Trans. Nonferrous Met. Soc. China 24(2014) 2660-2664
Mass transfer process in replacement-column purification device in zinc hydrometallurgy
Ping ZHOU, Dong-mei LI, Zhuo CHEN
School of Energy Science and Engineering, Central South University, Changsha 410083, China
Received 8 July 2013; accepted 4 November 2013
Abstract:
It is important to remove the impurities, such as copper and cadmium, from leaching solution in zinc hydrometallurgy. To improve purification efficiency, a replacement-column purification device was proposed and its mass transfer characteristics and purification efficiency were experimentally studied. The results show that purification efficiency increases with the decrease of the zinc powder diameter and decreases with the increase of solution velocity. If appropriate structure and operation parameters are used, it is possible to make purification efficiency more than 99%, but the diameter of zinc powder should be larger than 0.45 mm. For the velocity of 0.05-0.7 cm/s, mass transfer coefficient kc is in the range of 3.94×10-7-2.76×10-6 m/s, and increases with the decrease of zinc powder diameter and the increase of solution velocity. Moreover, it can be derived by mass transfer correlations of Sherwood number:Sh=0.1069Re0.5Sc0.33, for 0.3< Re<6.
Key words:
zinc hydrometallurgy; purification of copper and cadmium; replacement column; mass transfer behavior;
1 Introduction
In zinc hydrometallurgy, it is important to remove the impurities from leaching solution in which sulfuric zinc is the major content. Among the impurities, copper and cadmium are generally removed by replacement reaction. Currently, the removal process is usually completed by two kinds of equipment as suspended tank and mechanical stirred tank [1], and some problems usually exist during the purification process, such as dead zone, unevenness of flowing. Therefore, low purification efficiency, high equipment failure rate, and large consumption amount of zinc powder [2,3] are needed to be improved.
An alternative type of reactor that is replacement- column device was designed. It is filled with solid particles and of the characteristics of ion exchange column. Ion exchange column is a kind of pressure vessel column for ion exchange reaction, and is widely used in treating of industrial aqueous effluents, softening of water [4-6], decolorization and purification of food and drug [7]. It presents the same behavior as micro-reactor, such as simple structure, uniform reaction, low loss of solid powder and flexible control [8,9]. The investigation for the ion exchange column focuses on the mass transfer process whose influence factors involve packing size, gas and liquid superficial velocities and physical properties of the solution [10]. To obtain the mass transfer coefficient, some dimensionless number equations for Sherwood number and JD factor were established from a lot of experiments [6,11]. But the research on mass transfer behavior between metal ion and zinc powder in replacement column has not been seen in literatures.
In this work, an experimental device involving one replacement column, which is the basic unit of purification equipment, was built. The effect of parameters, involving the flow velocity of the leaching solution, particle diameter of replacement zinc powder and the length of the replacement column, on the mass transfer behavior of the device is experimentally studied. The experimental results will provide basis for the optimization of the device structure and operating conditions.
2 Experimental
2.1 Experiment device
A single replacement column was taken as the experimental device whose structure is shown in Fig. 1. Replacement column with inner diameter of 0.8 cm was made of plexiglass, and filled with zinc powder. Its lengths were respectively taken as 3.5, 4.4 and 6.0 cm, for diameters of zinc powders of 0.28-0.45, 0.45-0.71 and 0.71-1.00 mm. The physical characteristics of zinc powder were measured and are listed in Table 1. In order to improve measurement accuracy in experiment, CuSO4 solution with 10.24 g/L Cu2+ was approximately treated as the leaching solution and was injected from the entrance. When the solution flowed through the replacement column at ambient temperature, Cu2+ was replaced with the zinc powder. The solution was collected at the exit, and the concentration of Cu2+ was measured by inductively coupled plasma-atomic emission spectrometer.
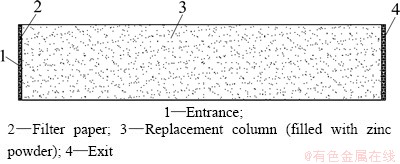
Fig. 1 Structure of a single replacement column
Table 1 Physical characteristics of zinc powder in experiment

2.2 Results
2.2.1 Purification efficiency
Purification efficiency, h, is an important index to evaluate the performance of purification device and is given by the following formula:
 (1)
(1)
where C0, C1 are the concentrations of Cu2+ at the entrance and exit of purification device, respectively.
2.2.2 Effects of diameter of zinc powder and solution velocity on purification efficiency
For the column with a constant length of 3.5 cm, the purification efficiencies in different diameter of zinc powder and solution velocity are calculated by formula (1), and shown in Fig. 2. The results show that the diameter of zinc powder and the solution velocity have a significant influence on purification efficiency which is in the range of 70%-99%. In general, purification efficiency increases with the decrease of the diameter of zinc powder, and decreases with the increase of solution velocity.
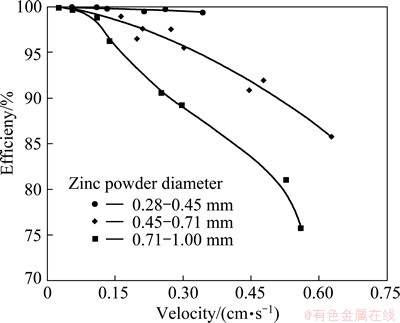
Fig. 2 Effects of zinc powder diameter and solution velocity on purification efficiency
When the solution velocity is very small (less than 0.1 cm/s), the purification efficiency is very high and up to more than 99% whether the diameter of zinc powder is large or small. Moreover, as to the zinc powder with smaller diameter, since purification efficiency is very high in the range of operating solution velocity, the influence of solution velocity on purification efficiency becomes relatively weak. Taking zinc powders with diameter of 0.28-0.45 mm as an example, purification efficiency can be sustained over 99% when the solution velocity is less than 0.4 cm/s. However, the purification efficiency sharply decreases when the solution velocity is over 0.4 cm/s, which can be seen in Fig. 3. This is because a larger solution velocity will lead to higher flow resistance inside replacement column [12]. And too high flow resistance may result in some problems such as decrease of porosity, unevenness of flow and even dead bed [13], thereby the abnormal operation of device takes place and the purification efficiency decreases. Therefore, to ensure the smooth operation, it is proposed that the zinc powder with the diameter less than 0.45 mm is not adopted in practice. If appropriate structure and operation parameters are used, it is possible to make purification efficiency more than 99%.
2.2.3 Effect of length of replacement column on purification efficiency
Purification efficiency in replacement column with different lengths (filled with zinc powders with diameter of 0.45-0.71 mm) is shown in Fig. 4. It is clear that purification efficiency is improved with the increase of the length of the replacement column because the longer replacement column provides longer reaction time. However, when the solution velocity is less than 0.1 cm/s, the length of replacement column has little influence on the purification efficiency, which means that the length of 3.5 cm offers enough time to finish the replacing reaction in this case. Therefore, it can be inferred that when solution velocity is larger than 0.1 m/s, high purification efficiency can be obtained by increasing the length of replacement column.
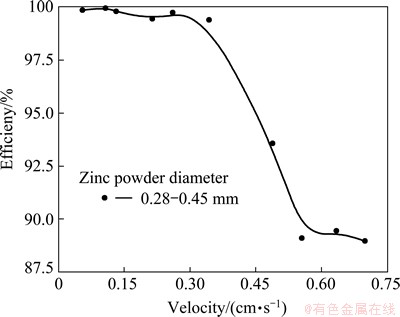
Fig. 3 Purification efficiency vs velocity with zinc powders with diameter 0.28-0.45 mm

Fig. 4 Effects of length of replacement column on purification efficiency
3 Mass transfer coefficient
3.1 Mass transfer coefficient in replacement column
The replacing reaction between zinc powder and solution involves two steps. One is the diffusion of Cu2+ toward the surface of zinc powder and ZnSO4 toward the leaching solution. The other is the chemical reaction substituting for Cu2+ with zinc. Based on the relative importance of diffusion resistance and chemical reaction resistance, the replacing reaction process is divided into three kinds, diffusion control, chemical reaction control, and mixed chemical-diffusion control [14]. A large number of literatures demonstrate that the reaction between zinc powders and metal ions such as copper and cadmium belongs to diffusion control [15]. So, the purification rate of leaching solution is determined by mass transfer coefficient kc in diffusion process.
Considering the small diameter of the replacement column and then ignoring radial diffusion, the mass transfer in replacement column is approximated as one-dimensional model, and its control equation is given by [16]
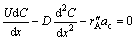 (2)
(2)
where U is the solution velocity of the axial direction; C is the molar concentration of Cu2+; 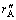 is the reaction rate based on the surface area of the zinc powder, in the experiment ,
is the reaction rate based on the surface area of the zinc powder, in the experiment , 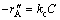 [14]; ac is the surface area per unit volume of the zinc powder (referring to Table 1); x is a coordinate along the axial direction.
[14]; ac is the surface area per unit volume of the zinc powder (referring to Table 1); x is a coordinate along the axial direction.
The molecular diffusion term, i.e. the second term in left hand in Eq. (2) is in the order of 10-5, and is greatly less than the convective term with the order of 100 [17]. Ignoring the molecular diffusion term, control equation can be approximated as
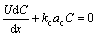 (3)
(3)
The concentration distribution is solved from Eq. (3) and written below [14]
 (4)
(4)
For the replacement of column with the length L, the Cu2+ concentrations at entrance (x=0) and exit (x=L) are C0 and C1, respectively. Then, mass transfer coefficient kc is derived from Eq. (4) as follows:
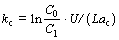 (5)
(5)
C0, C1 and U were measured during the experiment. According to Eq. (5), kc in different solution velocities and zinc powder diameters are calculated and shown in Fig. 5.
Figure 5 shows that kc is in the range of 3.94×10-7-2.76×10-6 m/s, and increases with the decrease of zinc powder diameter and the increase of solution velocity. Therefore, higher solution velocity and smaller zinc powder diameter are advantageous to improve purification rate. But as mentioned in the former section, the solution velocity and zinc powder diameter are limited by the purification device in practice.
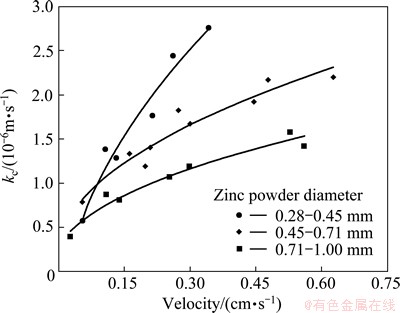
Fig. 5 kc vs solution velocity in different zinc powder diameter
3.2 Dimensionless number equation for mass transfer
The Sherwood number Sh is a dimensionless number used in mass-transfer operation, represents the ratio of convective to diffusive mass transport and is defined as [16]
Sh=f(Re, Sc) (6)
where Sherwood number Sh=kcd/D, Reynolds number Re=Ud/v, Schmidt number Sc=v/D; d is characteristic length that is the zinc powder diameter; D is the molecular diffusion coefficient; v is the kinematic viscosity.
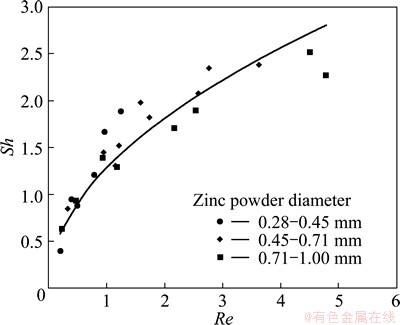
Fig. 6 Sh vs Re with different zinc powder diameters
Analog to heat transfer correlations of the Nusselt number in terms of the Reynolds number and Prandtl number, for a given geometry, a mass transfer correlation of Eq. (5) can be expressed as [16]
Sh=kRe0.5Sc0.33 (7)
Sh is obtained according to the mass transfer coefficient kc in Fig. 5, Re is calculated in terms of the experimental conditions, and Sc is given by a constant of 1968.50 for the experimental condition [17]. k in Eq. (7) is fitted based on the experimental data, and then mass transfer correlation of Sherwood number in terms of the Reynolds number and Schmidt number under the experimental conditions is obtained as follows:
Sh=0.1069Re0.5Sc0.33(0.3<RE<6) (8)
4 Conclusions
1) During the experiment, purification efficiency is in the range of 70%-99%. In general, it increases with the decrease of the zinc powder diameter and the lengthening of the replacement column, but decreases with the increase of solution velocity. The diameter of zinc powder is proposed to be larger than 0.45 mm to ensure the smooth operation.
2) For the velocity of 0.05-0.7 cm/s,, kc is in the range of 3.94×10-7-2.76×10-6 m/s, and increases with the decrease of zinc powder diameter and the increase of solution velocity.
3) As to the replacement column filled with zinc powder, the mass transfer correlation of Sherwood number in terms of the Reynolds number and Schmidt number is obtained as Sh=0.1069Re0.5Sc0.33, for 0.3 References [1] GUO Tian-li, GAO Liang-bin. Status and prospect of the purification technology of zinc sulphate solution in China [J]. Non-ferrous Mining and Metallurgy, 2006, 22(1): 26-28. (in Chinese) [2] YANG Ru-zhong, WU Jiang, GUO Tian-li, GAO Liang-bin. Application of large ratio between height and diamter mechanical agitation trough in leaching process of zinc hydrometallurgy [J]. Energy Saving of Non-ferrous Metallurgy, 2005, 10(5): 27-42. (in Chinese) [3] HE Chun-hai. The reform design of agitation system in zinc leach tanks [J]. Hunan Nonferrous Metals, 2003, 19(3): 48-51. (in Chinese) [4] VERMA R, KUMAR R, PANDEY D M, VERMA N. Hydrodynamic study on radially cross-flow fluidized bed multi-staged ion-exchange column [J]. Chemical Engineering and Processing: Process Intensification, 2010, 49(11): 1199-1204. [5] BORBA C E, SILVA E A, SPOHR S, SANTOS G H F, GUIRARDELLO R. Application of the mass action law to describe ion exchange equilibrium in a fixed-bed column [J]. Chemical Engineering Journal, 2011, 172(1): 312-320. [6] VERMA R, SRIVASTAVA G, VERMA N. Novel multi-staged radially cross-flow fluidized bed ion-exchange column [J]. Chemical Engineering and Processing: Process Intensification, 2009, 48(1): 396-407. [7] JUDITH K, REINHOLD C, DIETMAR R K. Adsorption and ion exchange: Basic principles and their application in food processing [J]. J Agric Food Chem, 2011, 51(1): 22-42. [8] GAVRILIDIS A, ANGELI P, CAO E, YEONG K K, WAN Y S S. Technology and applications of microengineered reactors [J]. Chemical Engineering Research and Design, 2002, 80(1): 3-29. [9] STONE H A, KIM S. Microfluidics: Basic issues, applications, and challenges [J]. AIChE Journal, 2001, 47(6): 1250-1254. [10] SEDAHMED G H, EL-TAWEEL Y A, KONSOWA A H, ABDEL-AZIZ M H. Effect of packing geometry on the rate of mass and heat transfer at a vertical tube imbedded in fixed bed under single and two phase flow [J]. International Communications in Heat and Mass Transfer, 2013, 48(11): 149-154. [11] SOLTAN E A, NOSIER S A, SALEM A Y, MANSOUR I A S, SEDAHMED G H. Mass transfer behaviour of a flow-by fixed bed electrochemical reactor under different hydrodynamic conditions [J]. Chemical Engineering Journal, 2003, 91(1): 33-44. [12] MELDA O, CARPINLIOGLU, EMRAH O. A simplified correlation for fixed bed pressure drop [J]. Powder Technology, 2008, 187(1): 94-101. [13] PAN Yang. Optimization for catalyst loading for fixed bed reactors [J]. Sino-global Energy, 2007, 12(6): 76-79. (in Chinese) [14] FOGLER H S. Elements of chemical reaction engineering [M]. New Jersey: Prentice Hall Professional Technical Reference, 1998: 500-650. [15] PENG Rong-qiu. Metallurgy of lead and zinc [M]. Beijing: Science Press, 2003: 381-382. (in Chinese) [16] ZHOU Ping, ZHOU Nai-jun, JIANG Ai-hua, CHEN Hong-rong. Principle and numerical simulation of transport processes [M]. Changsha: Central South University Press, 2006: 262-271. (in Chinese) [17] LI Jian, ZHU Hong-bo, ZHU Zhu-ze. One of the physicochemical property of copper electrolyte: The diffusion coefficient of Cu2+ in electrolyte [J]. Non-ferrous Metallurgy, 2004, 21(1): 26-31. (in Chinese). 周 萍,李冬梅,陈 卓 中南大学 能源科学与工程学院,长沙 410083 摘 要:浸出液中铜、镉的净化是湿法炼锌中的重要步骤。提出一种置换柱式净化装置,并对其净化规律及传质系数进行实验研究。结果表明,净化效率随着锌粉粒径的减小及浸出液流速的减小而增大,若结构参数和操作参数使用恰当,净化率可达99%,但所使用的锌粉粒径必须大于0.45 mm。当流速范围为0.05~0.7 cm/s时,置换柱内传质系数kc为3.94×10-7~2.76×10-6 m/s,且kc随着锌粉粒径的减小及浸出液流速的增大而增大,满足传质准数方程:Sh=0.1069Re0.5Sc0.33 (0.3< Re<6)。 关键词:湿法炼锌;铜镉净化;置换柱;传质规律 (Edited by Hua YANG) Foundation item: Project (Y2010-1-005) supported by the Collaborative Fund of Hunan Nonferrous Metals Holding Group-Central South University, China Corresponding author: Zhuo CHEN; Tel: +86-13974891750; E-mail: chenzhuo@csu.edu.cn DOI: 10.1016/S1003-6326(14)63396-3湿法炼锌置换柱式净化装置传质过程
Abstract: It is important to remove the impurities, such as copper and cadmium, from leaching solution in zinc hydrometallurgy. To improve purification efficiency, a replacement-column purification device was proposed and its mass transfer characteristics and purification efficiency were experimentally studied. The results show that purification efficiency increases with the decrease of the zinc powder diameter and decreases with the increase of solution velocity. If appropriate structure and operation parameters are used, it is possible to make purification efficiency more than 99%, but the diameter of zinc powder should be larger than 0.45 mm. For the velocity of 0.05-0.7 cm/s, mass transfer coefficient kc is in the range of 3.94×10-7-2.76×10-6 m/s, and increases with the decrease of zinc powder diameter and the increase of solution velocity. Moreover, it can be derived by mass transfer correlations of Sherwood number:Sh=0.1069Re0.5Sc0.33, for 0.3< Re<6.


