文章编号:1004-0609(2008)S1-0279-05
钴掺杂锰酸锂的合成与性能
王志兴,李新海,郭华军,彭文杰,张云河,胡启阳,刘久清
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:
通过X射线衍射(XRD)、扫描电镜(SEM)、粒度分析以及充放电性能测试对固相烧结法制备的LiCoxMn2-xO4(x=0,0.05,0.10,0.15,0.20)结构、形貌进行表征,并对电化学性质进行研究。研究结果表明,LiCoxMn2-xO4(x=0,0.05,0.10,0.15,0.20)均为单一尖晶石结构,无杂相存在;晶格常数随着掺杂量x的增大而线性减小;钴掺杂有助于LiCoxMn2-xO4晶体更规则地生长,使一次颗粒呈现八面体结构;掺钴对LiCoxMn2-xO4的平均粒径无明显影响;纯LiMn2O4在循环过种中容量衰减快,钴掺杂明显地改善了LiMn2O4充放电循环性能,且大电流放电能力提高;随着掺钴量的提高,大电流充放电性能与循环过程中容量的保持率也提高。
关键词:
中图分类号:TM 912.9 文献标识码:A
Preparation and electrochemical characterization of Co-doping lithium manganese oxide
WANG Zhi-xing, LI Xin-hai, GUO Hua-jun, PENG Wen-jie, ZHANG Yun-he, HU Qi-yang, LIU Jiu-qing
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Properties of LiCoxMn2-xO4(x=0, 0.05, 0.10, 0.15, 0.20) synthesized by solid state reaction were studied in the aspects of structure, and electrochemistry using X-ray diffractometry(XRD), scanning electronic microscopy(SEM), distribution of particle size and charge-discharge measurement. The results show that the pure phase exists with the same structure as spinel to all samples with and without cobalt-doping, not containing other minor impurity. The linear relationship of lattice parameter and Co-doping amount for LiCoxMn2-xO4 is determined by powder XRD refinement. The more regular octahedral morphology of primary particles is observed under scanning electron microscope, which indicates the doping of cobalt is beneficial to the growth of crystal. However, the particle size is not affected by cobalt doping. The cycleability and the rate capability are greatly improved by the introduction of cobalt to the lattice. The more the amount of manganese is substituted by cobalt, the higher the capacity retention and more excellent rate capability are obtained.
Key words: lithium ion batteries; lithium manganese oxide; spinel; cobalt doping; cathode materials
目前,锂离子电池在小型电器上被作为供电电源得到广泛应用[1]。迄今为止,商品化的锂离子电池正极材料主要采用LiCoO2[2]。但因为钴资源紧缺和价格昂贵,制约了这种材料在大型动力电池中的应用。在众多的锂离子电池用正极材料中,锰酸锂是一种很有应用前景的材料。由于合成锰酸锂的资源来源广泛,价格低廉,对环境友好及具有潜在的优良充放电特性等优点,近年来成为锂离子电池研发的焦点。
尖晶石LiMn2O4具有三维隧道结构,可快速充放电的特点[3]。在实际应用中,其可逆容量可达120 mA?h/g以上[4],能量密度达到LiCoO2的70%~80%。但其最大的缺点是,在循环过程中,容量衰减较快。
这是由于在充放电过程中,存在结构上的相变,且在目前的电解液体系中,发生Mn3+的歧化反应而溶解于电解液中,破坏了材料的结构与表面性能[5-6]。为了解决材料循环性能差的问题,人们对此进行了广泛研究。掺杂和表面处理是2种有效改善材料循环性能的手 段[7-9]。在以往的研究中,多采用锂作负极进行电化学性能测试,由于锰酸锂在充放电过程中存在锰的溶解现象,而这些溶出的锰在放电过程中可能在负极表面沉积,若采用炭负极,则其表面SEI膜将被改变,因此,在实际应用中必须考虑这种因素。本研究中,采用实际电化学体系对锰酸锂的电化学性能进行研究。
1 实验
将Li2CO3,MnO2和Co3O4按LiCoxMn2-xO4 (x=0,0.05,0.10,0.15,0.20)的化学计量比进行配料、混合,在马弗炉中于500 ℃反应5 h后,再升温到750 ℃反应10 h,完成第一次烧结。对得到的样品再过筛混合,然后,于750 ℃反应10 h,得到最终样品。
用日本Rigaku公司的X射线衍射仪对样品进行物相分析和晶格常数测量,用JEOL公司的JSM-5600LV 型扫描电子显微镜观察样品的表面形貌,用英国Malvern公司的Mastersizer-2000型激光粒度分析仪分析样品的平均粒径及粒径分布。采用全电池检测方法对电化学性能进行测试:将合成的正极活性物质LiCoxMn2-xO4(86 g),AB(乙炔黑,5 g)和溶解于NMP(n-甲基吡咯烷酮)的PVDF(聚偏二氟乙烯)(9 g)调成浆料。将浆料用极片涂布机涂敷在铝箔上制成正极片。本实验用于测试的电池为063048方型电池,以石墨作为负极,正负极之间用微孔聚丙稀和聚乙烯复合隔膜(型号Celgard 2400)隔开。电解液是溶于体积比为1?1?1的EC/DMC/EMC混合溶剂的LiPF6溶液,其浓度为1 mol/L。
采用BTS-51800型电化学测试系统(深圳市嵩和实业有限公司)对电池的电化学性能进行测试。采用恒流/恒压方法充电,而在恒流下进行放电。在(20±5)℃,以1C倍率充电至4.2 V后,改为恒压(4.2 V)充电,直至充电电流小于10 mA为止。放电速率特性测试中,分别以0.2C,1C和3C倍率分别对电池进行放电,终止电压为2.75 V。在循环性能测试中,将电池以1C倍率进行充放电,循环50次。
2 结果与讨论
图1所示为样品的X射线衍射谱。可以看出,它们均为正尖晶石结构,且不存在任何杂相,对比标准图谱可知,所得样品的空间点群均属于尖晶石结构的![]() (卡片号PDF35-0782)。通过粉末X射线衍射法精确测量得到样品的晶格常数,如图2所示。可以看出,LiCoxMn2-xO4的晶格常数随着x的增大而线性减小。这是因为掺杂过程中Co3+取代Mn3+,而Co3+的离子半径比Mn3+的离子半径小(高自旋和低自旋的Co3+离子半径分别为0.61×10-10 m和0.545×10-10 m,高自旋的Mn3+离子半径为0.645×10-10 m)[10],从而导致材料的晶格常数变小。
(卡片号PDF35-0782)。通过粉末X射线衍射法精确测量得到样品的晶格常数,如图2所示。可以看出,LiCoxMn2-xO4的晶格常数随着x的增大而线性减小。这是因为掺杂过程中Co3+取代Mn3+,而Co3+的离子半径比Mn3+的离子半径小(高自旋和低自旋的Co3+离子半径分别为0.61×10-10 m和0.545×10-10 m,高自旋的Mn3+离子半径为0.645×10-10 m)[10],从而导致材料的晶格常数变小。
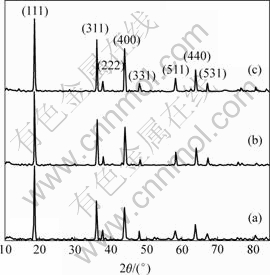
图1 LiCoxMn2-xO4的X射线衍射谱
Fig.1 XRD patterns of LiCoxMn2-xO4: (a) LiMn2O4; (b) LiCo0.1Mn1.9O4; (c) LiCo0.2Mn1.8O4
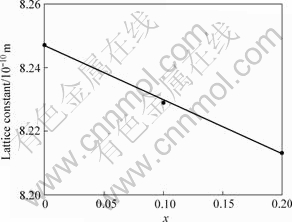
图2 LiCoxMn2-xO4的晶格常数与掺钴量x的关系
Fig.2 Relationship between lattice constant and Co-doped amounts x of LiCoxMn2-xO4
通过粒度分析,得到原材料与样品平均粒径,如表1所示。从表1可以看出,所有样品的粒径均大于原料MnO2的粒径,但随着钴取代量的提高,粒径的变化很小,从而可以推断,合成的样品粒径与样品中钴含量无关。
表1 MnO2和LiCoxMn2-xO4的平均粒径
Table 1 Average particle size of MnO2 and LiCoxMn2-xO4 (μm)
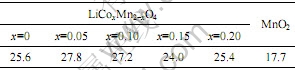
图3所示为样品与原料的扫描电镜分析结果。从低倍像(图3(a)、(b)和(c))看出,原料和样品的二次颗粒表面形貌无明显变化。但从高倍SEM像(图3(d)、(e)和(f))来看,LiMn2O4样品和LiCo0.15Mn1.85O4样品的一次颗粒粒度都比原料MnO2的大,且其晶粒呈现规则的形状。尤其从掺钴LiCo0.15Mn1.85O4的二次颗粒与LiMn2O4样品的二次颗粒来看,掺钴后呈现出规则八面体结构,表明掺钴有利于尖晶石晶体的生长。
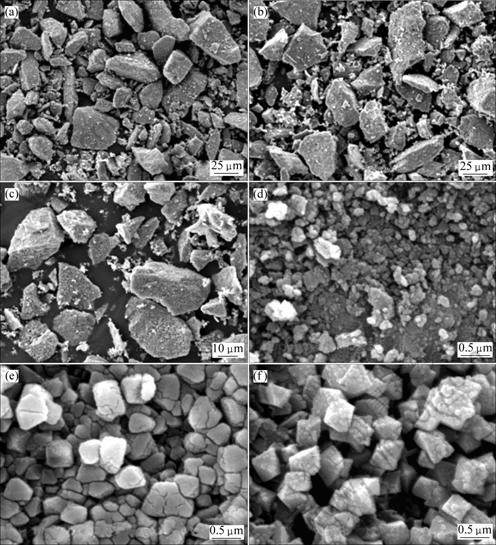
图3 MnO2和LiCoxMn2-xO4的SEM形貌
Fig.3 SEM images of MnO2 and LiCoxMn2-xO4: (a) MnO2; (b) x=0; (c) x=0.15; (d) MnO2; (e) x=0; (f) x=0.15
通过测试电池的放电性能,得到不同掺钴量的材料的放电曲线,如图4所示。从图4可以发现,LiMn2O4在高倍率下放电时,放电容量比低倍率下放电容量明显降低。为了进一步说明各材料的放电特性,根据图4得出放电性能的相关数据,见表2。
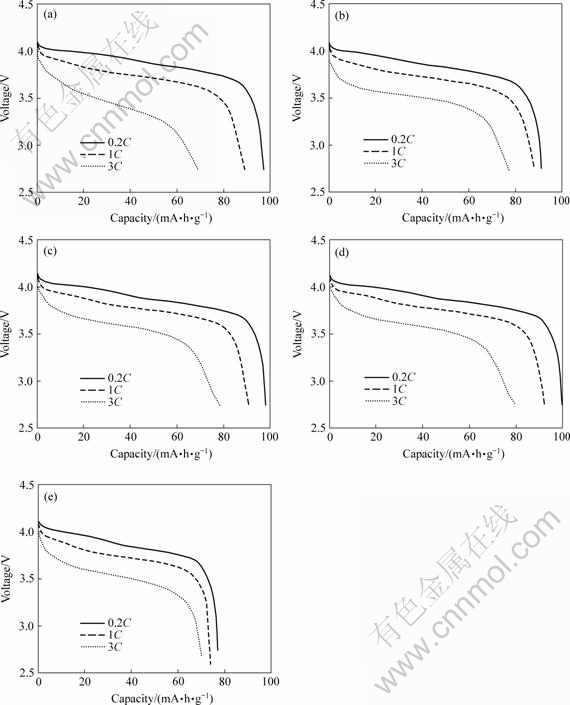
图4 不同倍率下LiCoxMn2-xO4的放电曲线
Fig.4 Discharge curves of LiCoxMn2-xO4 at different rates: (a) x=0; (b) x=0.05; (c) x=0.10; (d) x=0.15; (e) x=0.20
表2 不同倍率下LiCoxMn2-xO4放电性能对比
Table 2 Discharge performance of LiCoxMn2-xO4 at different rates
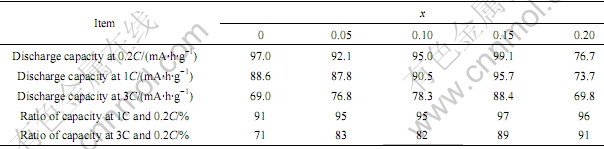
通过对比表2中1C,3C与0.2C倍率的容量之比可以看出,LiMn2O4的大电流放电性能不如掺钴后的性能,而且随着钴含量的提高,高倍率放电能力有增强的趋势。
图5所示为LiCoxMn2-xO4 的容量与循环次数的关系。可以看出,纯LiMn2O4经50次循环后容量损失率很高,达到31%;Co掺杂后电池的容量保持率提高。造成纯尖晶石LiMn2O4容量衰减快的主要原因之一是在充放电过程中存在Jahn-Teller效应,其来自于LiMn2O4中的Mn3+周围正八面体晶体场发生的畸 变[11]。掺钴后提高了电池的循环性能的主要原因是Co3+离子取代了其中部分的Mn3+,降低了Jahn-Teller效应造成的影响,使立方晶体结构在循环过程得以保持[8]。已有研究结果表明,掺钴后还可以减少锰的溶解[12]。
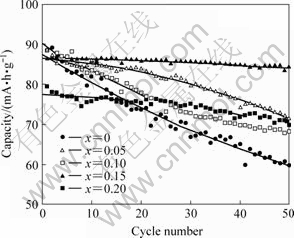
图5 LiCoxMn2-xO4的循环寿命曲线
Fig.5 Cycling performance of LiCoxMn2-xO4
3 结论
1) 对掺杂钴的锰酸锂的结构分析表明,LiCox- Mn2-xO4(x=0,0.05,0.10,0.15,0.20)均为单一尖晶石结构,且无杂相存在。晶格常数随着掺杂量x的增大而线性减小。
2) 钴掺杂有助于固相烧结法制备的LiCox- Mn2-xO4晶体更规则地形成,使一次颗粒呈现八面体结构。掺钴对LiCoxMn2-xO4的平均粒径无明显影响。
3) 检测由LiCoxMn2-xO4 (x=0,0.05,0.10,0.15,0.20)制成的电池的性能可知,纯锰酸锂的容量衰减快。钴掺杂明显地改善了LiMn2O4充放电循环性 能,且大电流放电能力提高。随着掺钴量的提高,大电流性能与循环过程中容量的保持率也越高。
4) 综合各性能指标,认为LiCo0.15Mn1.85O4是一种性能较好的锂离子电池正极材料。
REFERENCES
[1] NISHI Y. Lithium ion secondary batteries: Past 10 years and the future[J]. Journal of Power Sources, 2001, 100: 101-106.
[2] TANAKA T, OHTA K, ARAI N. Year 2000 R&D status of large-scale lithium ion secondary batteries in the national project of Japan[J]. Journal of Power Sources, 2001, 97/98: 2-6.
[3] THACKERAY M M. From gems to lithium battery electrodes: The significance of the diamond, ruby (sapphire), spinel and peridot structures[J]. Journal of Power Sources, 2001, 97/98: 7-12.
[4] THACKERAY M M, DAVID W I F, BRUCE P G, GOODENOUGH J B. Lithium insertion into manganese spinels[J]. Material Research Bullet, 1983, 18(4): 461-472.
[5] THACKERAY M M, HORN Y S, KAHAIAN A J, KEPLER K D, SKINNER E, VAUGHEY J T, HACKNEY S A. Structural fatigue in spinel electrodes in high voltage (4 V) Li/LixMn2O4 cells[J]. Electrochemical and Solid-state Letters, 1998, 1(1): 7-9.
[6] THACKERAY M M, JOHNSON C S, KAHAIAN A J, KEPLER K D, VAUGHEY J T, SHAO-HORN Y, HACKNEY S A. Stabilization of insertion electrodes for lithium batteries[J]. Journal of Power Sources, 1999, 81/82: 60-66.
[7] LI G, IKUTA H, UCHIDA T, WAKIHARA M. The spinel phases LiMyMn2-yO4(M=Co, Cr, Ni) as the cathode for rechargeable lithium ion batteries[J]. Journal of the Electrochemical Society, 1996, 143(1): 178-182.
[8] WANG Z, IKUTA H, UCHIMOTO Y, WAKIHARA M. Preparation and electrochemical properties of Stoichiometric and Nonstoichiometric LiCoxMn2-xO4-δ[J]. Journal of the Electrochemical Society, 2003, 150(9): A1250-A1254.
[9] KANNAN A M, MANTHIRAM A. Surface/chemically modified LiMn2O4 cathodes for lithium-ion batteries[J]. Electrochemical and Solid-State Letters, 2002, 5(7): A167-A169.
[10] SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides[J]. Acta Cryst, 1976, A32: 751-767.
[11] WAKIHARA M, YAMAMOTO O. Lithium ion batteries[M]. Kodansha Ltd, and Wiley-VCH Verlag GmbH, 1998: 30-31.
[12] SONG D, IKUTA H, WAKIHARA M. Cyclability and dissolution of manganese in substituted stoichiometric and non-stoichiometric lithium manganese oxides at high temperature[J]. Electrochemistry, 2000(6): 460-464.
基金项目:国家重点基础研究发展计划(973计划)项目资助(2007CB613607)
通讯作者:王志兴,教授,博士;电话:0731-8836633;E-mail: zxwang@mail.csu.edu.cn
(编辑 陈灿华)


