DOI:10.19476/j.ysxb.1004.0609.2019.08.20
球磨与助剂强化选择性回收电解锰渣中的锰
蓝际荣1, 2,孙 燕1, 2,潘 滢1,李卓蔓1,杜冬云1, 2
(1. 中南民族大学 资源与环境学院/湖北省重金属污染防治工程技术研究中心,武汉 430074;
2. 中南民族大学 催化转化与能源材料化学教育部重点实验室,武汉 430074)
摘 要:
摘 要:电解锰渣(Electrolytic manganese residues, EMRs)是电解锰工业产生的废渣,但电解锰渣还含有部分的Mn。目前,对电解锰渣的处理大多为露天堆放,不仅造成严重的环境问题还造成Mn资源的浪费。针对长期堆放的库存电解锰渣,采用球磨的方式浸出常规方法无法浸出的Mn。试验探究了固液比、转速、料球比、填充系数、球磨时间和不同化学助剂下等因素对Mn浸出率的影响。结果表明:当固液比为1:5、转速为200 r/min、料球比为0.5、填充系数为0.06、球磨时间为60 min,在化学助剂柠檬酸三钠添加量为2.2 g/kg或二水合草酸添加量为2.0 g/kg时,Mn的浸出率可达99%以上。球磨能够实现对堆放较久的库存电解锰渣中Mn的高效选择性浸出并减少Cr的干扰,为库存电解锰渣中Mn的回收处理提供了新的方法。
关键词:
文章编号:1004-0609(2019)-08-1749-07 中图分类号:TD981 文献标志码:A
锰元素广泛应用于有色金属冶炼、化工、医药、食品的分析和科研等方面是国民经济中不可缺少的重要基础物资[1-2]。在我国,金属锰的生产主要是通过电解法[3]。目前我国也已成为世界上最大的电解锰生产国、消费国与出口国[4]。金属锰作为一种重要的化工以及冶金原料[5],虽然为我国的工业发展和地区经济建设做出了巨大的贡献[6],但是同时也造成了严重的环境污染和破坏,而首当其冲的要数电解锰渣(EMRs)造成的污染[7]。
EMRs是电解锰生产过程中菱锰矿经浓硫酸酸浸、氨水中和、板压滤机压滤等工序后产生的废渣[8]。由于历史和技术的原因,我国现存露天堆存的EMRs高达5000万t[9],已成为环境和安全的一大隐患。随着锰矿资源的日益消耗,锰矿品位不断下降,导致每生产 1 t电解锰就要排放高达10~15 t的EMRs [10],进一步加重了EMRs处置的难度和环保压力。EMRs中主要含有 Si、S、Mn、Fe、Al、Zn、Ca、Cr、Ni、Co 等元素[11]。目前,EMRs的处理方法主要是安全堆放和填埋[12]。在长期的堆放过程中,EMRs中易溶性元素及重金属元素会迁移到周边地表水、地下水和土壤中,对当地环境造成严重污染[13]。首先是锰的污染,EMRs中一般含有4%~5%的锰[14],浸出量达800~2000 mg/L[15],锰是生物体必须的微量元素,但过量的锰对生物和环境具有毒害作用[16]。常规的堆放处理方法不仅对锰资源造成浪费还对环境造成影响。
已有研究表明,EMRs经长时间的露天堆放后,其物理化学性质产生了较大变化[17]。传统的浸出方法很难实现库存EMRs中锰元素的高效浸出[18]。EMRs在长期堆放过程中,里面的锰在空气或微生物的氧化作用下转化为更高价的锰氧化物,这些锰元素难以通过水浸出。现有的研究发现,通过酸浸[19]、超声[20]、电场作用[21]、辅助剂[22]、高温加热[23]等方式能够提高库存EMRs中锰的浸出率,但以上方法还存在以下不足:一是锰的浸出率不高,浸出率仅有50%~78%[24];二是浸出条件苛刻,需要多种方法结合[25];三是由于以前未改进的电解锰生产工艺需要铬钝化这一步骤,这使得库存EMRs中Cr含量较高,常规的回收方法会使得部分的Cr进入浸出液中[26]。这些不足都进一步加大了经济成本和后续处理的难度。目前,添加一些绿色化学助剂在球磨机作用下高效选择性回收库存EMRs中的锰还没有报道过。针对上述情况,本文以水作为浸取剂,在球磨作用下浸取库存EMRs以达到锰资源的最大回收利用并为EMRs的进一步利用提供解决方法。
1 实验
1.1 EMRs
试验所用EMRs取自于广西中信大锰矿业有限责任公司,是该公司利用菱锰矿(主要成分为MnCO3)通过硫酸浸取大量锰元素后的堆放于渣场的库存废渣。EMRs的物理特性表现为含水率高(23%~28%)、颗粒细小(40~250 μm)的灰黑色板结块状物。将自然风干的EMRs研磨过筛,利用电感耦合等离子体质谱仪分析电解锰废渣的主要元素构成为Si、S、Ca、Fe和Mn,其结果如表1。
表1 电解锰废渣主要组成成分
Table 1 Main components of EMR (mass fraction, %)

1.2 试验方法
溶液中锰的纯度(即杂质重金属的浸出率)对后续的回收有一定影响,为了探究锰的最佳选择性浸出工艺,对该过程进行了单因素试验和在化学助剂下的浸出情况试验。研究发现,影响锰浸出因素的条件有七个:固液比(m(EMRs):V(H2O),kg/L),料球比(H2O与钢球体积比),填充系数(钢球的堆积体积与罐体容积比),转速(r/min),球磨助剂添加情况CTAB(十六烷基三甲基溴化铵),柠檬酸三钠,二水合草酸。利用XQM立式行星球磨机(湘潭湘仪仪器有限公司生产)进行电解锰渣中的锰选择性浸出实验,具体操作为:根据试验设计好的浸出条件,用分析天平称取一定量EMR或球磨助剂,用量筒量取一定量的水并和一定量的钢球(以总体积计)装入反应罐中,加盖密封。按试验条件要求设定好时间和转速开始球磨浸出。浸出结束后,开盖取出反应体系进行钢球、废渣和液体三者的分离,将滤渣和滤液样品用于分析。
1.3 分析方法
浸出液中Cr的含量用Agilent 7900电感耦合等离子体质谱仪测定;浸出液中Mn的含量利用《水质 锰的测定 高碘酸钾分光光度法》(GB 11906—89)测定;矿样处理前后表而形貌用JSM-6610LV型扫描电镜分析。
2 结果与讨论
2.1 固液比对Mn和Cr浸出的影响
保持料球比为0.5、转速为200 r/min、填充系数为0.09不变,探究固液比对EMRs中Mn和Cr的浸出影响情况。当球磨时间为60 min时,试验结果如图1所示。

图1 固液比对Mn和Cr浸出率的影响
Fig. 1 Effect of solid-liquid ratio on leaching rate of Mn and Cr
由图1可知,固液比对Mn的浸出率影响相对较小,固液比为1:1时对应最小浸出率89.61%;固液比为1:5时,其浸出率最大,最大浸出率为91.31%。而Cr的浸出率在整个过程中,其最大浸出率为31.72%,最小浸出率为25.70%。Mn的浸出率均远高于Cr的浸出率,这说明在球磨作用下,以水作为浸出剂球磨对EMRs中的锰具有选择性浸出作用。然而,浸取剂量过大不仅会造成固液分离的困难,还会增加后续废水处理的难度。从本研究来看,在固液比为1:5浸出率就达到了90%以上,这比已有研究结果[27]需要1:10甚至更多液体的结果要好。这可能是由于球磨过程钢球和物料剧烈碰撞罐体内产生了高温,加大了Mn的浸出量也能加快浸出过程。
2.2 料球比对Mn和Cr浸出的影响
控制固液比为1:5、转速为200 r/min、填充系数为0.09不变,探究料球比对EMRs中Mn和Cr的浸出影响情况。当球磨时间为60 min时,试验结果如图2所示。
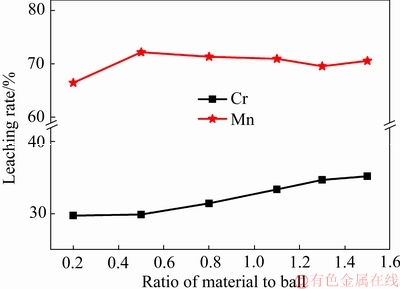
图2 料球比对Mn和Cr浸出的影响
Fig. 2 Effect of ratio of material to ball on leaching rate of Mn and Cr
由图2可知,不同料球比条件下对Mn的浸出效果均优于Cr的浸出。在料球比为0.5时其浸出率达到最大值,最大浸出率为72.19%,此时,Cr的浸出率为31.19%。物料过多则会减少与钢球的碰撞摩擦机会,造成EMRs球磨不彻底,但是过少又会使得球磨效率不高。因此,需要寻找一个恰当的配比,试验得出料球比为0.5时已经达到浸出的较好结果。
2.3 转速对Mn和Cr浸出的影响
保持固液比为1:5、料球比为0.5、填充系数为0.09不变,探究转速对EMRs中Mn和Cr的浸出影响情况。当球磨时间为60 min时,试验结果如图3所示。
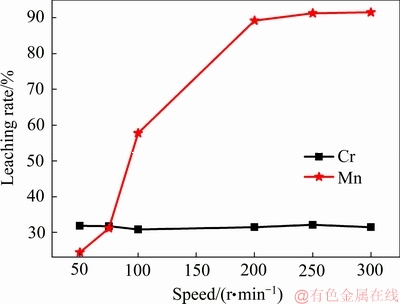
图3 转速对Mn和Cr浸出的影响
Fig. 3 Effect of rotational speed on leaching rate of Mn and Cr
从图3可知,当转速低于100 r/min时,Mn的浸出率低于Cr的浸出率;而当转速逐渐增大时,Mn的浸出率也逐渐增大;当转速达到200 r/min时达到最大浸出率,其最大浸出率为89.22%。而Cr的浸出率受转速的影响相对较小,其最大最小浸出率分别为32.10%和30.81%。当转速过低时EMRs与浸取剂无法充分混合,从而限制了Mn的浸出。但是,转速过高可能造成机械的磨损和能耗过大,因此200 r/min为最佳转速。
2.4 填充系数对Mn和Cr浸出的影响
设置固液比为1:5、转速为200 r/min、料球比为0.5不变,探究填充系数对EMRs中Mn和Cr的浸出影响情况。当球磨时间为60 min时,试验结果如图4所示。

图4 填充系数对Mn和Cr浸出的影响
Fig. 4 Effect of filling coefficient on leaching rate of Mn and Cr
如图4所示,不管填充系数为多少,Mn的浸出率整体远高于Cr的,且在填充系数为0.06时, Mn的浸出率达到90.89%,而Cr的浸出率仅为34.78%。当填充系数再增加时,对Mn的浸出影响不大这说明在填充系数为0.06时已经达到最佳浸出效果。
2.5 时间对Mn和Cr浸出的影响
控制固液比为1:5、转速为200 r/min、料球比为0.5、填充系数为0.06不变,探究球磨时间对EMRs中Mn和Cr的浸出影响情况,如图5所示。
从图5可知,随着时间的增加EMRs中Mn的浸出率逐渐增大,当时间达到60 min时,浸出率为88.63%。球磨时间越长,对EMRs的研磨越充分,对一些含Mn化合物的晶体结构破坏得更彻底,从而促进了Mn 的浸出。当时间大于60 min时,Mn的浸出效果增加不明显,但球磨时间越长能耗越大,所以并不是球磨时间越长越好。而随时间的增加,Cr的浸出率的变化不是很大,其浸出率基本在26.5%~27.5%之间。
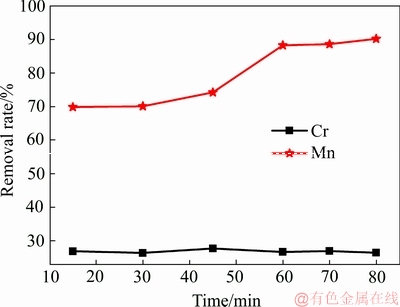
图5 时间对Mn和Cr浸出的影响
Fig. 5 Effect of time on leaching rate of Mn and Cr
2.6 CTAB含量对Mn和Cr浸出的影响
一些表面活性剂或者化学试剂往往对矿物中的浸出效果有着显著影响。保持固液比为1:5、转速为200 r/min、料球比为0.5、填充系数为0.06不变,球磨时间为60 min,探究表面活性剂十六烷基三甲基溴化铵(CTAB)对EMRs中Mn和Cr的浸出影响情况,如图6所示。

图6 CTAB含量对Mn和Cr浸出的影响
Fig. 6 Effect of CTAB content on leaching rate of Mn and Cr
由图6可知,随着CTAB含量的增加,Mn的浸出率逐渐降低,说明CTAB对于Mn的浸出具有抑制作用。不添加CTAB时,Mn和Cr的浸出率分别为77.23%和26.68%,而当体系中CTAB含量为8 g/kg时,两者的浸出率分别为38.81%和35.42%。这说明CTAB对于Mn的浸出具有抑制作用而对于Cr的浸出具有一定的促进。由于CTAB是阳离子型表面活性剂,震荡时会产生大量泡沫具有乳化作用,其极性基将吸附在矿石表面, 非极性基朝外, 对EMRs表面润湿作用不利。另外CTAB的加入会使得钢球表面更加光滑不适于对EMRs的碰撞和挤压。因此要想进一步增大EMRs中Mn的浸出需要增加矿物表面湿润并增加机械强度。
2.7 柠檬酸三钠对Mn和Cr浸出的影响
柠檬酸三钠具有很好的络合性能,除了用于化工食品行业,因其绿色环保还用于洗涤助剂。控制固液比为1:5、转速为200 r/min、料球比为0.5、填充系数为0.06不变,球磨时间为60 min,探究柠檬酸三钠对EMRs中Mn和Cr的浸出影响情况,如图7所示。
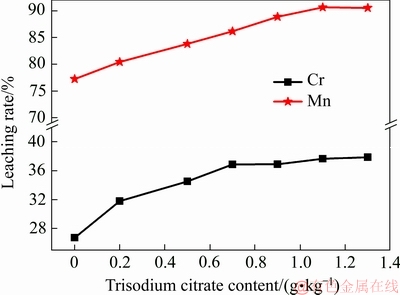
图7 柠檬酸三钠对Mn和Cr浸出的影响
Fig. 7 Effect of trisodium citrate on leaching rate of Mn and Cr
由图7可知,Mn和Cr的浸出率都随柠檬酸三钠含量的增加而增加,且Mn的浸出率始终高于Cr的浸出率。当柠檬酸三钠的添加量为1.1g/kg时,Mn的浸出率达到最大,为90.67%,此时Cr的浸出率为35.83%。已有的研究表明,柠檬酸钠应用于去污的时候能够加强物体表面的增湿作用。在体系中加入柠檬酸钠能够促进Mn的浸出,其机理可能在于其加速液体在EMRs表面的浸润从而加强Mn的浸出。
2.8 二水合草酸对Mn和Cr浸出的影响
控制固液比为1:5、转速为200 r/min、料球比为0.5、填充系数为0.06不变,球磨时间为60 min,考查二水合草酸对EMRs中Mn和Cr的浸出影响情况,如图8所示。

图8 二水合草酸对Mn和Cr浸出的影响
Fig. 8 Effect of oxalic acid dihydrate on leaching rate of Mn and Cr
由图8可知, 随着二水合草酸含量的增加,Mn和Cr的浸出率都逐渐增大。当二水合草酸含量大于1.0 g/kg时,Mn的浸出率趋于稳定,此时Mn的浸出率为99.78%。当二水合草酸含量大于0.7 g/kg时,Cr的去除率逐渐趋于稳定,此时Cr的去除率为39.02%。这是由于草酸具有还原性,可促进MnO2还原浸出。本研究利用球磨产生的高热使草酸与EMRs中的MnO2发生 还原反应促进该部分Mn的浸出从而提高浸出效率。草酸在水溶液中可电离出H+降低体系pH进一步促进Mn2+的浸出。
其反应可能主要为以下3部分:
MnO2+2H2C2O4  MnC2O4+2CO2+2H2O (1)
MnC2O4+2CO2+2H2O (1)
MnC2O4 MnO + CO+CO2 (2)
MnO + CO+CO2 (2)
MnO + 2H+ Mn2+ + H2O (3)
Mn2+ + H2O (3)
3 结论
1) 球磨能够实现对堆放较久的库存EMRs中Mn的高效选择性浸出且减少Cr的干扰。
2) 球磨浸出EMRs中Mn的最佳工艺参数为:固液比为1:5,转速为200 r/min,料球比为0.5,填充系数为0.06不变,球磨时间为60 min。此时Mn的浸出率可达91.09%以上。
3) 化学助剂柠檬酸三钠和二水合草酸能够提高Mn的选择性浸出,在少量的化学助剂作用下,Mn的浸出率可达99%以上。
REFERENCES
[1] 闫国孟, 彭 兵, 柴立元, 闵小波, 彭 宁, 雷 杰, 刘 琴, 张 强, 胡 明. 锰渣的理化特性及煅烧特性[J].中南大学学报(自然科学版), 2015, 46(7): 2419-2425.
YAN Guo-meng, PENG Bing, ZHAI Li-yuan, MIN Xiao-bo, PENG Ning, LEI Jie, LIU Qing, ZHANG Qiang, HU Ming. Physicochemical and calcination characteristics of manganese residue[J]. Journal of Central South University (Science and Technology), 2015, 46(7): 2419-2425.
[2] 陈红亮, 张玉涛, 张秋云, 李 琳. 酸法还原浸出电解锰渣中锰和铁的工艺条件和动力学分析[J]. 硅酸盐通报, 2017, 36(8): 2844-2849.
CHENG Hong-liang, ZHANG Yu-tao, ZHANG Qiu-yun, LI Lin. Technology conditions and kinetics analysis of manganese and iron ions leaching from electrolytic manganese residue by acid reduction[J]. Bulletin of the Chinese Ceramic Society, 2017, 36(8): 2844-2849.
[3] FENG L U, CHEN M, CHEN L L. Characteristics of heavy metal pollution in manganese residues of electrolytic manganese in Songtao, Guizhou and its toxic effects on plant growth[J]. Science Technology & Engineering, 2018, 18(5): 124-129.
[4] 罗 乐, 降林华, 段 宁. 电解锰废渣的浸出毒性及生石灰固化技术[J]. 环境工程, 2017, 35(12): 139-143.
LUO Le, JIANG Lin-hua, DUAN Ning. A EMR solidification technology based on quicklime and leaching toxicity. Environmental Engineering, 2017, 35(12): 139-143.
[5] 陈红亮. 电解锰渣中锰稳定化与氨氮控制的方法研究[D]. 重庆: 重庆大学, 2016.
CHEN Hong-liang. Stabilization of manganese and control of ammonia-nitrogen from electrolytic manganese residue[D]. Chongqing: Chongqing University, 2016.
[6] 王家伟. 电解锰渣预处理除氨硫锰的研究[J]. 矿产综合利用, 2018(1): 115-118.
WANG Jian-wei. Research on the removal of ammonia sulphur and manganese from manganese tailing slag by pretreatment[J]. Multipurpose Utilization of Mineral Resources, 2018(1): 115-118.
[7] 陆 凤, 陈 淼, 陈兰兰.贵州松桃某电解锰企业锰渣重金属污染特征及对植物生长的毒性效应[J]. 科学技术与工程, 2018, 18(5): 124-129.
LU Feng, CHEN Miao, CHEN Lan-lan. Characteristics of heavy metal pollution in manganese residues of electrolytic manganese in Songtao, Guizhou and its toxic effects on plant growth[J]. Science Technology and Engineering, 2018, 18(5): 124-129.
[8] 阳富强, 刘广宁, 刘晓霞, 黄贤煜. 机械力活化诱导硫化矿石自燃的热分析动力学[J]. 中国有色金属学报, 2016, 26(9): 1976-1981.
YANG Fu-qiang, LIU Guang-ning, LIU Xiao-xia, HUANG Xian-yu. Thermal analysis kinetics of mechanically activated sulfide ores for spontaneous combustion[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(9): 1976-1981.
[9] 田 磊, 张廷安, 吕国志, 刘 燕, 周 双, 张伟光, 张国权. 机械活化对闪锌矿物化性质及焙烧动力学的影响[J]. 中国有色金属学报, 2015, 25(12): 3535-3542.
TIAN Lei, ZHANG Ting-an, Lü Guo-zhi, LIU yan, ZHOU Shuang, ZHANG Wei-guang, ZHANG Guo-quan. Effect of mechanical activation on physical and chemical properties and roasting kinetics of sphalerite[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(12): 3535-3542.
[10] 牛莎莎, 王志兴, 郭华军, 李新海, 彭文杰, 胡启阳, 张云河. 电解锰阳极渣还原浸出锰[J]. 中国有色金属学报, 2012, 22(9): 2662-2666.
NIU Sha-sha, WANG Zhi-xing, GUO Hua-jun, LI Xin-hai, PENG Wen-jie, HU Qi-yang, ZHANG Yun-he. Reductive leaching of manganese from manganese anode slag[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(9): 2662-2666.
[11] SHU J, LIU R, LIU Z. Solidification/stabilization of electrolytic manganese residue using phosphate resource and low-grade MgO/CaO[J]. Journal of Hazardous Materials, 2016, 317: 267-274.
[12] XIN B, CHEN B, DUAN N. Extraction of manganese from electrolytic manganese residue by bioleaching[J]. Bioresource Technology, 2011, 102(2): 1683-1687.
[13] LI C, ZHONG H, WANG S, XUE J, ZHANG Z. Removal of basic dye (methylene blue) from aqueous solution using zeolite synthesized from electrolytic manganese residue[J]. Journal of Industrial & Engineering Chemistry, 2015, 23: 344-352.
[14] CHEN H, LIU R, LIU Z. Immobilization of Mn and NH4(+)-N from electrolytic manganese residue waste[J]. Environmental Science & Pollution Research, 2016, 23(12): 12352-12361.
[15] SHU J, WU H, LIU R, LIU Z, LI B, CHEN M, TAO C. Simultaneous stabilization/solidification of Mn(2+) and NH4(+)-N from electrolytic manganese residue using MgO and different phosphate resource[J]. Ecotoxicology & Environmental Safety, 2018, 148(4): 220-224.
[16] LI J, DU D, PENG Q. Activation of silicon in the electrolytic manganese residue by mechanical grinding-roasting[J]. Journal of Cleaner Production, 2018. 192(10): 347-353
[17] 李明强. 电解锰渣中锰元素的浸取研究[D]. 重庆: 重庆大学, 2015.
LI Ming-qiang. The leaching of manganese from electrolytic manganese residues[D]. Chongqing: Chongqing University, 2015.
[18] SHU J, LIU R, LIU Z. Simultaneous removal of ammonia and manganese from electrolytic metal manganese residue leachate using phosphate salt[J]. Journal of Cleaner Production, 2016, 135: 468-475.
[19] 盘 俊, 谢能银, 明宪权, 王雨红, 粟海锋. 锰矿浸渣中可溶锰离子的稳定化处理研究[J]. 广西大学学报(自然科学版), 2015, 40(3): 551-557.
PAN Jun, XIE Neng-yin, MING Xian-quan, WANG Yu-hong, LI Hai-feng. The stabilizing treatment for the soluble manganese in manganese leaching slag[J]. Journal of Guangxi University (Nat Sci Ed), 2015, 40(3): 551-557.
[20] LU J, DREISINGER D, GLüCK T. Electrolytic manganese metal production from manganese carbonate precipitate[J]. Hydrometallurgy, 2016, 161: 45-53.
[21] 陈红亮. 新鲜电解锰渣和长期堆存渣的矿物成分和毒性特征的差异分析[J]. 贵州师范大学学报(自然科学版), 2016, 34(2): 32-36.
CHEN Hong-liang. Differences analysis of minerals compositions and toxicity characteristics between the fresh electrolytic manganese residue and the stockpiling residue[J]. Journal of Guizhou Normal University (Natural Sciences), 2016, 34(2): 32-36.
[22] 车丽诗. 电解锰渣的特性表征及含锰废水中锰离子的去除研究[D]. 长沙: 湖南农业大学, 2016.
CHE Li-shi. Characterization of manganese slag and removal of manganese ions in waste[D]. Changsha: Hunan Agricultural University, 2016.
[23] 周亚武, 陆谢娟, 高明刚, 吴晓晖, 周 旻. 电解锰渣固结体中重金属浸出毒性及其在模拟酸雨下的淋溶特性分析[J]. 武汉科技大学学报, 2018, 41(2): 127-132.
ZHOU Ya-wa, LU Xie-juan, GAO Ming-gang, WU Xiao-hui, ZHOU Ming. Leaching toxicity and leaching properties in simulated acid rain of heavy metals in solidified electrolytic manganese residue[J]. Journal of Wuhan University of Science and Technology, 2018, 41(2): 127-132.
[24] 杨 磊.电解锰渣中可溶性锰固化及氨氮脱除的研究[D]. 淮南: 安徽理工大学, 2016.
YA Lei. The research of curing soluble Mn2+ and removing NH3-N in electrolytic manganese residue[D]. Huainan: Anhui University of Science and Technology, 2106.
[25] 陈 哲, 夏柳荫, BRIAN H A R T, 池汝安, 余军霞. 球磨介质及尺寸对铜锌矿矿浆化学性质及矿物表面化学性质的影响[J]. 中国有色金属学报, 2017, 27(8): 1701-1707.
CHEN Zhe, XIA Liu-yin, BRIAN H A R T, CHI Ru-an, YU Jun-xia. Influence of ball mill medium and size on properties of copper-zinc ore pulp and mineral surface[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(8): 1701-1707.
[26] 黄 川, 史晓娟, 龚 健, 陈绍杨. 碱激发电解锰渣制备水泥掺合料[J]. 环境工程学报, 2017, 11(3): 1851-1856.
HUANG Chuan, SHI Xiao-juan, GONG Jian, CHEN Shao-yang. Alkali-activated electrolytic manganese residue preparation of cement admixture[J]. Chinese Journal of Environmental Engineering, 2017, 11(3): 1851-1856.
[27] 赵博超, 王雪婷, 朱克松, 苑喜男, 窦广玉, 潘涔轩. 洗涤方式对电解锰渣中锰回收效率及无害化处理的影响[J]. 环境工程学报, 2017, 11(11): 6103-6108.
ZHAO Bo-chao, WANG Xue-ting, ZHU Ke-song, YUAN Xi-nan, DOU Guang-yu, PAN Cen-xuan. Effects of washing methods on recovery efficiency of manganese from manganese residue and harmless treatment[J]. Chinese Journal of Environmental Engineering, 2017, 11(11): 6103-6108.
[28] LIN Q, BARKER D J, DOBSON K J, LEE P D, NEETHLING S J. Modelling particle scale leach kinetics based on X-ray computed micro-tomography images[J]. Hydrometallurgy, 2016, 162(11): 25-36.
[29] XUE J, ZHONG H, WANG S, LI C, LI J, WU F. Kinetics of reduction leaching of manganese dioxide ore with Phytolacca americana in sulfuric acid solution[J]. Journal of Saudi Chemical Society, 2016, 20(4): 437-442.
[30] WEI L I U, TANG M T, TANG C B, HE J, YANG S H, YANG J C. Dissolution kinetics of low grade complex copper ore in ammonia- ammonium chloride solution[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 910-917.
Ball milling and auxiliary enhancement for selective recovery of manganese in EMRs
LAN Ji-rong1, 2, SUN Yan1, 2, PAN Ying1, LI Zhuo-man1, DU Dong-yun1, 2
(1. Hubei Engineering Research Center for Control & Treatment of Heavy Metal Pollution, College of Resources and Environmental Science, South-Central University for Nationalities, Wuhan 430074, China;
2. Key Laboratory of Catalysis Conversion and Energy Materials Chemistry, Ministry of Education, South-Central University for Nationalities, Wuhan 430074, China)
Abstract: Electrolytic manganese residues (EMRs) contained some of manganese. At present, EMRs were treated by open-air stacking, which caused serious environmental problems and made waste of Mn resources. This research provided a method for recovering Mn from EMRs by using ball milling. Solid-liquid ratio, rotation speed, material ball ratio, filling factor, ball milling time and different chemical additives were explored for effects of the Mn leaching. The results show that the leaching rate of Mn reaches more than 99% when the solid-liquid ratio is 1:5, the rotation speed is 200 r/min, the material ball ratio is 0.5, the fill factor is 0.06, the ball milling time is 60 min, the trisodium citrate is 2.2 g/kg or the oxalic acid dihydrate is 2.0 g/kg. By ball milling, manganese can be efficiently and selectively leached from the old EMRs which stacks for a long time and reduces the interference of Cr.
Key words: electrolytic manganese residue; selectivity; recovery; manganese; resource
Foundation item: Projects(2015BAB01B01, 2015BAB01B03) supported by the National Science and Technology Pillar Program, China; Project(CZY17016) supported by the Fundamental Research Funds for the Central Universities, China; Project(GCX1841) supported by National Innovation Training Program for College Students of China
Received date: 2018-07-12; Accepted date: 2018-11-04
Corresponding author: DU Dong-yun; Tel: +86-13871538801; E-mail: dydu666@mail.scuec.edu.cn
(编辑 王 超)
基金项目:国家科技支撑计划资助项目(2015BAB01B01,2015BAB01B03);中央高校基本科研业务费专项(CZY17016);国家级大学生创新训练计划资助项目(GCX1841)
收稿日期:2018-07-12;修订日期:2018-11-04
通信作者:杜冬云,教授,博士;电话:13871538801;E-mail:dydu666@mail.scuec.edu.cn
[2] 陈红亮, 张玉涛, 张秋云, 李 琳. 酸法还原浸出电解锰渣中锰和铁的工艺条件和动力学分析[J]. 硅酸盐通报, 2017, 36(8): 2844-2849.
[4] 罗 乐, 降林华, 段 宁. 电解锰废渣的浸出毒性及生石灰固化技术[J]. 环境工程, 2017, 35(12): 139-143.
[5] 陈红亮. 电解锰渣中锰稳定化与氨氮控制的方法研究[D]. 重庆: 重庆大学, 2016.
[6] 王家伟. 电解锰渣预处理除氨硫锰的研究[J]. 矿产综合利用, 2018(1): 115-118.
[7] 陆 凤, 陈 淼, 陈兰兰.贵州松桃某电解锰企业锰渣重金属污染特征及对植物生长的毒性效应[J]. 科学技术与工程, 2018, 18(5): 124-129.
[8] 阳富强, 刘广宁, 刘晓霞, 黄贤煜. 机械力活化诱导硫化矿石自燃的热分析动力学[J]. 中国有色金属学报, 2016, 26(9): 1976-1981.
[9] 田 磊, 张廷安, 吕国志, 刘 燕, 周 双, 张伟光, 张国权. 机械活化对闪锌矿物化性质及焙烧动力学的影响[J]. 中国有色金属学报, 2015, 25(12): 3535-3542.
[10] 牛莎莎, 王志兴, 郭华军, 李新海, 彭文杰, 胡启阳, 张云河. 电解锰阳极渣还原浸出锰[J]. 中国有色金属学报, 2012, 22(9): 2662-2666.
[17] 李明强. 电解锰渣中锰元素的浸取研究[D]. 重庆: 重庆大学, 2015.
[19] 盘 俊, 谢能银, 明宪权, 王雨红, 粟海锋. 锰矿浸渣中可溶锰离子的稳定化处理研究[J]. 广西大学学报(自然科学版), 2015, 40(3): 551-557.
[21] 陈红亮. 新鲜电解锰渣和长期堆存渣的矿物成分和毒性特征的差异分析[J]. 贵州师范大学学报(自然科学版), 2016, 34(2): 32-36.
[22] 车丽诗. 电解锰渣的特性表征及含锰废水中锰离子的去除研究[D]. 长沙: 湖南农业大学, 2016.
[23] 周亚武, 陆谢娟, 高明刚, 吴晓晖, 周 旻. 电解锰渣固结体中重金属浸出毒性及其在模拟酸雨下的淋溶特性分析[J]. 武汉科技大学学报, 2018, 41(2): 127-132.
[24] 杨 磊.电解锰渣中可溶性锰固化及氨氮脱除的研究[D]. 淮南: 安徽理工大学, 2016.
[26] 黄 川, 史晓娟, 龚 健, 陈绍杨. 碱激发电解锰渣制备水泥掺合料[J]. 环境工程学报, 2017, 11(3): 1851-1856.
[27] 赵博超, 王雪婷, 朱克松, 苑喜男, 窦广玉, 潘涔轩. 洗涤方式对电解锰渣中锰回收效率及无害化处理的影响[J]. 环境工程学报, 2017, 11(11): 6103-6108.


