J. Cent. South Univ. (2016) 23: 3051-3059
DOI: 10.1007/s11771-016-3368-6
Effects of minor Zn content on microstructure and corrosion properties of Al-Mg alloy
ZHAO Jing-wei(赵经纬)1, LUO Bing-hui(罗兵辉)1, HE Ke-jian(何克坚)2, BAI Zhen-hai(柏振海)1,
LI Bin(李彬)1, CHEN Wei(陈维)1
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. Advanced Research Centre, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
The effects of different Zn contents in Al-Mg alloy on the microstructure characterizations were observed by advanced electron microscopy and the corrosion properties were investigated by the inter-granular corrosion tests, the exfoliation corrosion tests, and the Potentiodynamic polarizaion tests. The τ phase (Mg32 (Al, Zn)49 ) forms on the pre-existing Mn-rich particles and at the grain boundaries. According to the theory of binding energy, the formation of τ phase is much easier than that of β phase (Al3Mg2), somehow replacing β phase and reducing the possibility of β phase precipitation. This change dramatically decreases the susceptibility of corrosion. The Zn addition increases the corrosion resistance of Al-Mg alloy with an optimal value of 0.31%. When the Zn addition is increased to 0.78%, however, the corrosion resistance of alloy decreases once again but it is still better than that of the alloy without Zn addition.
Key words:
Al-Mg alloy; Zn addition; τ phase (Mg32 (Al, Zn)49 ); corrosion;
1 Introduction
Al-Mg alloys are widely used in many applications like transport and vehicle industries thanks to their reasonable corrosion resistance, good weld-ability, formability and high specific strength [1-3]. The mechanical properties of Al-Mg alloys are mainly based on magnesium solid solution strengthening and strain hardening [4]. However, the Al-Mg alloys with Mg over 3.5% tend to be susceptible to the inter-granular corrosion (IGC) and stress corrosion cracking (SCC) [5]. The Al3Mg2 precipitate, also called β phase, with a corrosion potential of about -1.29 V (SCE) [6], is more active than the standard 5083 matrix (-0.73 V), and continuously or near continuously precipitates preferentially at grain boundaries during sensitization treatment [7]. This anodic phase is preferentially dissolved when exposed to a conductive medium due to a galvanic coupling between the matrix and the β phase. Besides that, β phase also leads to a higher susceptibility of pitting corrosion. The β phase forms both on the pre-existing Mn-rich particles in matrix and at grain boundaries [8]. The pits occur mainly in the alloy matrix surrounding Al 6(Mn, Fe) [9], where the cathodic reactions occur since the Al 6(Mn, Fe) phase has a higher value of corrosion potential compared with aluminum [10].
Regulating the annealing process can improve the corrosion resistance because it discontinues the distribution of β phase at grain boundaries [11]. OGUOCHA et al [12] demonstrated that annealing a AA5083-H116 alloy at 80°C for 672 h led to a better resistance towards IGC. LUO et al [13] demonstrated that annealing a 5083 alloy at 200°C and 250°C for 40 min led to a uniform distribution of spherical β phase throughout the structure. BENSAADA et al [14] chose three different temperatures of aging process (160°C, 220°C, and 270°C), and displayed that aging at 220°C discontinued the precipitation developed at grain boundaries.
Another effective method to increase the corrosion resistance of Al-Mg alloys is adding some suitable alloying elements to alloy. The addition of Er into Al-Mg alloys results in the precipitation of Al3Er particles which can retain the deformed microstructure and restrain recrystallization process, exhibiting a good corrosion resistance [15]. The addition of Zr and Sc to Al-Mg alloys can also increase the SCC resistance for it hinders the recrystallization of the alloy [16]. ALIL et al [17] showed that the Cu content affected the migration of Mg solutes toward grain boundaries and thus prevented the formation of β phase. Adding Zn (0.68%-0.7%) to 5083 alloys could improve the corrosion resistance of Al-Mg alloys due to a new stable ternary τ phase (Mg32 (Al, Zn)49 ) that forms at grain boundaries and precludes the formation of β phase [18-19]. BERGMAN et al [20] uncovered much of the characteristic structure of the τ phase (Mg32 (Al, Zn) 49) which has a BCC structure (space group Im ) with 162 atoms per unit cell. The lattice parameter amounts to a=14.16 .
.
Previous studies demonstrated that the formation of τ phase with a Zn addition can improve the corrosion resistance of Al-Mg alloys. For the τ phase, however, the details of its formation mechanism and its effects on corrosion resistance are still indistinct. In this work, the effects of Zn element, especially the Zn content, on the microstructure and corrosion properties have been investigated. In order to have a better understanding of the characteristics of morphology and distribution of τ phase precipitated at grain boundaries and in matrix, advanced electron microscopic studies have been carried out, including transmission electron microscopy (TEM), scanning transmission electron microscopy (STEM), and energy dispersive X-ray (EDX) elemental mapping. The formation mechanism of τ phase and its effects on enhancing corrosion resistance are discussed in detail.
2 Experimental procedure
2.1 Materials
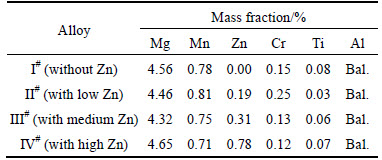
Four Al-Mg alloys used in this work were cast in our laboratory and their chemical compositions are listed in Table 1. The ingots were homogenized at 480°C for 24 h to modify the as-cast structure. After that, the alloys were hot rolled at 470°C with a reduction of 60% and then cold rolled with a reduction of 20%. Finally, stabilizing treatment was carried out at 200°C. In order to accelerate the precipitation of secondary particles, all the specimens were sensitized for 7 d.
Table 1 Analyzed chemical composition of investigated alloys
2.2 Microstructure characterization
The microstructures were carried out by a SIRION 200 SEM. Advanced TEM characterizations were conducted using a Titan G2 60-300 TEM with an image spherical aberration corrector and a Super-X detector, operating at 300 kV. The microstructural characteristics of the secondary phase precipitates including compositional information were studied by TEM bright field (BF) and dark field (DF) imaging, selected area electron diffraction, Z-contrast imaging, also termed as high-angle annular dark-field (HAADF) imaging and EDX elemental mapping. In HAADF imaging mode, the brighter regions correspond to heavier atoms, as the scattering cross-section is approximately proportional to Z2 [21]. The TEM samples were prepared by mechanically grinding, polishing, dimpling and finally ion-milling using a Gatan PIPS 691.
2.3 Inter-granular corrosion tests
The IGC tests were performed according to the Standard GB/T 7998-2005 [22] on the Al-Mg alloys with different Zn contents that had been immersed in an acidified salt solution (0.5 mol/L NaCl, 0.1 mol/L HCl) for 24 h at (35±2)°C. The corrosion susceptibility, including the maximum corrosion depth and average corrosion depth, was evaluated on 3 cm long metallographic cross sections normal to the rolling direction by using a Polyvar-MET optical microscope. The average depth of each alloy has been estimated from 15 deepest depths in 3 parallel samples.
2.4 Exfoliation corrosion (EXCO) tests
According to the standard of the GB/T 22639-2008 [23] specification, the Al-Mg alloys with different Zn contents were immersed in EXCO solution (1.53 g/mL NH4Cl, 1.73 g/mL NH4NO3, 1.60 g/mL (NH4)2C4H4O6, 1.11 g/mL H2O2) for 24 h at (65±1)°C. After immersion, the corrosion morphologies of alloys were examined by a digital camera and the surface microstructure was revealed by scanning electron microscope (SEM) fitted with energy dispersive spectroscopy (EDS).
2.5 Electrochemical tests
Electrochemical tests were conducted in a 3.5% NaCl electrolyte via IM6E potentiostat with a conventional three-electrode system. The experimental set-up was comprised of a saturated calomel reference electrode, 1 cm2 of the alloy as working electrode, and a platinum counter electrode. Potentiodynamic polarization tests were carried out to explore the corrosion resistance of samples by stepping the potential at open-circuit with a scanning rate of 2 mV/s from -1.2 V (SCE) to+0.2 V (SCE).
3 Results
3.1 Microstructure characteristics
Figure 1 shows the HAADF images of III# alloy within the grain and at the grain boundary. The morphology of τ phase formed surrounding the Mn-rich particle is found to be equiaxed, whereas that formed at grain boundaries is found to be needle-liked and rod-liked. And the size of τ phase is ranged from around 50 to 500 nm. The corresponding EDS elemental mapping images indicate that the τ phase contains a relatively large amount of Zn element. The τ phase nucleates and grows on pre-existing Mn-rich particles and at grain boundaries so it can be inferred that Zn has a tendency to form τ phase even if the quantity of Zn addition is quite small. Figure 2 shows the presence of τ phase at grain boundary in Al-Mg alloy.
Figure 3 shows the distribution of precipitates at grain boundary by TEM observation. For I# alloy, the secondary particles precipitated at grain boundary were proved to be β phase by many authors [24-25]. In the Al-Mg alloy without Zn addition, the β phases are continuously precipitated at grain boundaries. With the Zn addition to Al-Mg alloy, the ternary τ phase precipitates and sporadically distributes at triple junctions in II# alloy and at grain boundaries in III# alloy, respectively. However, when the Zn content is excessively added and reaches 0.78% (IV# alloy), the amount of τ phase increases to such an extent that the τ phase continuously precipitates at grain boundaries once again.
3.2 IGC behaviors
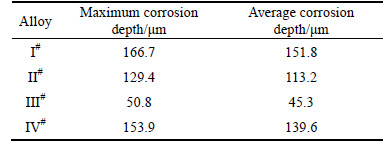
Optical micrographs of the surface microstructures of the IGC after the corrosion testing are shown in Fig. 4. The corroded microstructures of four samples are very different. It can be seen that the grain boundaries of I#, II#, and IV# alloy are seriously damaged, while the III# alloy only suffers a slightly attack. The maximum inter- granular depths and the average inter-granular depths of the specimens are given in Table 2. It is clear that both the maximum corrosion depth and the average depth of the alloys with a Zn addition are reduced compared with I# alloy. The maximum corrosion depth is reduced sharply from 166.7 μm without Zn addition to 50.8 μm with a 0.31% Zn additions. In contrast, the III# alloy shows a better corrosion resistance towards IGC.
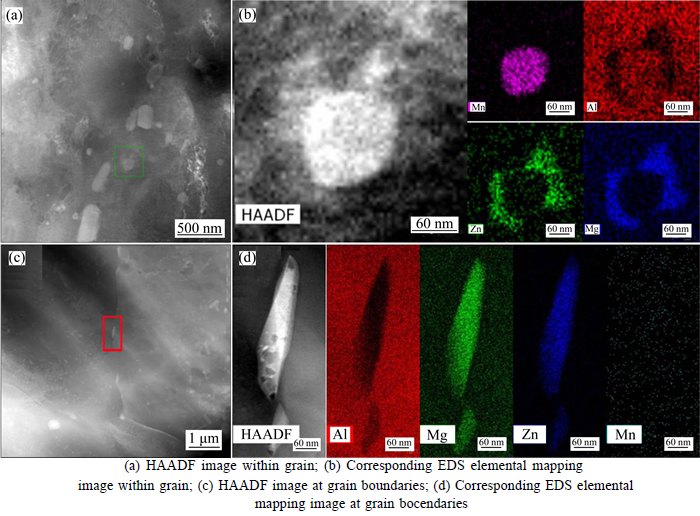
Fig. 1 HAADF images of precipitates in III# alloy:(brighter areas indicate heavier atoms)
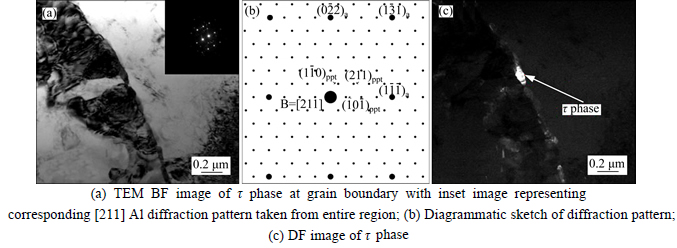
Fig. 2 TEM images and diffraction pattern:
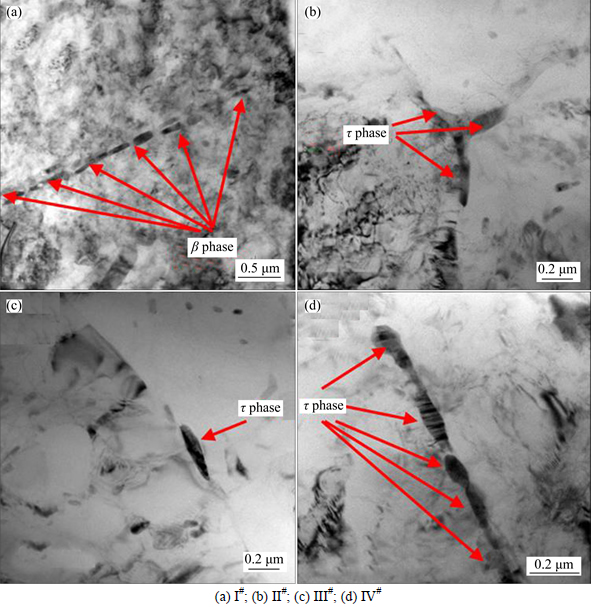
Fig. 3 TEM BF images of precipitation features at grain boundary:
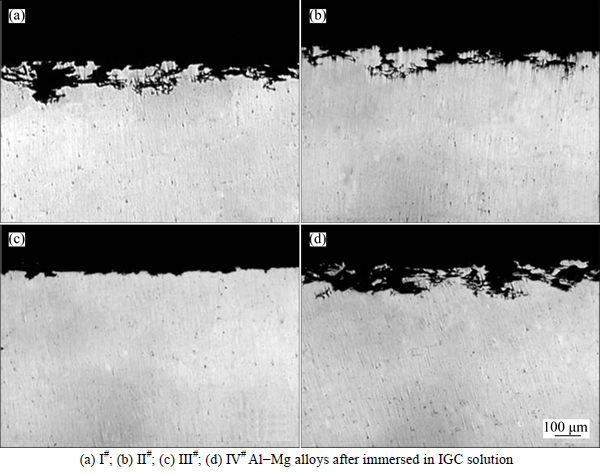
Fig. 4 Optical microstructures:
Table 2 IGC results of Al-Mg alloys
3.3 EXCO behaviors
The exfoliation corrosion morphologies and the corresponding corrosion ratings of Al-Mg alloys are shown in Figs. 5-6 and Table 3, respectively.Figures 5(a), (b), (c) and (d) present the exfoliation corrosion morphologies of four Al-Mg alloys, respectively. There is no obvious exfoliation corrosion that can be observed in these four alloys, but the III# alloy presents a lower degree of corrosion due to its smaller number and shallower depth of pitting holes. Figures 6(a)-(c) show the SEM images of specimens after the exfoliation corrosion tests. It can be seen that the grain boundaries of III# alloy are not very distinct, while the grains of I# alloy can be clearly distinguished.
Meanwhile, there are many corrosion pits mainly found within matrix. Figure 6(d) presents the EDS spectrum of corrosion pit in matrix. The large particle is enriched with Fe and Mn, which indicates that this intermetallic phase is Al6 (Mn, Fe). The pitting corrosion attack is mainly focused in matrix around Al6 (Mn, Fe) precipitates. All the Al-Mg alloys with a Zn addition show better EXCO corrosion resistance compared with I# alloy (without Zn addition), indicating that the Zn addition to the alloy does reduce the susceptibility of EXCO corrosion. With increasing Zn content, the EXCO corrosion resistance of alloy improves, and the optimal value is 0.31%. With the addition of Zn increased to 0.78%, however, the corrosion resistance decreases.
3.4 Electrochemical polarization curves
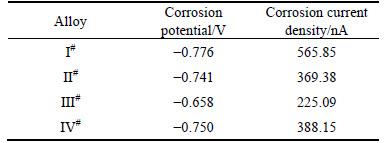
Figure 7 shows potentiodynamic polarization curves, whereas Table 4 lists the corrosion potentials (φcorr) and corrosion current densities (Jcorr), obtained by extrapolating the linear Tafel segments of the alloys’ polarization curves. In general, the samples with lower Jcorr and higher φcorr show better values of corrosion resistance. And the Jcorr value can more accurately reflect the corrosion rate than φcorr. The decreased Jcorr corresponds to the significantly lower anodic kinetics and higher cathode kinetics. With increasing Zn content, the corrosion potential shifts positively and Jcorr decreases. The III# alloy possesses the best corrosion resistance for its highest φcorr and lowest Jcorr. The discretely distributed τ phase at grain boundaries in III# alloy polarizes the alloy. With the addition of Zn increased to 0.78%, however, φcorr shifts negatively and Jcorr increases. The corrosion resistance of IV# alloy decreases once again but still better than that of I# alloy. It can be inferred that Jcorr decreases by adding minor Zn, and the corrosion resistance increases.
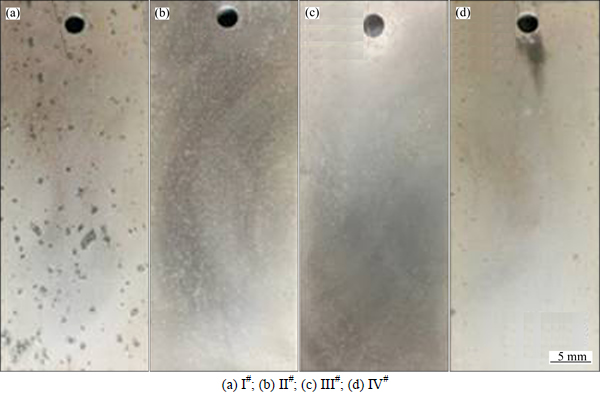
Fig. 5 Exfoliation corrosion morphologies of Al-Mg alloys:
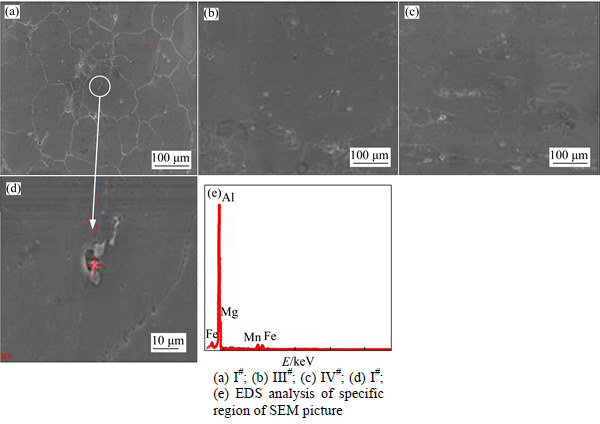
Fig. 6 SEM micrographs of surface after exfoliation test:
Table 3 Exfoliation corrosion ratings of Al-Mg alloys with different Zn contents

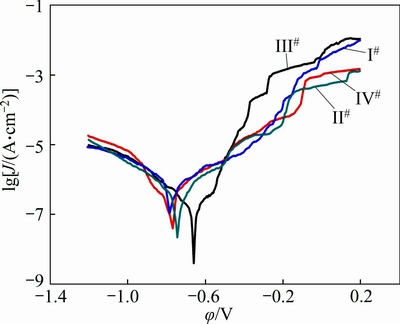
Fig. 7 Anodic polarization curves of Al-Mg alloys in a 3.5% NaCl solution at room temperature
Table 4 Corrosion potentials and corrosion current densities of different polarization curves
4 Discussion
4.1 Effects of Zn addition on precipitation
HAADF observation shown in Fig. 1 shows that the τ phase nucleates and grows on pre-existing Mn-rich particles and at grain boundaries, implying that these Mn-rich particles and grain boundaries are favorable sites for Al-Mg-Zn ternary phase nucleation. The secondary phase precipitates nucleate preferentially on the inoculant particles, since these non-equilibrium defects improve the free energy of system, decreasing the energy of nucleation. The relation of critical nucleation work between homogeneous and heterogeneous nucleation can be expressed as [26]:
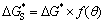 (1)
(1)
where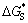 is the critical nucleation work of heterogeneous nucleation; △G* is the critical nucleation work of homogeneous nucleation, and f(θ), determined by the wetting angle θ, equals (2-3cosθ+cos3θ)/4. Because f(θ) is always less than 1, the nucleation of secondary phase precipitates on the heterogeneous sites is much easier than on the homogeneous nucleation. The precipitates of τ phase always forms on the Mn-rich particles and at grain boundaries, indicating that they are efficient heterogeneous nucleation sites.
is the critical nucleation work of heterogeneous nucleation; △G* is the critical nucleation work of homogeneous nucleation, and f(θ), determined by the wetting angle θ, equals (2-3cosθ+cos3θ)/4. Because f(θ) is always less than 1, the nucleation of secondary phase precipitates on the heterogeneous sites is much easier than on the homogeneous nucleation. The precipitates of τ phase always forms on the Mn-rich particles and at grain boundaries, indicating that they are efficient heterogeneous nucleation sites.
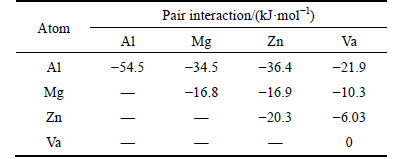
According to the theory of binding energy, the parameters of utilized pair interactions are given in Table 5 [27], the formation mechanism of τ phase can be analyzed.
Table 5 Parameters of pair interaction in Al between same atom species, between different atom species, and between a solute atom and a vacancy utilized in this work
The energies released after binding can be calculated by
 (2)
(2)
The existing form of Mg and Zn atoms in Al matrix is substitutional solid solution. The formation of Zn-Mg cluster can be explained by vacancy exchange mechanism. Figure 8 shows the diagrammatic sketch showing the clustering process of Zn atom and Mg atom.
According to Fig. 8, the binding energy difference, after Zn atom and Mg atom are clustered, can be calculated by
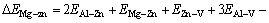
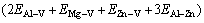 =7.9 kJ/mol (3)
=7.9 kJ/mol (3)
In a similar way, the binding energy differences of Mg-Mg and Zn-Zn are 6.1 kJ/mol and 0.23 kJ/mol, respectively. The energy difference, after Mg-Zn atoms are combined, is relatively larger, indicating that the solid solution tends to be in a lower energy state, which means that the formation of Mg-Zn cluster is relatively stable. After the addition of Zn element, Mg atoms are, therefore, more easily combined with Zn atoms and thus form the τ phase with Al atoms rather than the β phase.
4.2 Effects of Zn addition on corrosion behavior
According to the results of the corrosion tests, Zn addition to the Al-Mg alloy does improve the corrosion resistance, and the Al-Mg alloy with 0.31% Zn (III# alloy) performs the best in the corrosion property. With the Zn content increased to 0.78%, however, the corrosion resistance of alloy decreases once again but it is still better than that of the alloy without Zn addition.
One of the reasons why an aluminum alloy can be corroded is that there is a potential difference between a secondary precipitate and matrix. Previous studies have indicated that the corrosion potential of τ phase (-0.813 V) is quite similar to that of matrix (-0.73 V) [28], compared with the gap between β phase (-1.29 V) and matrix, which reduces the electrochemical force between secondary phase precipitates and matrix. In Al-Mg alloys, β phase is extremely anodic compared with the matrix. With Zn addition, however, the ternary τ phase forms, which replaces β phase and narrows the electrochemical gap. According to the potentiodynamic polarization curves as Fig. 7 shows, a higher corrosion potential of τ phase, compared with that of β phase, elevates the corrosion potential of Al-Mg alloy.
According to the theory of binding energy analysis, the formation of Mg-Zn cluster is more stable than Mg-Mg cluster, which means that the formation of τ phase is much easier and more stable than that of β phase. The β phase formed both on the pre-existing Mn-rich particles in matrix and at grain boundaries [8]. As Fig. 1 shows, τ phase is also nucleated on these sites. In addition, the growth of τ phase needs the diffusion of Mg atoms from matrix. The nucleation and growth of τ phase competes with β phase to get the Mg atoms and reduces the degree of solid solubility of Mg elements in matrix. The formation of τ phase is, therefore, somehow replacing β phase and decreasing the probability of β phase precipitation. In this work, with the Zn content added to 0.31%, the τ phase is discontinuously distributed at grain boundaries, dramatically decreasing the susceptibility of IGC. With the Zn content increased to 0.78%, the amount of τ phase is, however, increased to such an extent that it continuously precipitates at grain boundaries. The continuous τ phase forms the corrosion path as Fig. 4(d) shows, behaving the same as the distribution of β phase in Al-Mg alloy, so the corrosion resistance of alloy is decreased once again but still better than that of the alloy without Zn addition.
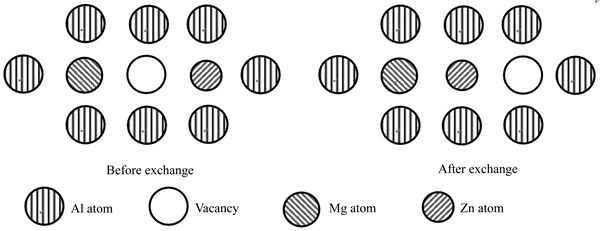
Fig. 8 Diagrammatic sketch of binding between Zn and Mg atoms
For the EXCO tests, there is no obvious layered corrosion taking place on these alloys as Figs. 5 and 6 show, but the grain boundaries of I# and IV# alloys have almost been corroded, while the Al-Mg alloy without Zn addition presents a higher degree of pitting corrosion. In EXCO tests, the pits occurred mainly in the alloy matrix surrounding Al 6(Mn, Fe), since the Al 6(Mn, Fe) phase, where the cathodic reactions occur, had a higher value of corrosion potential compared with aluminum [11]. Pitting usually occurs at isolated regions by local attack, where the passivity has been broken. Both β and τ phases nucleated on pre-existing Al6(Mn, Fe) particles play a role as anode in our alloys. The decreasing of electrochemical imbalance between the secondary phase precipitates and matrix indicates a better corrosion resistance against pitting corrosion.
5 Conclusions
1) Both pre-existing Mn-rich particles and grain boundaries are efficient heterogeneous nucleation sites for the formation of τ phase (Mg32 (Al, Zn)49 ). According to the experimental results and the binding energy theory analysis, the formation of τ phase is much easier than that of β phase (Al3Mg2), somehow replacing β phase and reducing the possibility of β phase precipitation.
2) The formation of τ phase elevates the corrosion potential of Al-Mg alloy. A proper Zn addition to Al-Mg alloy can preclude the precipitation of β phase at grain boundaries, which can dramatically decrease the susceptibility of pitting corrosion and IGC and thus improve the corrosion resistance of Al-Mg alloy, for which the optimal Zn content is 0.31%. With the Zn content increased to 0.78%, however, the amount of τ phase is increased to such an extent that the τ phase continuously precipitates at grain boundaries, so the corrosion resistance of alloy is decreased once again but still better than that of the alloy without Zn addition.
References
[1] KAIBYSHEV R, MUSIN F, LESUER D R, NIEH T G. Superplastic behavior of an Al–Mg alloy at elevated temperatures [J]. Materials Science and Engineering A, 2003, 342(1/2): 169-177.
[2] HOEVEN J A V D, ZHUANG L, SCHEPERS S B P D, BAEKELANDT J P. A new 5xxx series alloy developed for automotive applications [J]. Aluminium, 2002, 78: 750-754.
[3] KIM S J, JANG S K, KIM J I. Investigation on optimum corrosion protection potential of Al alloy in marine environment [J]. Materials Science, 2008, 26(3): 779-786.
[4] JONES R H, BAER D R, DANIELSON M J, VETRANO J S. Role of Mg in the stresscorrosion cracking of an Al–Mg alloy [J]. Metallurgical and Materials Transactions A, 2001, 32(7): 1699-1711.
[5] CHANG J C, CHUANG T H. Stress-corrosion cracking susceptibility of the superplastically formed 5083 aluminum alloy in 3.5 pct NaCl solution [J]. Metallurgical and Materials Transactions A, 1999, 30(12): 3191-3199.
[6] BESSONE J B, SALINAS D R, MAYER C E, EBERT M, LORENZ W J. An EIS study of aluminium barrier-type oxide films formed in different media [J]. Electrochimica Acta, 1992, 37(12): 2283-2290.
[7] SEARSUS J L, GOUMA P I, BUCHHEIT R G. Stress corrosion cracking of sensitized AA5083 (Al-4.5Mg-1.0Mn) [J]. Metallurgical and Materials Transactions A, 2001, 32(11): 2859-2867.
[8] ZHU Ya-kun, CULLEN D A. Evaluation of Al3Mg2 precipitates and Mn-rich phase in aluminum-magnesium alloy based on scanning transmission electron microscopy imaging [J]. Metallurgical and Materials Transactions A, 2012, 43(13): 4933-4939.
[9] YASAKAU K A, ZHELUDKEVICH M L. Role of intermetallic phases in localized corrosion of AA5083 [J]. Electrochimica Acta, 2007, 52(27): 7651-7659.
[10] BARBUCCI A, CERISOLA G, BRUZZONE G, SACCONE A. Activation of aluminium anodes by the presence of intermetallic compounds [J]. Electrochimica Acta, 1997, 42(15): 2369-2380.
[11] BIRBILIS N, ZHANG R, LIM M L C, GUPTA R K, DAVIES C H J, LYNCH S P, KELLY R G, SCULLY J R. Quantification of sensitization in AA5083-H131 via imaging Gaembrittled fracture surfaces [J]. Corrosion, 2012, 69(4): 396-402.
[12] OGUOCHA I N A, ADIGUN O J, YANNACOPOULOS S. Effect of sensitization heat treatment on properties of Al–Mg alloy AA5083-H116 [J]. Journal of Materials Science, 2008, 43(12): 4208-4214
[13] LUO Bing-hui, SHAN Yi-min, BAI Zhen-hai, Effect of annealing temperature on microstructure and corrosive properties of cold-rolled 5083 aluminum alloy after quenching [J]. Journal of Central South University: Science and Technology, 2007, 38(5): 802-808. (in Chinese)
[14] BENSAADA S, BOUZIANE M T, MOHAMMEDI F. Effect of the temperature on the mechanism of the precipitation in Al–8% mass.Mg alloy [J]. Materials Letters, 2011, 65(17/18): 2829-2832.
[15] LIN Shuang-pin, NIE Zuo-ren, HUANG Hui, LI Bo-long. Annealing behavior of a modified 5083 aluminum alloy [J]. Materals and Design, 2010, 31(3): 1607-1612.
[16] FULLER C B, KRAUSE A R, DUNAND D C, SEIDMAN D N, Microstructure and mechanical properties of a 5754 aluminum alloy modified by Sc and Zr additions [J]. Materials Science and Engineering A, 2002, 338(1/2): 8-16.
[17] ALIL A, POPOVIC M, RADETIC T. Influence of annealing temperature on the baking response and corrosion properties of an Al-4.6% Mg alloy with 0.54% Cu [J]. Journal of Alloys and Compounds, 2015, 625: 76-84.
[18] CARROLL M C, GOUMA P I, MILLS M J, DAEHN G S, DUNBAR B R. Effects of Zn additions on the grain boundary precipitation and corrosion of Al-5083 [J]. Scripta Materialia, 2000, 42(4): 335-340.
[19] MENG Chun-yan, ZHANG Di, HUA Cui, ZHUANG Lin-zhong, ZHANG Ji-shan. Mechanical properties, intergranular corrosion behavior and microstructure of Zn modified Al-Mg alloys [J]. Journal of Alloys and Compounds, 2014, 617: 925-932.
[20] BERGMAN G, WAUGH J L T, PAULING L. The crystal structure of the metallic phase Mg32(Al, Zn)49 [J]. Acta Crystallographica, 1957, 10: 254-259.
[21] MULLER D A. Structure and bonding at the atomic scale by scanning transmission electron microscopy [J]. Nature Materials, 2009, 8: 263-270.
[22] GB/T 22639-2008. Test method of exfoliation corrosion for wrought aluminium and aluminium alloys [S]. 2008. (in Chinese)
[23] GB/T 7998-2005. Test method for intergranular corrosion of aluminium alloy [S]. 2005. (in Chinese)
[24] GOSWAMI R, SPANOS G, PAO P S, HOLTZ R L. Precipitation behavior of the β phase in Al-5083 [J]. Materials Science and Engineering A, 2010, 527: 1089-1095.
[25] HAMANA D, BOUCHEAR M, BETROUCHE M, DERAFA A, ROKHMANOV N Y. Comparative study of formation and transformation of transition phases in Al–12% Mg alloy [J]. Journal of Alloys and Compounds, 2001, 320(1): 93-102.
[26] ZHENG Zi-qiao. Fundamentals of materials science [M]. Changsha: Central South University Press, 2005: 365-368. (in Chinese)
[27] HIROSAWA S, SATO T, KAMIO A. Classification of the role of microalloying elements in phase decomposition of Al based alloys [J]. Acta Materialia, 2000, 48(8): 1997-1806.
[28] YANG Lei, LUO Bing-hui, ZHAN Ge. Effect of addition of Zn on microstructure and corrosion property of 5083Al alloy [J]. Journal of Central South University: Science and Technology, 2012, 43(12): 4666-4670. (in Chinese)
(Edited by FANG Jing-hua)
Foundation item: Project(2011-006) supported by the State Administration of Science, Technology and Industry for National Defence, China
Received date: 2015-11-27; Accepted date: 2016-03-17
Corresponding author: LUO Bing-hui, Professor, PhD; Tel: +86-731-88830333; E-mail: luobinghui@csu.edu.cn
Abstract: The effects of different Zn contents in Al-Mg alloy on the microstructure characterizations were observed by advanced electron microscopy and the corrosion properties were investigated by the inter-granular corrosion tests, the exfoliation corrosion tests, and the Potentiodynamic polarizaion tests. The τ phase (Mg32 (Al, Zn)49 ) forms on the pre-existing Mn-rich particles and at the grain boundaries. According to the theory of binding energy, the formation of τ phase is much easier than that of β phase (Al3Mg2), somehow replacing β phase and reducing the possibility of β phase precipitation. This change dramatically decreases the susceptibility of corrosion. The Zn addition increases the corrosion resistance of Al-Mg alloy with an optimal value of 0.31%. When the Zn addition is increased to 0.78%, however, the corrosion resistance of alloy decreases once again but it is still better than that of the alloy without Zn addition.


