Surface treatment of NiTi shape memory alloy by
modified advanced oxidation process
CHU Cheng-lin(储成林)1, WANG Ru-meng(王如萌)1, YIN Li-hong(尹立红)2,
PU Yue-pu(浦跃朴)2, DONG Yin-sheng(董寅生)1, GUO Chao(郭 超)1,
SHENG Xiao-bo(盛晓波)1, LIN Ping-hua(林萍华)1, CHU Paul-K(朱剑豪)3
1. Jiangsu Key Laboratory for Advanced Metallic Materials, School of Materials Science and Engineering,
Southeast University, Nanjing 211189, China;
2. School of Public Health, Southeast University, Nanjing 210096, China;
3. Department of Physics and Materials Science, City University of Hong Kong, Hong Kong, China
Received 7 November 2008; accepted 4 January 2009
Abstract:
A modified advanced oxidation process(AOP) utilizing a UV/electrochemically-generated peroxide system was used to fabricate titania films on chemically polished NiTi shape memory alloy(SMA). The microstructure and biomedical properties of the film were characterized by scanning electron microscopy(SEM), X-ray photoelectron spectroscopy(XPS), inductively-coupled plasma mass spectrometry(ICPMS), hemolysis analysis, and blood platelet adhesion test. It is found that the modified AOP has a high processing effectiveness and can result in the formation of a dense titania film with a Ni-free zone near its top surface. In comparison, Ni can still be detected on the outer NiTi surface by the conventional AOP using the UV/H2O2 system. The depth profiles of O, Ni, Ti show that the film possesses a smooth graded interface structure next to the NiTi substrate and this structure enhances the mechanical stability of titania film. The titania film can dramatically reduce toxic Ni ion release and also improve the hemolysis resistance and thromboresistance of biomedical NiTi SMA.
Key words:
NiTi shape memory alloy; titania film; advanced oxidation process(AOP); biomedical properties;
1 Introduction
Deposition of a titania film improves the biological safety of NiTi shape memory alloys(SMA) used in cardiovascular products[1-4] because the titania film as a barrier layer can commendably block leaching of toxic nickel ions from the NiTi substrate in patients[5] and can also improve the blood compatibility of NiTi SMA by preventing thrombosis formation[6]. There have hitherto been a number of reports on the fabrication of titania films on NiTi SMA by different methods, including sol-gel technique[7], heat oxidation[8], laser oxidation [9], H2O2 oxidation[10], etc.
Recently, we have reported that the surface structure of NiTi SMA can be modified by advanced oxidation processes(AOP) in a Fe2+/H2O2 system[11] or an ultraviolet (UV)/H2O2 photocatalytic system[12], where hydroxyl radicals (·OH) are generated by direct decomposition of H2O2 with Fe2+ ions or UV irradiation. Such a nonselective and highly reactive oxidant has been utilized to remove organic pollutants from water[13-14]. Since ·OH (with oxidation potential of 2.8 V) is a stronger oxidant than H2O2 (oxidation potentials equal 1.80 and 0.87 V at pH=0 and 14, respectively), it can be used to oxide NiTi SMA to form a titanium oxide layer on NiTi substrate.
However, in our previous studies, it is also found that during the AOP of NiTi SMA, the advanced oxidation reaction in the UV/H2O2 system is not stable because the quantity of ·OH produced by catalytic decomposition of H2O2 under UV irradiation decreases due to continuous consumption of H2O2. This intrinsic problem can be overcome by a modified AOP utilizing a UV/electrochemically-generated peroxide system, in which H2O2 can be produced continuously at the cathode under rather mild conditions by the reduction of oxygen. The electrochemically generated H2O2 subsequently reacts with UV to supply ·OH continuously[13]. In this case, there is a stable ·OH source for the formation of the titania film on NiTi substrate by AOP.
The aim of this work is to fabricate a titania film on chemically polished NiTi SMA by this modified AOP in a UV/electrochemically-generated peroxide system. The microstructure and biomedical properties of the film are determined by scanning electron microscopy(SEM), X-ray photoelectron spectroscopy(XPS), inductively- coupled plasma mass spectrometry (ICPMS), hemolysis analysis, and blood platelet adhesion test.
2 Experimental
2.1 Fabrication of titania film on NiTi SMA by modified AOP
A commercially available NiTi (50.8% Ni, molar fraction) SMA plate for medical applications with a martensite initiation temperature (Ms) of -12.8 ℃ and austenite finish temperature (Af) of 33.4 ℃ was cut into small rectangular blocks with dimensions of 10 mm×10 mm×1 mm. The samples were chemically polished with a solution containing H2O, HF, and HNO3 in a ratio of 5:1:4 for 5 min. The samples were then ultrasonically washed in acetone for 10 min and deionized water for 10 min. They were divided into two groups. The first group was used as the control (denoted as the CP). The second group was treated by the modified AOP to form a surface titania film at a constant current of 0.3 A for 60 min in an electrolytic cell consisting of a graphite cathode and the NiTi sample as the anode. The electrolyte consisted of a 0.02 mol/L Na2SO4 aqueous solution at pH=3.0. During the modified AOP, fresh air was continuously blown into the Na2SO4 electrolyte near the graphite cathode. The electrolyte was irradiated by UV (254 nm) vertically, leading to photocatalytical decomposition of the electrochemically-generated H2O2. Afterwards, the samples were ultrasonically washed in acetone for 10 min and deionized water for 10 min (denoted as the modified AOP).
2.2 Microstructures
The surface morphology was assessed by a field-emission type SEM (Sirion 2000, FEI Co.) at 20 kV accelerating voltage after the surface was coated with gold. The samples were analyzed by XPS on a VG Scientific ESCALAB 5 spectrometer with monochromatic Al Ka (1486.6 eV) X-ray radiation. The base pressure in the analysis chamber was better than 10-6 Pa. High-resolution Ti 2p, O 1s and Ni 2p spectra were acquired at a 20 eV pass energy to determine the chemical states and concentrations. The XPS depth profiles were obtained by using a rastered 3 keV Ar+ ion beam. The argon pressure during depth profiling was about 10-5 Pa and the sputtering rate was estimated to be about 20 nm/min.
2.3 Ni release
Two samples of each type were immersed in 25 mL simulated blood fluid(SBF) in a polypropylene bottle. The bottles were closed tightly and incubated in a thermostatic chamber at (37±0.1)℃ for 2 weeks and 5 weeks, respectively. At each time point for each group, the SBF was taken out and analyzed by inductively- coupled plasma mass spectrometry(ICPMS) to determine the average amount of Ni leached from the 4 specimens in two bottles.
2.4 Hemolysis
8 mL of fresh blood was collected from a rabbit and then diluted with 10 mL 0.9% saline. Each sample was put into a test tube with 10 mL saline and incubated at 37℃ for 30 min. Afterwards, 0.2 mL of the diluted blood was added to each test tube and incubation continued for another 60 min. After incubation, the suspension was centrifuged at 2 500 r/min for 5 min. The absorbance of the supernatant fluid was measured by a spectrophotometer (UV240, China). The positive control was a mixture of blood and deionized water, and the negative control was a mixture of blood and saline. The hemolysis results were averages of three measurements.
2.5 Blood platelet adhesion
The samples were put into a 24-well tissue culture plate. A 3.8% (mass fraction) citrate acid solution was added to the blood with a blood to citrate acid volume ratio of 9:1. The solution was centrifuged to form a platelet-rich plasma(PRP) and erythrocyte. 0.1 mL of the PRP was added to each well. After incubation at 37 ℃ for 3 h, the PRP was taken out from the wells. A phosphate buffer solution(PBS) was added to the wells and gently rinsed 2-3 times to get rid of platelets adsorbed loosely on the sample surface. The samples were then soaked in 2.5% glutaraldehyde at room temperature for 12 h to fix the adhered platelets. The samples were subsequently dehydrated in 50%, 75%, 90%, and 100% ethanol for 10 min sequentially. After dehydration, the residual alcohol on the samples was cleaned off in 50%, 75%, 90% and 100% isoamyl acetate water solutions for 10 min. After critical point drying, the samples were coated with thin gold films. And the distribution and morphology of the platelets were observed by SEM.
3 Results
SEM photographs (Fig.1(a)) show that the surface of the CP NiTi SMA is relatively smooth and a white phase that appears in the parent phase is confirmed to be Ti2Ni according to energy-dispersive X-ray analysis (EDS) performed in conjunction with SEM. In contrast, a dense oxide film is formed on the modified AOP sample, as indicated in Fig.1(b). The typical XPS survey spectra in Fig.2 show that the dominant elements on the CP surface are Ni, Ti, O and C whereas Ti, O and C on the modified AOP one. The Ni concentration on the CP sample surface can reach as high as 11.4% (molar fraction) but there is no detectable Ni on the surface of the modified AOP sample. The results suggest that the latter is covered by a dense titanium oxide film that has no detectable Ni near its top surface. The presence of C results from physical adsorption of carbon-containing molecules onto the surface.

Fig.1 Surface morphologies of NiTi SMAs: (a) CP sample; (b) Modified AOP sample

Fig.2 XPS survey spectra of surface of NiTi SMAs: (a) CP sample; (b) Modified AOP sample
High resolution XPS spectra were taken to investigate the Ti and Ni binding energies on the surface of the modified AOP sample. The Ti 2p XPS spectrum exhibits two dominant peaks that can be identified to be Ti4+ (TiO2) 2p3/2 at 458.8 eV and Ti4+ (TiO2) 2p1/2 at 464.6 eV, as displayed in Fig.3. In contrast, no Ni in any chemical state can be seen from the Ni 2p XPS spectrum (not shown here), further confirming the absence of Ni in the outermost surface of the modified AOP sample. The high resolution O 1s XPS spectrum acquired from the surface of the modified AOP one is shown in Fig.4 together with the fitted curves. The dominant peak is also at 530.5 eV that can be assigned to oxygen in metal oxides. Other oxygen states such as adsorbed water and Ti—OH can also be detected.
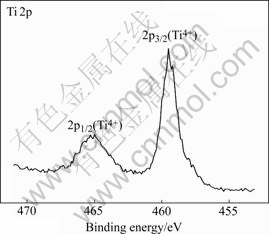
Fig.3 Ti 2p high resolution XPS spectrum of surface on modified AOP sample

Fig.4 O 1s high resolution XPS spectrum of surface on modified AOP sample
The XPS depth profiles of Ni, Ti and O acquired from the surface of the modified AOP sample are shown in Fig.5. The oxygen profile shows a peak close to the top surface whereas the Ti profile shows a minimum concentration. The Ni concentration on the outermost surface is below the XPS detection limit. Afterwards, the O concentration decreases to zero and the Ni concentration increases to a steady-state value of about 50% after 14 min sputtering. If the oxide thickness is estimated by taking the depth where the O signal drops to 50% of the maximum value, the thickness of the titania film on the modified AOP NiTi SMA is about 200 nm.
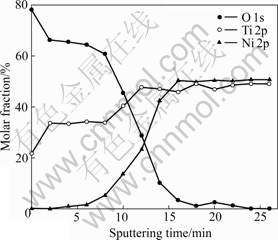
Fig.5 XPS depth profiles of composition changes of surface on modified AOP sample
Table 1 summarizes the ICPMS results of the Ni ion concentrations in SBF for different immersion time and hemolysis ratios of NiTi SMAs. The surface titania film on the modified AOP sample can significantly reduce Ni release from the NiTi substrate. For the two immersion times (2 weeks and 5 weeks), the amounts of Ni leached from the modified AOP NiTi are only about 2% of those leached from the CP NiTi. Obviously, the titania film formed by modified AOP can mitigate out-diffusion of Ni from the substrate.
Table 1 Concentrations of release Ni ions in SBF for different immersion times and hemolysis ratios of NiTi SMAs
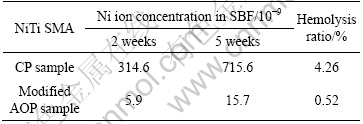
The hemolytic activity is assessed by determining hemoglobin release under static conditions. The hemolysis ratios determined from the CP and modified AOP sample are 4.26% and 0.52%, respectively. A lower hemolysis ratio means that less hemolysis occurs on the surface of the modified AOP sample. The results show that the hemolysis resistance of NiTi can be improved by the formation of a titania film using the modified AOP.
The morphologies of the adhered blood platelets on the NiTi SMAs after 3 h of incubation are shown in Fig.6. The number of adherent platelets on the modified AOP NiTi is much less than that on the CP one. Accumulation and pseudopodium of platelets are also serious on the CP sample as indicated by the three-dimensional structures connected by pseudopodium in Fig.6(a). In contrast, the platelets on the modified AOP sample are isolated (Fig.6(b)). There is no sign of accumulation and only slight pseudopodium can be observed. Hence, platelet adhesion is reduced remarkably on the NiTi SMA after the modified AOP and the thromboresistance of NiTi SMA can also be improved by fabrication of a titania film.

Fig.6 SEM morphologies of adherent platelets on surface of NiTi SMAs after 180 min incubation in PRP: (a) CP sample; (b) Modified AOP sample
4 Discussion
The conventional AOP using a UV/H2O2 system is unstable because the quantity of produced ·OH diminishes continuously on account of continuous consumption of H2O2. During the modified AOP, H2O2 can be produced continuously at the cathode under the acidic conditions by the reduction of oxygen from air blown into the electrolyte[13]:
O2+2H2O+2e→H2O2+2OH- (1)
Under UV irradiation, the electrochemically- generated H2O2 reacts to continuously replenish ·OH according to the following reactions[13], which can provide a stable ·OH source for the formation of the titania film on NiTi substrate by oxidation:
![]() (2)
(2)
As discussed in Ref.[11], Ti has a stronger affinity to O chemisorptions than Ni because the formation enthalpy of TiO2 (-956 kJ/mol) is four times that of NiO (-241 kJ/mol)[15]. Therefore, Ti on the NiTi surface can be oxidized by ·OH to form TiO2, whereas Ni may remain unchanged and can be removed from the Ni-Ti matrix due to acidic etching in the aqueous solution. Moreover, the formation of a titania film can be further accelerated by anodic oxidation of the NiTi substrate serving as the anode. The exact in-situ formation mechanism of the titania film on NiTi SMA during the modified AOP is studied in more details and new findings will be reported in due course.
As indicated by these results, the modified AOP can result in the formation of a dense titania film with a Ni-free zone near its top surface, whereas a trace of Ni can still be detected on the upper NiTi surface after the conventional AOP in a UV/H2O2 system, as reported previously[12]. The titanium oxide platforms of both O and Ni last for the same sputtering time of about 8 min, during which the molar ratio of O to Ti is stable near 2:1. This suggests that the titanium oxide film is mainly composed of TiO2. The XPS depth profiles also show that the titania film has a graded structure at the interface with the NiTi substrate. It is likely that this graded interface structure improves the bonding strength of the titania film. SEM and XPS results obviously reveal the formation of a dense titania film of about 200 nm in thickness after the modified AOP for 1 h, which indicates that the modified AOP has a higher processing effectiveness than the conventional AOP in a given UV/H2O2 system[12].
Our results indicate the titania film produced by the modified AOP method is more effective in impeding the out-diffusion of Ni from NiTi SMA during the entire five-week immersion period. Platelet adhesion is also reduced remarkably on the NiTi SMA after the modified AOP method and the thromboresistance of NiTi SMA can also be improved by fabrication of titania film. The improvement may be related to the intrinsic electrical characteristics of the titania film[7].
5 Conclusions
1) A modified AOP conducted in a UV/ electrochemically generated peroxide system has a higher processing effectiveness and can result in the formation of a dense titania film with a Ni-free zone near its top surface.
2) A trace amount of Ni can still be detected from the NiTi surface after undergoing the conventional AOP in a given UV/H2O2 system.
3) The titania film can dramatically reduce Ni release and also improve the hemolysis resistance and thromboresistance of biomedical NiTi SMA.
4) There is a smooth graded interface structure enhancing the mechanical stability of the titania film.
References
[1] Cheng Y, Cai W, Li H T, Zheng Y F, Zhao L C. Surface characteristics and corrosion resistance properties of TiNi shape memory alloy coated with Ta [J]. Surface and Coatings Technology, 2004, 186(3): 346-352.
[2] Otsuka K, Wayman C M. Shape memory materials [M]. Cambridge: Cambridge University Press, 1998.
[3] Duerig T, Pelton A, Stockel D. An overview of nitinol medical applications [J]. Mater Sci Eng A, 1999, A273/275: 149- 160.
[4] Chen M F, Yang X J, Hu R X, Cui Z D, Man H C. Bioactive NiTi shape memory alloy used as bone bonding implants [J]. Mater Sci Eng C, 2004, 24: 497-502.
[5] Rondelli G, Vicentini B. Localized corrosion behaviour in simulated human body fluids of commercial Ni-Ti orthodontic wires [J]. Biomaterials, 1999, 20: 785-792.
[6] Baier RE, Dutton RC. Initial events in interaction of blood with foreign surfaces [J]. J Biomed Mater Res, 1969, 3: 191-206.
[7] Liu J X, Yang D Z, Shi F, Cai Y J. Sol-gel deposited TiO2 film on NiTi surgical alloy for biocompatibility improvement [J]. Thin Solid Films, 2003, 429: 225-230.
[8] FIRSTOV G S, VITCHEV R G, KUMAR H, BLANPAIN B, HUMBEECK J V. Surface oxidation of NiTi shape memory alloy [J]. Biomaterials, 2002, 23: 4863-4871.
[9] Cheng F T, Shi P, Pang G K H, Wong M H, Man H C. Microstructural characterization of oxide film formed on NiTi by anodization in acetic acid [J]. Journal of Alloys and Compounds, 2007, 438(1/2): 238-242.
[10] Hu T, Chu C L, Yin L H, Pu Y P, Dong Y S, Guo C, Sheng X B, Chung C Y, Chu P K. In vitro biocompatibility of titanium-nickel alloy with titanium oxide film by H2O2 oxidation [J]. Trans Nonferrous Met Soc China, 2007, 17(3): 553-557.
[11] Chu C L, Hu T, Wu S L, Wang R M, Dong Y S, Lin P H, Chung C Y, Chu P K. In situ synthesis of nanostructured titania film on NiTi shape memory alloy by Fenton’s oxidation method [J]. Trans Nonferrous Met Soc China, 2007, 17(5): 902-906.
[12] Wang R M, Chu C L, Hu T, Dong Y S, Guo C, Sheng X B, Lin P H, Chung C Y, Chu P K. Surface XPS characterization of NiTi shape memory alloy after advanced oxidation processes in UV/H2O2 photocatalytic system [J]. Applied Surface Science, 2007, 253: 8507-8512.
[13] Neyens E, Baeyens J. A review of classic Fenton’s peroxidation as an advanced oxidation technique [J]. Journal of Hazardous Materials, 2003, B98: 33-50.
[14] Bergendahl J A, Thies T P. Fenton’s oxidation of MTBE with zero-valent iron [J]. Water Research, 2004, 38: 327-334.
[15] Weast R C, Astle M J. CRC Handbook of chemistry and physics [M]. Boca Raton, Florida: CRC Press, 1982: 36.
Foundation item: Project(NCET-06-0464) supported by the Program for New Century Excellent Talents in University of Ministry of Education of China; Project(BK2007515) supported by the Natural Science Foundation of Jiangsu Province, China; Project(2006AA03Z445) supported by the National High-tech Research and Development Program of China; Project supported by Nippon Sheet Glass Foundation for Materials Science and Engineering (NSG Foundation); Project(7001999) supported by SRG Grant from the Research Committee of the CityU of HK
Corresponding author: CHU Cheng-lin; Tel: +86-25-52090683; E-mail: clchu@seu.edu.cn
DOI: 10.1016/S1003-6326(08)60315-5
(Edited by YANG Bing)
Abstract: A modified advanced oxidation process(AOP) utilizing a UV/electrochemically-generated peroxide system was used to fabricate titania films on chemically polished NiTi shape memory alloy(SMA). The microstructure and biomedical properties of the film were characterized by scanning electron microscopy(SEM), X-ray photoelectron spectroscopy(XPS), inductively-coupled plasma mass spectrometry(ICPMS), hemolysis analysis, and blood platelet adhesion test. It is found that the modified AOP has a high processing effectiveness and can result in the formation of a dense titania film with a Ni-free zone near its top surface. In comparison, Ni can still be detected on the outer NiTi surface by the conventional AOP using the UV/H2O2 system. The depth profiles of O, Ni, Ti show that the film possesses a smooth graded interface structure next to the NiTi substrate and this structure enhances the mechanical stability of titania film. The titania film can dramatically reduce toxic Ni ion release and also improve the hemolysis resistance and thromboresistance of biomedical NiTi SMA.



