LI D, WANG Y, XIA Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays[J]. Nano Lett, 2003, 3: 1167-1171.
[81] LI D, WANG Y, XIA Y. Electrospinning nanofibers as uniaxiallly aligned arrays and layer-by-layer stacked films[J]. Adv Mater, 2004, 16: 361-366.
[82] LI D, OUYANG G, MCCANN J T, XIA Y. Collecting electrospun nanofibers with patterned electrodes[J]. Nano Lett, 2005, 5: 913-916.
[83] THERON A, ZUSSMAN E, YARIN A L. Electrostatic field-assisted alignment of electrospun nanofibres[J]. Nanotechnology, 2001, 12: 384-390.
[84] ZUSSMAN E, THERON A, YARIN A L. Formation of nanofiber crossbars in electrospinning[J]. Appl Phys Lett, 2003, 82: 973-975.
[85] XU C Y, INAI R, KOTAKI M, RAMAKRISHNA S. Aligned biodegradable nanofibrous structure: A potential scaffold for blood vessel engineering[J]. Biomaterials, 2004, 25: 877-886.
[86] MAI L, XU L, HAN C H, XU X, LUO Y, ZHAO S, ZHAO Y. Electrospun ultralong hierarchical vanadium oxide nanowires with high performance for lithium ion batteries[J]. Nano Lett, 2010, 10: 4750-4755.
[87] BAN C, CHERNOVA N A, WHITTINGHAM M S. Electrospun nano-vanadium pentoxide cathode[J]. Electrochem Communi, 2008, 11: 522-525.
[88] ZIABARI M, MOTTAGHITALAB V, HAGHI A K. Application of direct tracking method for measuring electrospun nanofiber diameter[J]. Braz J Chem Eng, 2009, 26: 53-62.
[89] LIU P, LEE S H, TRACY C E, YAN Y, TURNER J A. Preparation and lithium insertion properties of mesoprous vanadium oxide[J]. Adv Mater, 2002, 14: 27-30.
[90] AUGUSTYN V, DUNN B. Vanadium oxide aerogels: Nanostructured materials for enhanced energy storage[J]. Comptes Rendus Chimie, 2010, 13: 130-141.
[91] CAO A M, HU J S, LIANG H P, WAN L J. Self-assembled vanadium pentoxide (V2O5) hollow microspheres from nanorods and their application in lithium-ion batteries[J]. Angew Chem Int Ed, 2005, 44: 4391-4395.
[92] ODANI A, POL V G, POL S V, KOLTPIN M, GEDANKEN A, AURBACH D. Testing carbon-coated VOx prepared via reaction under autogenic pressure at elevated temperature as Li-insertion materials[J]. Adv Mater, 2006, 18: 1431-1436.
[93] HU Y S, LIU X, MULLER J O, SCHLOGL R, MAIER J, SU D S. Synthesis and electrode performance of nanostructured V2O5 by using a carbon tube-in-tube as a nanoreactor and an efficient mixed-conducting network[J]. Angew Chem Int Ed, 2009, 48: 210-214.
[94] SAKAMOTO J S, DUNN B. Vanadium oxide-carbon nanotube composite electrodes for use in secondary lithium batteries[J]. J Electrochem Soc, 2002, 149: A26-A30.
[95] DEPTUA A, DUBARRY M, NORET A, GAUBICHER J, OLCZAK T, ADA W, GUYOMARD D. A typical Li1.1V3O8 prepared by a novel synthesis route[J]. Electrochem Solid-State Lett, 2006, 9: A16-A18.
[96] CAO X, XIE L, ZHAN H, ZHOU Y. Large-scale synthesis of Li1.2V3O8 as a cathode material for lithium secondary battery via a soft chemistry route[J]. Mat Res Bull, 2009, 44: 472-277.
[97] LIU H, YANG H, HUANG T. Synthesis, structure and electrochemical properties of one-dimensional nanometer materials LiV3O8[J]. Materials Science and Engineering B, 2007, 143: 60-63.
[98] XU H Y, WANG H, SONG Z Q, WANG Y W, YAN H, YOSHIMURA M. Novel chemical method for synthesis of LiV3O8 nanorods as cathode materials for lithium ion batteries[J]. Electrochem Acta, 2004, 49: 349-353.
[99] XU J, ZHANG H, ZHANG T, PAN Q, GUI Y. Influence of heat-treatment temperature on crystal structure, morphology and electrochemical properties of LiV3O8 prepared by hydrothermal reaction[J]. J Alloys and Compounds, 2009, 467: 327-331.
[100] BRYLEV O A, SHLYAKHTIN O A, EGOROV A V, TRETYAKOV Y D. Phase formation and electrochemical properties of cryochemically processed Li1+xV3O8 materials[J]. J Power Sources, 2008, 164: 868-873.
[101] TRAN N, BRAMNIK K G, HIBST H, PROLB J, MRONGA N, HOLZAPFEL M, SCHEIFELE W, NOVAK P. Spray-drying synthesis and electrochemical performance of lithium vanadates as positive electrode materials for lithium batteries[J]. J Electrochem Soc, 2008, 155: A384-A389.
[102] JOUANNEAU S, VERBASERE A, LASCAUD S, GUYOMARD D. Improvement of the lithium insertion properties of Li1.1V3O8[J]. Solid State Ionics, 2006, 177: 311-315.
[103] REN M M, ZHOU Z, GAO X P, PENG W X, WEI J P. Core-shell Li3V2(PO4)3@C composites as cathode materials for lithium-ion batteries[J]. J Phys Chem C, 2008, 112: 5689-5693.
[104] WANG L, ZHANG L C, LIEBERWIRTH I, XU H W, CHEN C H. A Li3V2(PO4)3/C thin film with high rate capability as a cathode material for lithium-ion batteries[J]. Electrochem Communi, 2010, 12: 52-55.
[105] PAN A, LIU J, ZHANG J G, XU W, CAO G, NIE Z, AREY B W, LIANG S. Nano-structured Li3V2(PO4)3/carbon composite for high-rate lithium-ion batteries[J]. Electrochem Communi, 2010, 12: 1674-1677.
(编辑 陈卫萍)
基金项目:国家自然科学创新团队基金资助项目(50721003)
收稿日期:2011-05-28;修订日期:2011-07-16
通信作者:梁叔全,教授,博士;电话:0731-88876691;E-mail: sqliang@mail.csu.edu.cn
梁叔全教授简介
梁叔全,教授,博士生导师,享受国务院特殊津贴专家,教育部优秀骨干教师,湖南省人民政府芙蓉学者特聘教授。湖南大学学士、天津大学硕士、中南大学博士。1993—1994年Hungarian Academy of Science做访问学者。1996—1998年在Universiti Sains Malaysia任教。多年来,在国家“863”及国家自然科学基金等项目资助下,在新材料合成、评价表征及粉末注射成形流变等方面开展研究,发表学术论文100余篇,其中包括国际知名学术刊物,如《J Mater Chem》、《Electrochem Comm》、《J Am Ceram Soc》、《J Power Sources》等。被邀请为《ACS Nano》、《Electrochem Comm》等国际知名刊物(SCI)的审稿人。出版了《粉末注射成形流变学》专著。获国家科技进步二等奖1项、部级科技进步一等奖1项、省级科技进步二等奖1项、第五届国家图书奖提名奖、第十届全国优秀科技图书奖二等奖。


