
J. Cent. South Univ. (2020) 27: 2054-2067
DOI: https://doi.org/10.1007/s11771-020-4430-y
Swelling characteristics of East-Africa black cotton soil based on computer molecular simulation
ZHU Jun-qing(朱俊清)1, ZHANG Wei-guang(张伟光)1, ZHANG Yu-qing(张裕卿)2, 3, TANG Hao(唐皓)1
1. School of Transportation, Southeast University, Nanjing 210096, China;
2. National Engineering Laboratory of Highway Maintenance Technology, Changsha University of Science & Technology, Changsha 410114, China;
3. Aston Institute of Materials Research, Engineering Systems & Management Group, Aston University,Birmingham, B4 7ET, UK
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
Black cotton soil in East Africa is not a stable engineering material for highway and railroad projects. Its strong swelling potential when it absorbs water causes distresses in subgrade of highway and railroad, and thus leads to failures of the projects. This paper presents study on the swelling characteristics of black cotton soil in East Africa. Lab tests were conducted to obtain its basic engineering properties, and the results show that black cotton soil contains high amount of montmorillonite and exchangeable cations and is strong expansive soil. Molecular modelling was exploited to further investigate water absorption ability of montmorillonite. Three different molecular models of montmorillonite were constructed and used for simulations, among which Types I and II montmorillonite represent the expansive soil montmorillonite in China, and Types II and III montmorillonite represent black cotton soil montmorillonite in East Africa. The results showed that the interlayer cations of Type III montmorillonite possessed the strongest water absorption ability based on analysis of radial distribution function (RDF) of cations. Interlayer compensatory cations of Na+ enhance the hydration ability of the other major cations, thus resulting in the strong swelling potential of East-Africa black cotton soil.
Key words:
Cite this article as:
ZHU Jun-qing, ZHANG Wei-guang, ZHANG Yu-qing, TANG Hao. Swelling characteristics of East-Africa black cotton soil based on computer molecular simulation [J]. Journal of Central South University, 2020, 27(7): 2054-2067.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4430-y1 Introduction
The highway and railroad construction projects in East Africa are frequently faced with problems caused by local black cotton soils. The swelling characteristic of black cotton soils can cause serious engineering problems if not being taken care of. Replacement of black cotton soil is typically chosen as the treatment plan, which significantly increases the cost. The swelling characteristics of black cotton soil, however, has not been carefully studied by local agencies [1, 2].
Black cotton soil is a typical expansive soil. Studies have shown that its main mineral is montmorillonite. Montmorillonite is a 2:1 type layered aluminosilicate mineral which has a sandwiched layer of octahedral sheet of alumina between two layers of silica tetrahedral sheets [3, 4]. Its volume increases greatly when it absorbs water. In recent years, the researches and applications of computer molecular simulation technology have been used on the study of expansive soil owing to their special advantages [5-8]. As a typical expansive material, the characteristics of swell/shrink of montmorillonite have attracted wide attention [9-14]. The molecular simulation techniques applied to expansive soils mainly include Monte Carlo method (MC) and molecular dynamics method (MD) [15-17].
The objective of this study is to investigate the swelling characteristics of black cotton soil in East Africa in the molecular level using computer molecular simulation. Basic engineering properties of the soil samples were tested in the lab to evaluate its swelling potential. Molecular model of montmorillonite was carefully setup and constructed, and simulations were conducted. The water absorption characteristics of montmorillonite were investigated to explain its swelling characteristics.
2 Materials
Specimens of East-Africa black cotton soil were collected and tested in the lab to investigate its swelling properties. Standard test methods listed in Test Methods of Soils for Highway Engineering (JTG E40-2007) were followed in conducting the tests [18]. This section presents the lab test results.
2.1 Gradation and Atterberg limits
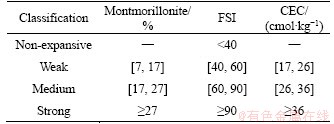
Table 1 presents gradation and Atterberg limits of East-Africa black cotton soil. East-Africa black cotton soil only contains sizes below 0.075 mm. For Specimens 3 and 4, clay contents (<0.002 mm) are as high as 64% and 84%, respectively. This shows the black cotton soil contains high amount of clay particles, which possesses high specific surface area and thus high swelling/shrinkage potential. Based on the Atterberg limits shown in Table 1, East-Africa black cotton soil is classified as typical expansive soil according to Test Methods of Soils for Highway Engineering (JTG E40-2007) [18].
2.2 Mineral composition
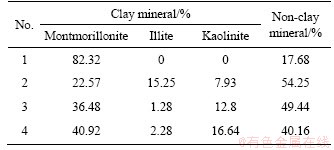
Mineral composition of East-Africa black cotton soil was obtained using X-ray diffractometer (XRD). The results are shown in Table 2. The classification of expansive soil based on montmorillonite content according to Code for Rock and Soil Classification of Railway Engineering (TB 10077-2001) and Test Methods of Soils for Highway Engineering (JTG E40-2007) are presented in Table 3 [18, 19]. East-Africa black cotton soil contains high clay mineral content, which is between 45.75% to 82.32% according to Table 2. Based on the classification method, it belongs to medium to strong expansive soil. Montmorillonite content varies among different specimens which were collected from different areas in East Africa. In Nairobi, Kenya (Specimen 1), the content of montmorillonite is as high as 82.32%. It is proven that montmorillonite directly affects the shrink-swell capacity of soil [20, 21].
2.3 Free swell index
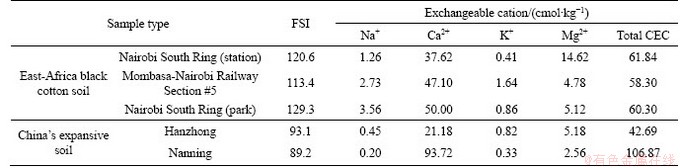
Free swell index (FSI) reflects the ability of soil to expand when wet and is one of the key indicators of expansive soil. The test results of FSI are presented in Table 4. Two types of China’s expansive soil were added as comparison. The classification of expansive soil based on FSI is presented in Table 3 [22]. Based on Tables 3 and 4, East-Africa black cotton soil is classified as strong expansive soil. Difference exists in types of exchangeable cations between China’s expansive soil and East-Africa black cotton soil. The East- Africa black cotton soil contains high percentage of exchangeable Mg2+. In the specimen from Nairobi South Ring (Station), the percentage of Mg2+ is as high as 23.64%. In addition, the percentage of Na+ in East-Africa black cotton soil is between 2.04% to 5.90%, while the percentage of Na+ in China’s expansive soil is between 0.19% to 1.05%.
Table 1 Gradation and Atterberg limits of East-Africa black cotton soil clay content
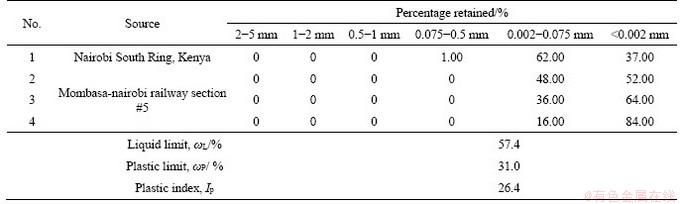
Table 2 Mineral composition of East-Africa black cotton soil (mass fraction)
2.4 Cation-exchange capacity
Cation-exchange capacity (CEC) is a measure of how many cations can be exchanged per mass of soil, usually measured in cmol/kg. It reflects the swelling potential of expansive soil. Standard test method of Test Method for Cation Exchange Capacity and Exchangeable Bases of Neutral Soil (NY/T 295-1995) was followed in conducting this test [23]. The test results of CEC are presented in Table 4. Two types of China’s expansive soil were added as comparison. The classification of expansive soil based on CEC is presented in Table 3 [19]. Based on Tables 3 and 4, East-Africa black cotton soil is classified as strong expansive soil.
Table 3 Classification of expansive soil based on CEC and FSI
Comparing China’s expansive soil with East- Africa black cotton soil, total CEC of Nanning expansive soil is higher than black cotton soil, while Hanzhong expansive soil is smaller than black cotton soil. Difference of exchangeable cation types exists between the two expansive soils. In East-Africa black cotton soil, exchangeable Mg2+ content is larger, accounting for up to 23.64% in the total CEC. Exchangeable Na+ content in East-Africa black cotton soil is not high but accounting for 2.04%-5.90% in the total CEC, while Na+ of exchangeable cation in China’s expansive soils accounts for only 0.19%-1.05%. On top of that, FSIs of China’s expansive soils are far less than East-Africa black cotton soils as shown in Table 4. Total CEC of Nanning expansive soil is as high as 106.87 cmol/kg, but its FSI is only 89.2%. By comparison, total CEC of the East-Africa black cotton soil (Nairobi South Ring Park) is 60.3 cmol/kg, but the FSI reaches 129.3%. This indicates that types and combinations of exchangeable cations significantly affect the water adsorption ability and swelling potential of expansive soil.
3 Molecular modelling
To further investigate the swelling characteristics of East-Africa black cotton soil,molecular simulation was conducted. In this study, Materials Studio from Accelrys of USA was used for molecular simulation, which offers a comprehensive simulation environment that enables researchers to construct, display and analyze structural models of molecules, solids, and surfaces, and to study and predict relative properties of materials.
Table 4 FSI and CEC of East-Africa black cotton soil and China’s expansive soil
3.1 Model setup
Since computer molecular simulation needs to be carried out under certain initial conditions and boundary conditions, this section briefly introduces the molecular simulation setup of black cotton soil swelling minerals (montmorillonite).
3.1.1 Potential model
For clay system, the potential energy model between water molecules mainly includes the Matsuoka-Clementi-Yoshimine (MCY) model of potential energy calculated from the ab initio algorithm of quantum mechanics, simple point charge (SPC), extended simple point charge (SPC/E) and transferable intermolecular potential 4-point (TIP4P) models calculated from the semi-empirical method [24-26]. The form of the MCY model is very complex and calculation time is very long. Most models used in the simulation are based on semi-empirical methods, which uses a simple potential energy function to represent the structure and properties of water [27-29]. The SPC/E model was used in this paper since it can well describe the equilibrium, structural and kinetic properties of water and is widely used in various systems of molecular simulation [30-32].
3.1.2 Force field and charge calculation
Universal force field (UFF) was selected in this study due to its wide applicability to different environments and suitability to the charge balance method (QEq) [33]. QEq was selected for charge calculation since it was developed together with UFF.
3.1.3 Boundary conditions
The periodic boundary condition was used since it met the requirements for simulating the interlayer adsorption characteristics of montmorillonite. The realization of this boundary condition is that when one particle passes through the hexagonal surface of the elementary molecular dynamics cell, the particle will pass through opposite surface at the same speed to reenter the molecular dynamics intracellular.
3.1.4 Initial conditions
The position pattern close to equilibrium must be used as the initial position for property-transition and time-dependent function calculation. The MC method achieves thermodynamic equilibrium very fast. Therefore, MC method and MD method are often used together in the simulation. The initial velocity of the molecule can be sampled according to the Maxwell-Boltzmann distribution, expressed as follows:
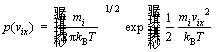 (1)
(1)
where vix is the velocity vector; m is the mass of the i-th particle; kB is the Boltzmann’s constant; T is the thermodynamic temperature.
The combination of prediction-correction method and Verlet algorithm was used in this study to solve the equations of motion in molecular dynamics.
3.2 Modelling of montmorillonite
The strong swelling potential of East-Africa black cotton soil mainly comes from the montmorillonite in its composition. This section establishes the model of montmorillonite minerals and water molecules in East-Africa black cotton soil based on the model setup presented in previous section.
3.2.1 Characteristics of montmorillonite
In the montmorillonite mineral structure, there are a wide range of isomorphic substitutions in tetrahedral and octahedral sheets. The silicon atoms in the tetrahedron are often replaced by aluminum atoms. Aluminum atoms in octahedrons are often replaced by magnesium atoms, iron atoms (tris valence or divalent), nickel atom, zinc atom, lithium atom plasma. The layer charge of montmorillonite mainly comes from the substitution of atoms in the octahedron, and only a very small part comes from the substitution of atoms in the tetrahedron.
The negative charges caused by the replacement of atoms in the tetrahedral and octahedral sheets are mainly balanced by the interlayer monovalent or divalent cations. Often these interlayer cations are hydrated. For the ideal tetrahedron, the interlayer cations are generally located in the tetrahedral hexagonal hole center, forming twelve coordination. For the Si4-yAly structure, especially the Si3.5Al0.5 superlattice, the interlayer cations may deviate from the center of the hexagonal holes, which may be located at the center of the tetrahedron bottom surface or in the tetrahedral space of the tetrahedron hole.
In the theoretical chemical composition of montmorillonite minerals, SiO2 accounted for 63.4%; Al2O3 accounted for 31.6%; H2O accounted for 5%. Due to isomorphism, the crystal structure of montmorillonite is Mx+(z+y)/x(H2O)n{(Al2-zMgz)· [(Si4-yAly)O10](OH)2}. Among them, Mx+ is an interlayer exchangeable cation, mainly including potassium (K+), calcium (Ca2+), sodium (Na+) and magnesium (Mg2+). In the crystal structural formula, H2O is written on the front, indicating that H2O is packed in the interlayer space with the exchangeable cation Mx+. Water in montmorillonite exists mainly in the form of interlayer water. The amount of interlayer water depends on the type of interlayer cations, temperature and humidity of the environment. Adsorption of water molecules exists in the structure layer in the form of sheet, up to four sheets. If the previous sheet is not full, a new water sheet won’t form typically. Illite is not considered in this study as its water absorption ability is far inferior to montmorillonite.
3.2.2 Construction of montmorillonite cell
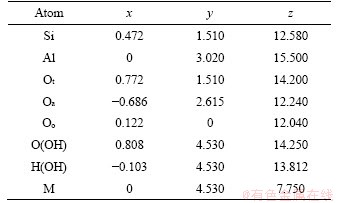
The basic parameters of the montmorillonite model are based on the Wyoming montmorillonite configuration of Skipper, Chang and Chavez-Paez [34-36]. The montmorillonite structure is monoclinic, the space group is C2/m and the symmetry type is L2PC. The lattice constants are as follows: a≈0.523 nm, b≈0.906 nm, c is variable, α=γ=90° and β=99°. C≈0.960 nm when there is no water between the structural layers. If there is water molecule between structural layers, the value of c will change in the range of 0.960-1.85 nm with the number of exchangeable cations and water molecules. Table 5 shows the spatial coordinates of each atom and interlayer cations in a unit cell with two-layer water molecule sheets constructed in this study, based on cell parameters of Skipper [34].
In this paper, the supercells are built as 4a×2b×c according to the actual need of atomic replacement, which means that the size of the supercell in x-o-y plane is 20.92  ×18.12
×18.12  . The isomorphism in the supercell is divided into two parts: one is that Si in the Si-O tetrahedron is replaced by Al, and the other is that Al in the Al-O octahedra is replaced by Mg. Considering that the majority of the host isomorphisms in montmorillonite occurs in the Al-O films, the adopted atomic substitution scheme is that one of every 32 Si atoms in the supercell is replaced by an Al atom, among which one of every eight Al atoms is replaced by a Mg atom. In addition, specific isomorphism operations in Materials Studio software are based on the following conditions: both octahedral and tetrahedral slices follow the Loewenstein rule that any two substitution sites of atoms are not adjacent. Due to isomorphism, the superlattice produces a layer charge of -6e and the layer charge is balanced by the interlayer cations. If the interlayer cations are monovalent, there will be six cations between the layers. The resulting crystal structure formula of the montmorillonite model is M0.75(H2O)n(Al3.5Mg0.5)(Si7.75Al0.25)O20(OH)4.Figure 1shows the constructed supercell cell model of sodium montmorillonite without water.
. The isomorphism in the supercell is divided into two parts: one is that Si in the Si-O tetrahedron is replaced by Al, and the other is that Al in the Al-O octahedra is replaced by Mg. Considering that the majority of the host isomorphisms in montmorillonite occurs in the Al-O films, the adopted atomic substitution scheme is that one of every 32 Si atoms in the supercell is replaced by an Al atom, among which one of every eight Al atoms is replaced by a Mg atom. In addition, specific isomorphism operations in Materials Studio software are based on the following conditions: both octahedral and tetrahedral slices follow the Loewenstein rule that any two substitution sites of atoms are not adjacent. Due to isomorphism, the superlattice produces a layer charge of -6e and the layer charge is balanced by the interlayer cations. If the interlayer cations are monovalent, there will be six cations between the layers. The resulting crystal structure formula of the montmorillonite model is M0.75(H2O)n(Al3.5Mg0.5)(Si7.75Al0.25)O20(OH)4.Figure 1shows the constructed supercell cell model of sodium montmorillonite without water.
Table 5 Space coordinates of each atom and interlayer cations in montmorillonite unit cell
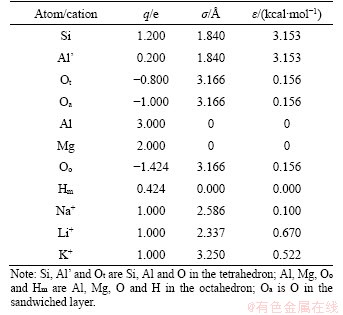
The Qeq charge balance method was used to optimize the charge in montmorillonite sheets and interlayer cations. The charge and Lennard-Jones potential energy parameters of different atoms and cations are shown in Table 6. This table fits nicely with atomic charge and Lennard-Jones potential energy parameters in the montmorillonite model obtained by Smith [37].
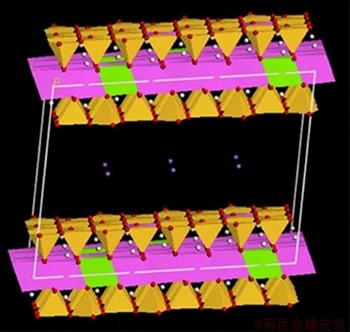
Figure 1 Supercell cell model of Na-montmorillonite in anhydrous state
Table 6 Charge and Lennard-Jones potential energy parameters for different atoms and cations in montmorillonite
After all parameters were determined, different montmorillonite configurations can be constructed accordingly. Previous study has pointed out that montmorillonites with different cation exchange capacity and compositions have different swelling potentials [38]. Therefore, to analyze the swelling characteristics of East-Africa black cotton soil and China’s expansive soil, representative montmorillonite structures were constructed.
Lab test results of FSI and CEC in the previous section have shown that type and combination of exchangeable cations have considerable impact on the water absorption capacity and the swelling potential of montmorillonite.
Therefore, in this study, three different montmorillonite configurations were established by setting up different interlayer exchangeable cations as shown in Table 7 to investigate the effect of high content of Mg2+ and certain amount of Na+ on the swelling potential of montmorillonite. The configuration of East-Africa black cotton soil is close to Types II and III and the China’s expansive soil is close to Types I and II.
Figure 2 illustrates the supercell of Type III montmorillonite without water. The pink octahedron represents the unaltered aluminum atom, the green octagon is the atomic position substituted with magnesium, and the yellow tetrahedron represents the unplaced position of the silicon atom. The pink tetrahedron is replaced by the aluminum atom, the azure, green and purple atoms in the middle are used to balance the negative charge of the interlayer calcium ions, magnesium ions and sodium ions.
3.2.3 Construction of water molecular model
The water molecules model was built directly in the Sketch atoms module in Materials Studio. Because water molecule was constructed manually, there is a big deviation of each bond energy. The Forceit module was used to optimize the water molecule with SPC\E energy model and UFF (Universal force field) mentioned above. The water molecule model after optimization is shown in Figure 3.
The total energy values before and after optimization as well as the charge and Lennard-Jones potential energy parameters of each atom after optimization are shown in Table 8. Total energy before optimization is 16.499 kcal/mol and is 0 after optimization. Data for charge q and Lennard-Jones potential energy parameters obtained in this table are very close to the results of Refs. [39, 40], which verifies the water molecule model in this paper.
4 Analysis of water absorption characteristics of montmorillonites
4.1 Water molecular absorption simulation
Sorption-fixed pressure module was used to simulate water absorption capability. Supercell c value is 1.55 nm in two-layer water molecules. Full-size water box was established in the cell interlayer. Metropolis method was used in the simulation with total simulation steps of 1×106 and balance steps of 1×105. Atom-based method was used for Van der Waals forces. The truncation radius was 0.9 nm. The bond tooth width was 0.1 nm and the buffer width was 0.05 nm. Ewald summation method was taken for electrostatic long-range action and the rejection radius was 6  . The results are shown in Table 9.
. The results are shown in Table 9.
Table 7 Composition of three configurations of montmorillonite

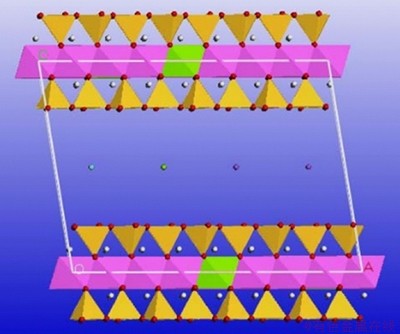
Figure 2 Type III montmorillonite supercell without water molecules
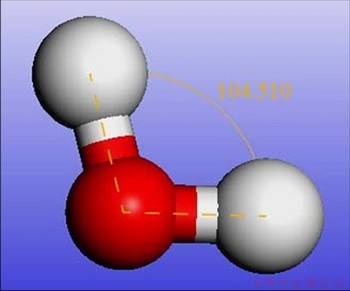
Figure 3 Water molecule model after optimization
As can be seen from Table 9, even if the layer spacing is fixed, there are still some differences in the amount of water molecules adsorbed by the three montmorillonite configurations. In the amount of water molecules adsorbed, Type III adsorbed the highest amount of water molecules and Type I adsorbed the least amount.
To further analyze the ability of adsorbing water molecules of Type III montmorillonite and Type I montmorillonite, statistical analysis of the adsorption energy distributions of water molecules in the two configurations was carried out. The results are shown in Figures 4 and 5. Comparing Figure 4 with Figure 5, it can be found that the adsorption energy of Type III montmorillonite is more sharply distributed with the maximum adsorption energy of 18 kcal/ mol and the average adsorption energy of 10 kcal/mol, while the maximum adsorption energy of Type I montmorillonite is only is 15.5 kcal/mol and the average adsorption energy is 9 kcal/mol.
The analysis shows that Type III montmorillonite adsorption capacity of water molecules is stronger and more stable. This also shows that the strong swelling potential of East-Africa black cotton soil comes from the strong water molecules absorption ability by high Mg2+ content and high Na+ content in montmorillonite.
4.2 Molecular mechanics optimization
In order to reasonably compare the structural properties of different montmorillonite configurations with the same quantity of adsorbed water molecules, the structural properties of three montmorillonite configurations in the two-layer water molecule state were studied. Studies have shown that one, two, and three layers of water are formed in the montmorillonite layered structure with 0.1, 0.2 and 0.3 kg water per kg of clay, respectively. The corresponding numbers of water molecules in the interlayer are about 32, 64 and 96, respectively, if converted into montmorillonite minerals established in this paper. 64 water molecules are inserted in the interlayer structure of montmorillonite by applying the sorption-fixed loading module. Then, montmorillonite superlattice containing two water molecules was obtained as shown in Figure 6. Montmorillonite is not at the natural state with the least energy, so it should be structurally optimized through molecular mechanics.
Table 8 Energy of water molecule before and after optimization and parameters of atoms in water
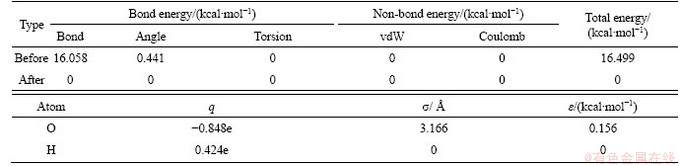
Table 9 Water molecules adsorption of three kinds of montmorillonite configuration

Forcite module was used to optimize the structure of montmorillonite unit cell through smart algorithm. The algorithm includes the conjugate gradient method, steepest descent method and Newton method. The software automatically assigns a suitable algorithm based on the model. Combined with the actual situation, clay layer is assumed as rigid, that is a, b, α, γ are fixed, and c and β are variable. It is worth mentioning that cation and water molecules are specified to move freely in the software. The parameters are set as follows for iteration accuracy of the geometric optimization: energy difference: 0.001 kcal/mol; root mean square (RMS) stress: 0.1 GPa; RMS force: 0.1 kcal/(mol· ); RMS displacement:0.003
); RMS displacement:0.003  ; the maximum number of iterations: 1000 or more (adjusted according to accuracy requirements); external pressure: 100 kPa, i.e.1 atm.
; the maximum number of iterations: 1000 or more (adjusted according to accuracy requirements); external pressure: 100 kPa, i.e.1 atm.
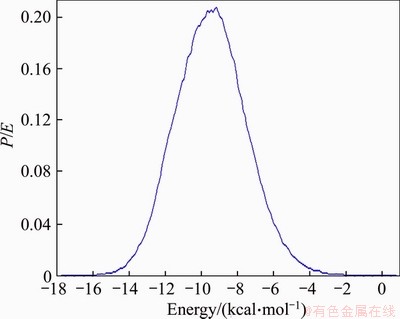
Figure 4 Type I montmorillonite water molecule adsorption energy distribution curve
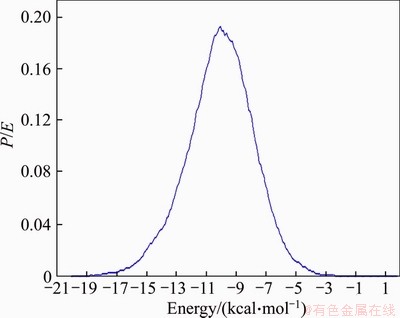
Figure 5 Type III montmorillonite water molecule adsorption energy distribution curve
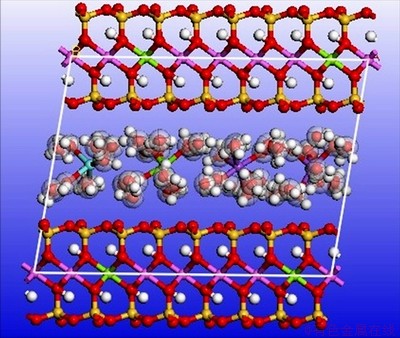
Figure 6 Superlattice of Type III montmorillonite with two hydration states
Figure 7 illustrates the variation of each deviation parameter in the process of molecular mechanics optimization of Type III montmorillonite. The optimized type III montmorillonite supercell with two hydration states is shown in Figure 8 below (only the lower half of the montmorillonite crystals is shown to make the image more intuitive).
Figures 6 and 8 show that coordination bonds are formed between the cations and the water molecules after adsorption of water molecules. The cations serve as coordination atoms and the water molecules serve as ligands. The coordination number is 6. The bonds did not break after optimization, indicating that stable complex ions were formed by water molecules and cations. The optimized state of montmorillonite model is closer to the actual state. The tetrahedral and octahedron became irregular polyhedrons after optimization. The positions of hydroxyl between the layers of montmorillonite are fixed and form the same angle with A-B plane before optimization. After optimization, angle between most of the hydroxyl and the A-B plane increase. There are also a few hydroxyls parallel to the A-B plane. This phenomenon indicates that strong interaction exists among the structural hydroxyl and the interlayer cations and water molecules. The results of this computer simulation are consistent with the experimental results. The energy values of each item before and after optimization of Type III montmorillonite are shown in Table 10. The following conclusions can be drawn from Table 10:
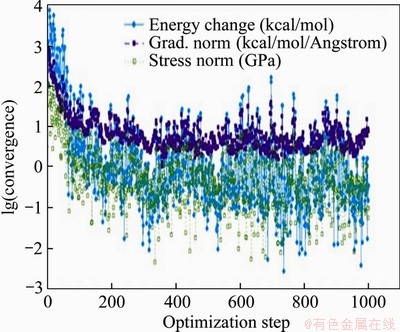
Figure 7 Type III montmorillonite during molecular mechanics optimization
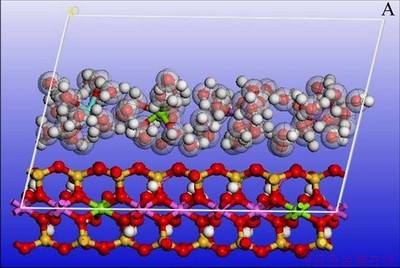
Figure 8 Optimized III-type montmorillonite with two hydration states
1) The total energy of the supercell of montmorillonite is greatly reduced compared with that before optimization.
2) After optimization, the energy of montmorillonite is dominated by the Coulomb force of non-valent bonds and the angular bending of the valence bonds, followed by the bond-bond stretching and Van der Waals forces (vdw).
3) During the energy optimization process, in addition to the slight increase of the structural distortion energy and Van der Waals energy, the remaining valent bond energy and non-valent bond energy are reduced by different degrees, in which bond-bond stretching of the valent bond energy and Coulomb force of non-valent bond energy decreases most greatly.
4) Other parameters such as RMS force, RMS stress, the maximum stress, the maximum atomic force and internal stress are reduced compared with those before optimization, which are not listed here.
5) The lab-tested density of montmorillonite containing a small amount of water (1 to 3 layers of water molecules), is 2.0-2.7 g/cm3. The calculated density of montmorillonite with two layers of water molecules in this paper is 2.4 g/cm3, which is very close to the experimental data. This also verifies the model built in this paper.
4.3 Molecular dynamics simulation
The purpose of molecular mechanics optimization is to find the most natural state of montmorillonite supercell as the initial state of molecular dynamics simulation to reduce the number of iterations and calculation time. The purpose of molecular dynamics simulation is to find the stable state of montmorillonite supercell movement of a certain ensemble.
The most important parameter in molecular dynamics simulation is the appropriate integration step, which ensures the optimal combination of computing time and accuracy. The integral step is typically less than one-tenth of the fastest motion cycle of the system. In order to reduce the iteration steps, NPT ensemble, which are amount of substance (N), pressure (P) and temperature (T), is used to find the natural energy state first in the molecular dynamics simulation. Then the final equilibrium structure simulated by the ensemble is used as the initial structure of the second NPT ensemble simulation. Finally, the structural and kinetic information of the system was statistically calculated by the second simulated hydration state of the montmorillonite molecular motion. The specific parameters and simulation process are as follows:
Table 10 Bond energy of Type III montmorillonite before and after optimization

1) NPT ensemble is used in the dynamics module to simulate at normal temperature and pressure (300 K, 1 atm). The simulation time is 200 ps. The time step is 0.5 fs and the total number of steps is 400000. Nose-Hoover is used as the temperature control function. The target temperature is 300 K. The relaxation time is 0.1 ps, which means that the Q ratio is 0.01. The pressure control function is Berendsen. The decay constant is 0.1 ps and the initial velocity is set to be random. The temperature curve fluctuates within 20°, as shown in Figure 9 below, indicating that the molecular dynamics simulation has reached convergence.
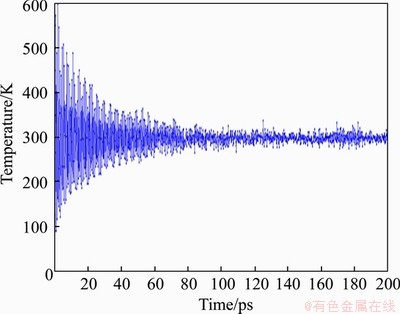
Figure 9 Temperature curve of molecular dynamics simulation
2) Based on the model obtained in the first step, NPT ensemble is used in the dynamics module, which is carried out under normal temperature and pressure (300 K, 1 atm). The simulation time is 200 ps, the time step is 0.5 fs and the total number of steps is 400000. The temperature control function is Nose-Hoover, the target temperature is 298 K, the relaxation time is 0.1 ps, which means that the Q ratio is 0.01. The pressure control function is Berendsen, the decay constant is 0.1 ps, the initial velocity velocities is Random. Simulation results calculate structural and kinetic information every 0.5 ps to analyze structural changes.
4.4 Hydration state of interlayer cations
The strength of montmorillonite swelling characteristics mainly depends on the adsorption capacity of montmorillonite cations on water molecules. To assess the interaction between cations and water molecules in the montmorillonite layer, it is necessary to analyze the hydration state of the cations therein. The hydration structure characteristics of the components in the interlayer of montmorillonite can be obtained according to the radial distribution function. Radial distribution function (RDF) refers to the spatially distribution probability (how far away from a given particle) of other particles after giving coordinates of a particle.
The definition expression of the radial distribution function is given in Eq. (2):
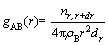 (2)
(2)
where nr,r+dr is the number of particle B surrounding particle A in a radius from r to r+dr in the shell; ρB is density of particle B.
Radical distribution function (RDF) analysis tools were used to obtain the radial distribution function between any two atoms in montmorillonite. Cations (Ca2+, Mg2+, Na+) and water molecular oxygen Ow in the three configurations of montmorillonite cells were analyzed and the results are shown in Figure 10. The data of the peak position value of the radial distribution function of cations and Ow are summarized in Table 11.
Figure 10 and Table 11 show that Na+-Ow forms the first peak near 2.225  ; Mg2+-Ow forms the first peak near 2.275-2.425
; Mg2+-Ow forms the first peak near 2.275-2.425  ; Ca2+-Ow forms the first peak near 2.375-2.625
; Ca2+-Ow forms the first peak near 2.375-2.625  . The peak position of Ca2+-Ow is the largest, followed by Mg2+-Ow, and Na+-Ow. This indicates that the ability of organizing oxygen atoms of different interlayer montmorillonite cations is different. Na+ is the easiest to organize oxygen atoms, followed by Mg2+, and Ca2+ is the hardest. The interaction between Na+ and water is the strongest (easiest to hydrate). In addition, the hydration radii of Mg2+ and Ca2+ in Type III montmorillonite are significantly lower than those of Types I and II montmorillonites, indicating that a small amount of Na+ promoted the hydration of Mg2+ and Ca2+. It shows that the hydration characteristics of interlayer compensatory cations have made a great contribution to the swelling characteristics of montmorillonite.
. The peak position of Ca2+-Ow is the largest, followed by Mg2+-Ow, and Na+-Ow. This indicates that the ability of organizing oxygen atoms of different interlayer montmorillonite cations is different. Na+ is the easiest to organize oxygen atoms, followed by Mg2+, and Ca2+ is the hardest. The interaction between Na+ and water is the strongest (easiest to hydrate). In addition, the hydration radii of Mg2+ and Ca2+ in Type III montmorillonite are significantly lower than those of Types I and II montmorillonites, indicating that a small amount of Na+ promoted the hydration of Mg2+ and Ca2+. It shows that the hydration characteristics of interlayer compensatory cations have made a great contribution to the swelling characteristics of montmorillonite.
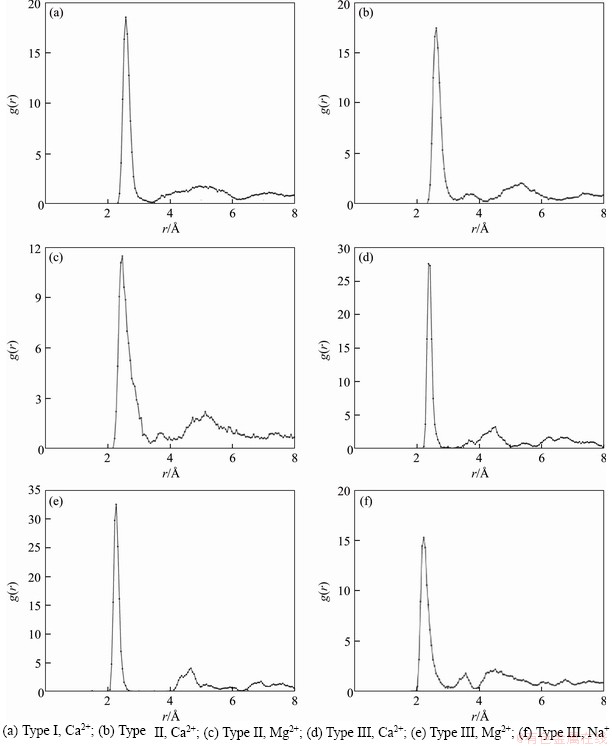
Figure 10 Radial distribution function of montmorillonite cation and water molecule oxygen:
Table 11 M-Ow peak positions of three configuration montmorillonite RDFs (A)
Based on the characterization of the cation RDFs of the three kinds of montmorillonite, the internal mechanism of the strong swelling potential of East-Africa black cotton soil can be further explained:
1) The montmorillonites in the East-Africa black cotton soil are mainly Types II and III montmorillonites, while the China’s expansive soils are mainly Types I and II montmorillonites. From the above analysis, it can be clearly seen that the cations in Types II and III montmorillonites have stronger hydration ability and more obvious solvating effect.
2) The main exchanging cations in the East-Africa black cotton soil is Ca2+, followed by Mg2+ and a small amount of Na+ as compensatory cation. However, Na+ promotes the hydration of Ca2+ and Mg2+, so the swelling potential of East-Africa black cotton soil is stronger.
5 Conclusions
The study focused on swelling characteristics of East-Africa black cotton soil. Lab test results of black cotton soil samples collected from East Africa were presented. Molecular model of montmorillonite was carefully setup and constructed. Three kinds of montmorillonite molecular cells were constructed according to the cation exchange capacity and type of East-Africa black cotton soil and expansive soil in China. Types II and III montmorillonite represent black soil montmorillonite in East-Africa, and Types I and II montmorillonite represent the expansive soil montmorillonite in China. Water molecular absorption simulation and optimization of molecular mechanics was conducted. The hydration state of interlayer cations was analyzed by radial distribution function (RDF). The following conclusions can be drawn from this study:
1) Lab test results show that East-Africa black cotton soil is typical strong expansive soil. It possesses high amount of clay, high liquid limit and plastic index, high free swell index, high amount of montmorillonite and exchangeable cations.
2) Water molecular adsorption simulation results show that Type III montmorillonite adsorb 10% more water molecules than Type I with the same layer distance. Molecular mechanics optimization successfully produced natural state of montmorillonite which is closer to actual state. The bonds did not break after optimization, indicating stable complex ions were formed by water molecules and cations.
3) Interlayer cations of Type III montmorillonite possesses the strongest water absorption ability based on analysis of RDFs of cations. Interlayer compensatory cations of Na+ enhances the hydration ability of the other major cations, thus resulting strong swelling potential of East-Africa black cotton soil.
References
[1] REDDY S S, PRASAD A, KRISHNA N V. Lime-staiblized black cotton soil and brick powder mixture as subbase material [J]. Advances in Civil Engineering, 2018: 5834685. DOI: 10.1155/2018/5834685.
[2] MALIK V, PRIYADARSHEE A. Compaction and swelling behavior of black cotton soil mixed with different non-cementitious materials [J]. International Journal of Geotechnical Engineering, 2018, 12(4): 413-419. DOI: 10.1080/19386362.2017.1288355.
[3] ZHAO Ping, XU Zheng-xuan, TANG Lin, ZENG De-li. Research on the black cotton soil swell-shrink characteristics and the depth of black cotton soil influenced by the atmosphere in Ethiopia [J]. Journal of Railway Engineering Society, 2014(4): 46-50. (in Chinese)
[4] ZHANG Jun-hui, DING Le, LI Feng, PENG Jun-hui. Recycled aggregates from construction and demolition wastes as alternative filling materials for highway subgrades in China [J]. Journal of Cleaner Production, 2020, 255: 120223. DOI: 10.1016/j.jclepro.2020.120223.
[5] KATTI D R, SRINIVASAMURTHY L, KATTI K S. Molecular modeling of initiation of interlayer swelling in Na–montmorillonite expansive clay [J]. Canadian Geotechnical Journal, 2015, 52(9): 1385-1395. DOI: 10.1139/cgj-2014-0309.
[6] AHMED H R, ABDULJAUWAD, S N. Nano-level constitutive model for expansive clays [J]. Geotechnique, 2016, 67(3): 187-207. DOI: 10.1680/jgeot.15.P.140.
[7] MA Tao, ZHANG De-yu, ZHANG Yao, WANG Si-qi. Simulation of wheel tracking test for asphalt mixture using discrete element modelling [J]. Road Materials & Pavement Design, 2018, 19(2): 367-384. DOI: 10.1080/14680629. 2016.1261725.
[8] ZHANG Jun-hui, PENG Jun-hui, LIU Wei-zheng, LU Wei-hua. Predicting resilient modulus of fine-grained subgrade soils considering relative compaction and matric suction [J]. Road Materials and Pavement Design, 2019. DOI: 10.1080/14680629.2019.1651756.
[9] FITYUS S, BUZZI O. The place of expansive clays in the framework of unsaturated soil mechanics [J]. Applied Clay Science, 2009, 43(2): 150-155. DOI: 10.1016/j.clay.2008. 08.005.
[10] WANG Gang, WEI Xing. Modeling swelling–shrinkage behavior of compacted expansive soils during wetting– drying cycles [J]. Canadian Geotechnical Journal, 2014, 52(6): 783-794. DOI: 10.1139/cgj-2014-0059.
[11] LIU Xian-feng, BUZZI O, YUAN Sheng-yang, MENDES J, FITYUS S. Multi-scale characterization of retention and shrinkage behaviour of four Australian clayey soils [J]. Canadian Geotechnical Journal, 2015, 53(5): 854-870. DOI: 10.1139/cgj-2015-0145.
[12] GAO You, SUN De-an, WU Ya-jun. Volume change behaviour of unsaturated compacted weakly expansive soils [J]. Bulletin of Engineering Geology and the Environment, 2017, 77: 837-848. DOI: 10.1007/s10064-017-1142-0.
[13] TANG Fan-long, MA Tao, GUAN Yong-sheng, ZHANG Zhi-xiang. Parametric modeling and structure verification of asphalt pavement based on BIM-ABAQUS [J]. Automation in Construction, 2020, 111: 103066. DOI: 10.1016/j.autcon. 2019.103066.
[14] ZENG Ling, XIAO Liu-yi, ZHANG Jun-hui, FU Hong-yuan. The role of nanotechnology in subgrade and pavement engineering: a review [J]. Journal of Nanoscience and Nanotechnology, 2020, 20(18): 4607-4618. DOI: 10.1166/ jnn.2020.18491.
[15] HANSEN E J M, TAMBACH T J, BLIEK A, SMIT B. Adsorption isotherms of water in Li-, Na-, and K- montmorillonite by molecular simulation [J]. The Journal of Chemical Physics, 2001, 115(7): 3322. DOI: 10.1063/ 1.1386436.
[16] ZHANG Yao, MA Tao, LING Meng, ZHANG De-yu, HUANG Xiao-ming. Predicting dynamic shear modulus of asphalt mastics by using the discretized element simulation and reinforcement mechanisms [J]. Journal of Materials in Civil Engineering, 2019, 31(8): 04019163. DOI: 10.1061/ (ASCE)MT.1943-5533.0002831
[17] MA Tao, WANG Hao, HE Liang, ZHAO Yong-li, HUANG Xiao-ming, CHEN Jun. Property characterization of asphalt and mixtures modified by different crumb rubbers [J]. Journal of Materials in Civil Engineering, 2017, 29(7): 04017036-1-10. DOI: 10.1061/(ASCE)MT.1943-5533. 0001890.
[18] Ministry of Transport of the People’s Republic of China. JTG E40-2007: Test methods of soils for highway engineering [S]. Beijing: China Communications Press, 2007. (in Chinese)
[19] Ministry of Railways of the People’s Republic of China. TB 10077-2001: Code for rock and soil classification of railway engineering [S]. Beijing: China Communications Press, 2001. (in Chinese)
[20] FATTAH M Y, SALIM N M, IRSHAYYID E J. Influence of soil suction on swelling pressure of bentonite-sand mixtures [J]. European Journal of Environmental and Civil Engineering, 2017: 1-15. DOI: 10.1080/19648189.2017. 1320236.
[21] DING Xun-hao, MA Tao, GU Lin-hao, ZHANG De-yu, HUANG Xiao-ming. Discrete element methods for characterizing the elastic behavior of the granular particles [J]. Journal of Testing and Evaluation, 2020, 48: 20190178. DOI: 10.1520/JTE20190178.
[22] Ministry of Transport of the People’s Republic of China. JTG C20-2011: Code for highway engineering geological investigation [S]. Beijing: China Communications Press, 2011. (in Chinese)
[23] Ministry of Agriculture of the People’s Republic of China. NY/T 295-1995: Test method for cation exchange capacity and exchangeable bases of neutral soil [S]. Beijing: China Communications Press, 1995. (in Chinese)
[24] LIE G C, CLEMENTI E. Molecular-dynamics simulation of liquid water with an ab initio flexible water-water interaction potential [J]. Physical Review A: General Physics, 1986, 33(4): 2679-2693. DOI: 10.1103/physreva.36.3935.
[25] BERENDSEN H J C, POSTMA J P M, GUNSTEREN W F V, HERMANS J. Interaction models for water in relation to protein hydration [J]. Intermolecular Forces, 1981: 331-342. DOI: 10.1007/978-94-015-7658-1_21.
[26] DING Xun-hao, CHEN Lu-chuan, MA Tao, MA Hai-xia, GU Lin-hao, CHEN Tian, MA Yuan, Laboratory investigation of the recycled asphalt concrete with stable crumb rubber asphalt binder [J]. Construction and Building Materials, 2019, 203: 552-557. DOI: 10.1016/j.conbuildmat.2019.01.114.
[27] ZHENG Y, ZAOUI A, SHAHROUR I. Evolution of the interlayer space of hydrated montmorillonite as a function of temperature [J]. American Mineralogist, 2010, 95(10): 1493-1499. DOI: 10.2138/am.2010.3541.
[28] ZHANG Yao, MA Tao, DING Xun-hao, HUANG Xiao-ming, XU Guang-ji. Impacts of air-void structures on the rutting tests of asphalt concrete based on discretized emulation [J]. Construction and Building Materials, 2018, 166: 334-344. DOI: 10.1016/j.conbuildmat.2018.01.141
[29] TANG Fan-long, MA Tao, ZHANG Jun-hui, GUAN Yong-sheng, CHEN Li-feng. Integrating three-dimensional road design and pavement structure analysis based on BIM [J]. Automation in Construction, 2020, 113: 103152. DOI: 10.1016/j.autcon.2020.103152.
[30] MARRY V, TURQ P. Microscopic simulations of interlayer structure and dynamics in bihydrated heteroionic montmorillonites [J]. Journal of Physical Chemistry B, 2003, 107(8): 1832-1839. DOI: 10.1021/jp022084z.
[31] CHEN Tian, LUAN Ying-cheng, MA Tao, ZHU Jun-qing, HUANG Xiao-ming, MA Si-jie. Mechanical and microstructural characteristics of different interfaces in cold recycled mixture containing cement and asphalt emulsion [J]. Journal of Cleaner Production, 2020, 258: 120674. DOI: 10.1016/j.jclepro.2020.121447.
[32] MA Tao, WANG Hao, ZHANG De-yu, ZHANG Yao. Heterogeneity effect of mechanical property on creep behavior of asphalt mixture based on micromechanical modeling and virtual creep test [J]. Mechanics of Materials, 2017, 104: 49-59. DOI: 10.1016/j.mechmat.2016.10.003.
[33] RAPPE A K, CASEWIT C J, COLWELL K S, GODDARD III W A, SKIFF W M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations [J]. Journal of the American Chemical Society, 1992, 114(25): 10024-10035. DOI: 10.1021/ja00051a040.
[34] HO T, CRISCENTI L J, GREATHOUSE J A. Revealing transition states during the hydration of clay minerals [J]. Journal of Physical Chemistry Letters, 2019, 10(13): 3704. DOI: 10.1021/acs.jpclett.9b01565.
[35] CHANG F R C, SKIPPER N T, SPOSITO G. Computer simulation of interlayer molecular structure in sodium montmorillonite hydrates [J]. Langmuir, 2002, 11(7): 2734-2741. DOI: 10.1021/la00007a064.
[36] CHAVEZ-PAEZ M, VAN WORKUM K, PABLO L. Monte Carlo simulations of Wyoming sodium montmorillonite hydrates [J]. Journal of Chemical Physics. 2001, 114(3): 1405-1413. DOI: 10.1063/1.1322639.
[37] SMITH D E. Molecular computer simulations of the swelling properties and interlayer structure of cesium Montmorillonite. Langmuir. 1998, 14(20): 5959-5967.
[38] LI Gui-li, ZHOU Chun-hui, FIORE S, YU Wei-hua. Interactions between microorganisms and clay minerals: New insights and broader applications [J]. Applied Clay Science, 2019, 177: 91-113. DOI: 10.1016/j.clay.2019.04. 025.
[39] ZHANG Fan. The modification of montmorillonite and its adsorption of arsenate and molecular simulation of hydrates [D]. Tianjin University, 2007.
[40] LI Bin. The effect of temperature on the interlayer structure of Na-, Cs- montmorillonite using molecular simulation [D]. Taiyuan: Taiyuan University of Technology, 2013. (in Chinese)
(Edited by ZHENG Yu-tong)
中文导读
东非黑棉土膨胀特性的计算机分子模拟
摘要:东非黑棉土是一种不稳定的公路铁路工程材料。由于黑棉土具有较强的膨胀性,易导致公路、铁路路基工程产生严重病害。本文对东非黑棉土的膨胀特性进行了深入研究。首先,对东非黒棉土原状土样的基本工程性质进行了室内试验和分析,结果表明,东非黑棉土的蒙脱石含量高,交换性阳离子数量大,为强膨胀性土。然后,采用分子模拟对蒙脱石的吸水特性进行了分析,根据东非黑棉土和我国膨胀土的层间阳离子特征建立了三种蒙脱石构型。径向分布函数分析结果表明,III型蒙脱石层间阳离子对水分子的吸附能力最强。层间补偿性阳离子增强了主要阳离子的水化能力,从而导致了东非黑棉土的强膨胀特性。
关键词:东非黑棉土;膨胀特性;蒙脱石;分子模拟;自由膨胀系数
Foundation item: Project(51878164) supported by the National Natural Science Foundation of China; Projects(BK20180149, BK20161421) supported by the Natural Science Foundation of Jiangsu Province, China; Project(KFJ170106) supported by Changsha University of Science & Technology via Open Fund of National Engineering Laboratory of Highway Maintenance Technology, China
Received date: 2020-03-25; Accepted date: 2020-04-16
Corresponding author: ZHU Jun-qing, PhD, Postdoctoral Researcher; Tel: +86-15150659102; E-mail: zhujunqing@seu.edu.cn; ORCID: 0000-0003-4134-4064
Abstract: Black cotton soil in East Africa is not a stable engineering material for highway and railroad projects. Its strong swelling potential when it absorbs water causes distresses in subgrade of highway and railroad, and thus leads to failures of the projects. This paper presents study on the swelling characteristics of black cotton soil in East Africa. Lab tests were conducted to obtain its basic engineering properties, and the results show that black cotton soil contains high amount of montmorillonite and exchangeable cations and is strong expansive soil. Molecular modelling was exploited to further investigate water absorption ability of montmorillonite. Three different molecular models of montmorillonite were constructed and used for simulations, among which Types I and II montmorillonite represent the expansive soil montmorillonite in China, and Types II and III montmorillonite represent black cotton soil montmorillonite in East Africa. The results showed that the interlayer cations of Type III montmorillonite possessed the strongest water absorption ability based on analysis of radial distribution function (RDF) of cations. Interlayer compensatory cations of Na+ enhance the hydration ability of the other major cations, thus resulting in the strong swelling potential of East-Africa black cotton soil.


