Trans. Nonferrous Met. Soc. China 25(2015) 4160-4166
Continuous changes in electrical conductivity of sodium aluminate solution in seeded precipitation
Gui-hua LIU, Zheng LI, Tian-gui QI, Qiu-sheng ZHOU, Zhi-hong PENG, Xiao-bin LI
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 18 January 2015; accepted 29 July 2015
Abstract:
The mechanism of seeded precipitation of sodium aluminate solution was studied by measuring the seeded-precipitation rate and electrical conductivity online, as well as calculating the activity and fraction of ion pair. The results show that the electrical conductivity of sodium aluminate slurry linearly decreases with increasing aluminum hydroxide addition. Moreover, both the electrical conductivity of slurry and the difference in electrical conductivity between sodium aluminate solution and slurry remarkably decline in the first 60 min before gradually increasing in the preliminary 10 h and finally reaching almost the same level after 10 h. In low Na2O concentration solution the activities of NaOH and NaAl(OH)4 in seeded precipitation are high, which can enlarge the difference in conductivity between slurry and solution. Additionally, more ion pairs exist in solution in preliminary seeded precipitation, and the adsorption of Na+Al(OH)4- on seed surface is likely to break the equilibrium of ion pair formation and to decrease the difference in conductivity in preliminary seeded precipitation.
Key words:
sodium aluminate solution; seeded precipitation; electrical conductivity; activity coefficient; ion pair;
1 Introduction
Seeded precipitation for preparing aluminum hydroxide (AH) is used in alumina refineries, including AH precipitated from pregnant sodium aluminate solution (Al(OH)4- Al(OH)3+OH-) and coarse AH crystallized by nucleation [1], agglomeration, and growth [2]. The mechanism of seeded precipitation was extensively studied by solubility of AH [3-5], electrical conductivity [6], surface tension [7], viscosity [8], activity [9] in the solution and the structure of aluminate ion. Based on kinetic studies [10], various methods, such as varying the concentration of caustic soda or alumina [11], adding active seed [12] and crystallization additives [13], irradiating with ultrasonic sound [14], and setting up an additional electric field or magnetic field [15] have been used to increase precipitation rate. However, the seeded-precipitation ratio in theory is greater than that in practice in the precipitation time range of 30-55 h. Low seeded- precipitation rate, long precipitation time, and low output of equipment are the disadvantages in alumina refineries for preparing sandy alumina. The main reason lies in the uncertainty of the mechanism in seeded precipitation with high seed content.
Al(OH)3+OH-) and coarse AH crystallized by nucleation [1], agglomeration, and growth [2]. The mechanism of seeded precipitation was extensively studied by solubility of AH [3-5], electrical conductivity [6], surface tension [7], viscosity [8], activity [9] in the solution and the structure of aluminate ion. Based on kinetic studies [10], various methods, such as varying the concentration of caustic soda or alumina [11], adding active seed [12] and crystallization additives [13], irradiating with ultrasonic sound [14], and setting up an additional electric field or magnetic field [15] have been used to increase precipitation rate. However, the seeded-precipitation ratio in theory is greater than that in practice in the precipitation time range of 30-55 h. Low seeded- precipitation rate, long precipitation time, and low output of equipment are the disadvantages in alumina refineries for preparing sandy alumina. The main reason lies in the uncertainty of the mechanism in seeded precipitation with high seed content.
The structure of aluminate ion is the key to understand the mechanism of seeded precipitation. Numerous researchers have studied the structure of aluminate ion in synthetic solution or solution from alumina refinery by filtration or centrifugation [16]. By measuring electrical conductivity, surface tension, viscosity or obtaining ultraviolet spectrum, infrared spectrum, Raman spectrum, or nuclear magnetic resonance (NMR) [17-19], the following conclusions about the aluminate ion structure can be drawn. Firstly, aluminate ion structure varies with the concentration, preparation method and hold time of solution [20-22]. Secondly, tetrahedral Al(OH)4- is the dominant form in sodium aluminate solution, though dimer ion [Al2O(OH)6]2- and other aluminate ion may exist in concentrated solution [6]. Thirdly, polymer aluminate anion, acting as growth units in precipitation, is aggregated from Al(OH)4- in seeded precipitation [23]. Fourthly, Na+, OH- or H2O also affect the forms of the aluminate ion because of the formation of ion hydration or ion pair [24,25]. All results suggest that various conversions among aluminate ions extremely affect the seeded precipitation. However, we often neglect the significant difference in aluminate ion structure in solution and slurry with high content of seed, and pay little attention on the influence of seed on the aluminate structure. Moreover, the changes in structure and mechanism of the preliminary period in seeded precipitation are rarely studied.
In this work, we investigated the influence of AH seed and solution composition on the electrical conductivity of sodium aluminate solution by detecting electrical conductivity online, discussed the variation of electrical conductivity based on the calculation of mean activity coefficient and ion pair, and further explored the mechanism of seeded precipitation. All results will benefit the development of new technology for seeded precipitation.
2 Experimental
2.1 Materials
Sodium aluminate solution was prepared with analytically pure AH and sodium hydroxide (Xilong Chemical Co., Ltd.). AH was added as the seed in precipitation. Seeded precipitation was conducted in an 1.5 L steel-tank which was heated by water bath. The electrical conductivity of solution was measured online by M300 conductivity meter (METTLER TOLEDO, Shanghai, Co., Ltd.).
2.2 Experimental procedures
1 L sodium aluminate solution with αk=1.41 (molar ratio of caustic soda (Na2O) to alumina (Al2O3) in sodium aluminate solution) was added into 1.5 L tank, heated to 55 °C and stirred at 150 r/min. AH was then added according to the seed coefficient Kr=3.4 (mass ratio of alumina in seed to alumina in solution). Meanwhile, the online conductivity meter was turned on, and the data were recorded simultaneously. Lastly, the slurry in seeded precipitation was obtained at a given time, and Na2O and Al2O3 concentrations were detected following the separation of solid and solution.
2.3 Methods
Na2O and Al2O3 concentrations in sodium aluminate solution were determined by titration. Afterwards, the seeded-precipitation rate (η) was calculated according to the following equation:
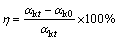 (1)
(1)
where subscripts 0 and t stand for initial time and time t in seeded precipitation, respectively.
The difference in conductivity value (△σ) in seeded precipitation was obtained by subtracting the electrical conductivity of the slurry from the electrical conductivity of the solution.
3 Results and discussion
3.1 Electrical conductivity of slurry in seeded precipitation at constant temperature
3.1.1 Influence of AH addition on electrical conductivity of slurry
The strong polarity of AH highly affects the electrical conductivity of sodium aluminate solution, as shown in Fig. 1.
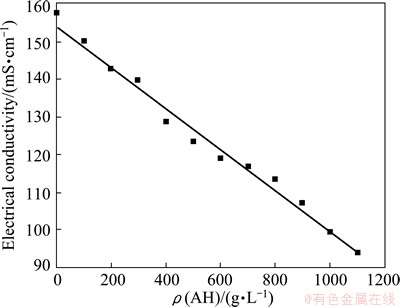
Fig. 1 Influence of AH addition on electrical conductivity of slurry
The test conditions are as follows: ρ(Na2O)=130 g/L; αk=1.45; seeded precipitation temperature 55 °C; stirring speed 150 r/min.
The electrical conductivity (σ) of the slurry linearly decreases with increasing AH concentration in the solution of 130 g/L Na2O (Fig. 1). The decrease of the electrical conductivity of the slurry can be attributed to the inversely induced-electrical field, resulting from the polar surface of AH. Afterwards, linear equation is obtained:
 (2)
(2)
where σ is the electrical conductivity, and ρ(AH) is the AH concentration, g/L. The correlation coefficient R2 is 0.985.
Based on Eq. (2), the influence of AH (seed and AH precipitated from solution) on the electrical conductivity of the slurry can be discussed.
3.1.2 Changes in electrical conductivity in preliminary seeded precipitation
The stability of the sodium aluminate solution is broken after adding seed. However, few researchers have reported changes in electrical conductivity in preliminary seeded precipitation. The electrical conductivity of sodium aluminate solution after adding seed in the first 60 s is shown in Fig. 2.
Interestingly, the electrical conductivity of the slurry sharply decreases at the first 30 s and then slowly increases, a minimum value at 37 s can be observed in Fig. 2. However, the seeded-precipitation rate is almost close to zero within 60 s because of good metastability of the sodium aluminate solution. This fact implies that aluminate ion structure remarkably changes in preliminary seeded precipitation.
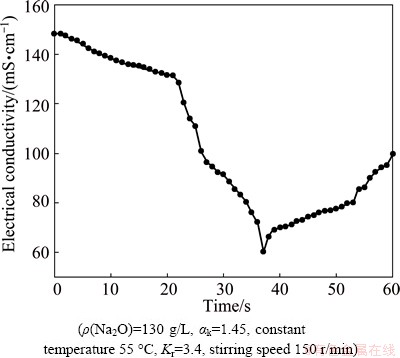
Fig. 2 Effect of time on electrical conductivity of slurry in preliminary precipitation
3.1.3 Influence of caustic soda concentration on seeded- precipitation rate and electrical conductivity of slurry
The effect of Na2O concentration and time on the seeded-precipitation rate at 55 °C is presented in Fig. 3.

Fig. 3 Effect of Na2O concentration on seeded-precipitation rate in sodium aluminate solution (αk=1.45, 55 °C, 150 r/min, Kr=3.4)
As shown in Fig. 3, the seeded-precipitation rate gradually increases with prolonging seeded precipitation time, and the seeded-precipitation rate in 130 g/L Na2O solution is greater than that in 200 g/L Na2O solution. However, the seeded-precipitation rate is only 0.62% in the 130 g/L Na2O solution at 20 min. Afterwards, significant increases with seeded-precipitation rate of 2.78% and 5.01% can be observed at 30 and 60 min, respectively. By contrast, the seeded-precipitation rates is less than 1% in sodium aluminate solutions of 170 and 200 g/L Na2O at 20 min, and low seeded-precipitation rates of 2% and 3% can be observed at 60 min for 170 and 200 g/L Na2O solution, respectively. Hence, few AH is precipitated in the first 1 h in the concentrated solution.
The electrical conductivity of the slurry in seeded precipitation is shown in Fig. 4.
Fig. 4 Effect of time on electrical conductivity of slurry in seeded precipitation (αk=1.45, 55 °C, 150 r/min, Kr=3.4)
Figure 4 indicates that the electrical conductivity of the slurry sharply increases at the first 5 h, and then almost levels off or increases very slowly in solution of 200 g/L Na2O, differing from the variation of seeded-precipitation rates in Fig. 3. Meanwhile, the conductivity in the dilute solution is greater than that in the concentrated solution, though more ions may exist in the concentrated solution. In addition, during the preliminary 60 min, electrical conductivity of the 130 g/L Na2O solution more remarkably increases than that of the 170 and 200 g/L Na2O solutions, which is in accordance with the seeded-precipitation rate in different solutions. The reason is mainly attributed to releasing free OH- ion in seeded precipitation according to the following equation:
Al(OH)4-=Al(OH)3+OH- (3)
Generally, Na+ concentration is constant in seeded precipitation at a given Na2O concentration, and the migration rate of OH- is faster than that of Al(OH)4- and Al2O(OH)62-. Thus, the electrical conductivity of the solution synchronously increases with the increase of the seeded-precipitation rate in theory. However, the electrical conductivity of the slurry sharply increases at the first 5 h, though all seeded-precipitation rates are less than 15%, and then slowly increase (almost levels off) after 10 h, though the seeded-precipitation rate still noticeably increases. All the above facts imply that the electrical conductivity is related to the variation of aluminate ions structure besides OH-.
3.2 Influence of AH addition on electrical conductivity of sodium aluminate solution
The results in Fig. 1 show a linear relationship between the electrical conductivity of slurry and AH concentration, but the effect of the seed on the electrical conductivity of the solution in seeded precipitation remains unknown. Therefore, the difference in electrical conductivity (Δσ) between slurry and solution is obtained according to the following procedures: obtaining the electrical conductivity of sodium aluminate solution at different concentrations, and subtracting the electrical conductivity from the electrical conductivity of the solution.
Electrical conductivity of sodium aluminate solution was measured according to an orthogonal design method at αk of 1.45-3.30, ρ(Na2O) of 123.03-205.60 g/L and 55 °C. The data are listed in Table 1.
Table 1 Electrical conductivities of sodium aluminate solution at 55 °C and different Na2O concentrations
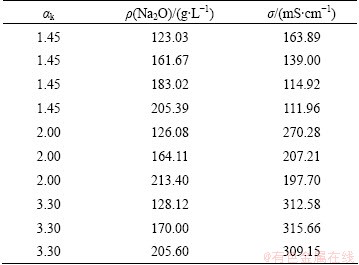
The following equation was obtained by data fitting:
σ=176.96-3.67ρ(Na2O)+249.21αk+0.0096ρ2(Na2O)-31.39αk2 (4)
The correlation coefficient R2 is 0.99.
Thus, the electrical conductivity of sodium aluminate solution in seeded precipitation was calculated according to Eq. (4) based on Na2O and Al2O3 concentrations in seeded precipitation. The difference in electrical conductivity (Δσ) in seeded precipitation is shown in Fig. 5.
Figure 5 shows that Δσ remarkably decreases before 2.5 h and then sharply increases, differing from the results in Fig. 4. Moreover, Δσ of 130 g/L Na2O solution increases more significantly after 25 h compared with that of the 170 and 200 g/L Na2O solutions. The increase in electrical conductivity of the solution is more than that of the slurry in seeded precipitation due to more OH- forming. Additionally, the sharp decrease of the electrical conductivity before 2.5 h is also attributed to notable changes in aluminate ion structure, confirming the significant influence of AH seed on the aluminate ion structure in Fig. 2. Both imply that aluminate ion complicatedly varies in seeded precipitation, which subsequently influences the mechanism of seeded precipitation.
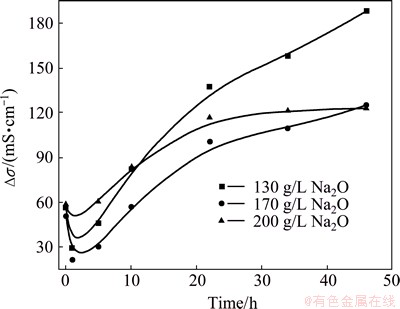
Fig. 5 Effect of time on electric conductivity difference in seeded precipitation (αk=1.45, 55 °C, 150 r/min, Kr=3.4)
3.3 Activity and fraction of ion pair for sodium aluminate in seeded precipitation
3.3.1 Activity of sodium aluminate and sodium hydroxide
Based on mean activity coefficient calculation model of sodium aluminate in NaOH-NaAl(OH)4-H2O system derived from Pitzer equation [3], changes in the mean activity coefficient of NaOH and NaAl(OH)4 in seeded precipitation can be determined. The activities of NaOH and NaAl(OH)4 are obtained according to Eq. (5).
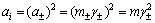 (5)
(5)
where subscript i stands for i substance, m and γ± represent mass molar concentration (mol/kg) and the mean activity coefficient, respectively. The results are presented in Fig. 6.
The results in Figs. 6(a) and (b) show that the mean activity coefficient of NaOH gradually decreases, whereas its activity slowly increases because of NaOH formation in seeded precipitation according to Eq. (3). Meanwhile, low concentration of Na2O increases the activity of NaOH compared with the electrical conductivity in 170 and 200 g/L Na2O solutions, which leads to the increase of the electrical conductivity and seeded precipitation. By contrast, the mean activity coefficient and activity of sodium aluminate increase in seeded precipitation, but its activity is lower than that of NaOH in seeded precipitation. Thus, the small increase of NaOH activity mainly is ascribed to the slow increase of electrical conductivity in Figs. 4 and 5.
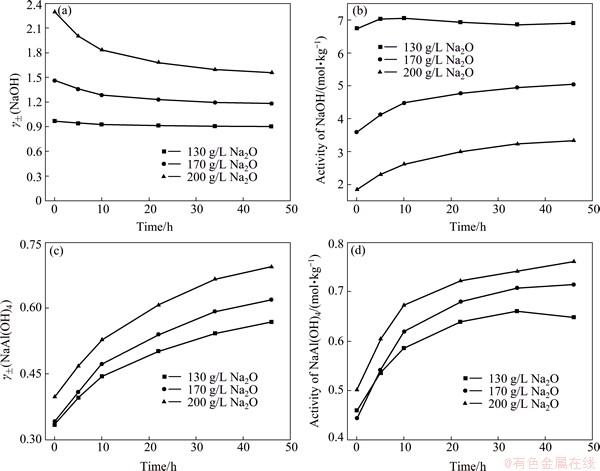
Fig. 6 Changes in mean activity coefficients and activities of sodium hydroxide and sodium aluminate in seeded precipitation
However, the remarkable decrease of Δσ in preliminary seeded precipitation may be caused by the formation of ion pair.
3.3.2 Changes in fraction of ion pair in seeded precipitation
Ion pair can be easily found in the concentrated solution. Given that various ion pairs may be formed in the sodium aluminate solution [26] and ion pair simplification is adopted, two kinds of ion pairs Na+OH- [3] and Na+Al(OH)4- [27] are calculated according to Bjerrum theory. The equation is written as follows:
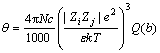 (6)
(6)
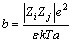 (7)
(7)
where θ is the ion associated degree, N is the number of ions, c is the molar concentration, Zi and Zj are ionic charges of ions i and j, respectively, ZiZj is associated with ionic charge number, e is the electric charge, ε is the dielectric constant of solution, and k is the Boltzmann constant, T is the thermodynamic temperature, a is the distance of ion pair.
The distances between Na+ and OH-, Na+ and Al(OH)4- are 3.31 and 3.595 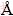 , respectively. Temperature is 328.15 K. Meanwhile, the total concentration of caustic soda (NaOHT) is equal to the sum of the concentration of sodium hydroxide c(NaOHf) and sodium aluminate, as shown in Eq. (8):
, respectively. Temperature is 328.15 K. Meanwhile, the total concentration of caustic soda (NaOHT) is equal to the sum of the concentration of sodium hydroxide c(NaOHf) and sodium aluminate, as shown in Eq. (8):
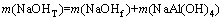 (8)
(8)
The fraction of ion pair is shown in Table 2.
Table 2 shows that the ion pair of Na+OH- is easily formed. Moreover, increasing the alumina concentration favors the formations of Na+OH- and Na+Al(OH)4-. The ion pairs of Na+OH- and Na+Al(OH)4- still occur in the later seeded precipitation because the fractions of ion pairs of Na+OH- and Na+Al(OH)4- in solution of ak=3.0 are 0.20 and 0.11, respectively. These results verify that ion pairs of Na+OH- and Na+Al(OH)4- in seeded precipitation affect the activity of aluminate ion and contribute to changes in Δσ.
Table 2 Fraction of ion pair for Na+OH- and Na+Al(OH)4- in different solutions
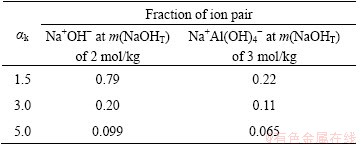
Ion pair of Na+Al(OH)4- exists in sodium aluminate solution, resulting in the low electrical conductivity as shown in Fig. 4. Meanwhile, AH surface is characterized by negative charge precipitated from sodium aluminate solution [27]. When AH seed is added, the adsorption of Na+Al(OH)4- on seeded surface also breaks up the following equilibrium: Na++Al(OH)4- Na+Al(OH)4-.
Na+Al(OH)4-.
Consequently, more free Na+ and Al(OH)4- in solution form Na+Al(OH)4- and subsequently decrease the electrical conductivity of the solution. Thus, significant decrease in Δσ in the preliminary period of seeded precipitation as mentioned in Fig. 6 can be observed. The finding provides a valuable reference in raising seeded precipitation rate. However, more evidences are required to support this conclusion.
4 Conclusions
1) The electrical conductivity of slurry linearly decreases with AH addition increasing in sodium aluminate solution; it sharply decreases at the preliminary stage and then remarkably increases, and almost levels off after 10 h in seeded precipitation.
2) The activities of NaOH and NaAl(OH)4 increase in seeded precipitation, and the high activity in dilute solution benefits the electrical conductivity. High concentration and low molar ratio facilitate the formation of ion pair, and ion pair still occurs in later seeded precipitation.
3) The activity of NaOH mainly contributes to the increase in electrical conductivity and difference electrical conductivity change in the seeded precipitation. The adsorption of Na+Al(OH)4- on seed surface may lead to the sharp decrease in electrical conductivity in preliminary seeded precipitation.
References
[1] SEYSSIECQ I, VEESLER S, BOISTELLE R, LAME'RANT J M. Agglomeration of gibbsite Al(OH)3 crystals in Bayer liquors: Influence of the process parameters [J]. Chemical Engineering Science, 1998, 53(12): 2177-2185.
[2] ZHOU Qiu-sheng, PENG Dian-jun, PENG Zhi-hong, LIU Gui-hua, LI Xiao-bin. Agglomeration of gibbsite particles from carbonation process of sodium aluminate solution [J]. Hydrometallurgy, 2009, 99(3-4): 163-169.
[3] LI Xiao-bin, YAN Li, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong. Thermodynamic model for equilibrium solubility of gibbsite in concentrated NaOH solutions [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(2): 447-455.
[4] LI Xiao-bin, YAN Li, ZHAO Dong-feng, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong, YANG Shuai-shuai, QI Tian-gui. Relationship between Al(OH)3 solubility and particle size in synthetic Bayer liquors [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1472-1479.
[5] BROWNE G R, FINN C W P. The effects of aluminum content, temperature and impurities on the electrical conductivity of synthetic bayer liquors [J]. Metallurgical Transactions B, 1981, 12(3): 487-492.
[6] WANG Ya-jing, ZHAI Yu-chun, TIAN Yan-wen, JI Zhi-ling, QU Xing-tao, YANG Xing-man. Mathematical model on surface tension of aluminate solution [J]. Nonferrous Metals, 2004, 56(3): 60-62. (in Chinese)
[7] LI J, PRESTIDGE C A, ADDAI-MENSAH J. Viscosity, density, and refractive index of aqueous sodium and potassium aluminate solutions [J]. Journal of Chemical and Engineering Data, 2000, 45(4): 665-671.
[8] WESOLOWSKI D J. Aluminum speciation and equilibria in aqueous solution: I. The solubility of gibbsite in the system Na-K-Cl-OH-Al(OH)4 from 0 to 100 °C [J]. Geochimica et Cosmochimica Acta, 1992, 56(3): 1065-1091.
[9] LI Xiao-bin, ZHAO Dong-feng, YANG Shuai-shuai, WANG Dan-qin, ZHOU Qiu-sheng, LIU Gui-hua. Influence of thermal history on conversion of aluminate species in sodium aluminate solution [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(10): 3348-3355.
[10] FARHADI F, BABAHEIDARY M B. Mechanism and estimation of Al(OH)3 crystal growth [J]. Journal of Crystal Growth, 2002, 234(4): 721-730.
[11] LIU Gui-hua, WANG Peng, QI Tian-gui, LI Xiao-bin, TIAN Lü, ZHOU Qiu-sheng, PENG Zhi-hong. Variation of soda content in fine alumina trihydrate by seeded precipitation [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(1): 243-249.
[12] ZENG Ji-shu, YIN Zhou-lan, CHEN Qi-yuan. Intensification of precipitation of gibbsite from seeded caustic sodium aluminate liquor by seed activation and addition of crown ether [J]. Hydrometallurgy, 2007, 89(1-2): 107-116.
[13] WU Yu-sheng, YU Hai-yan, YANG Yi-hong, BI Shi-wen. Effects of additives on agglomeration and secondary nucleation in seed precipitation in sodium aluminate solution [J]. Journal of Chemical Industry and Engineering, 2005, 56(12): 2434-2439. (in Chinese)
[14] ZHANG Bin, LI Jie, CHEN Qi-yuan, CHEN Guo-hui. Precipitation of Al(OH)3 crystals from supersaturated sodium aluminate solution irradiated with ultrasonic sound [J]. Minerals Engineering, 2009, 22(9-10): 853-858.
[15] LI Xiao-bin, WANG Dan-qin, ZHOU Qiu-sheng, LIU Giu-hua, PENG Zhi-hong. Influence of magnetic field on the seeded precipitation of gibbsite from sodium aluminate solution [J]. Minerals Engineering, 2012, 32: 12-18.
[16] GALE J D, ROHL A L, WATLING H R, PARKINSON G M. Theoretical investigation of the nature of aluminum-containing species present in alkaline solution [J]. Journal of Physical Chemistry B, 1998, 102(50): 10372-10382.
[17] CARREIRA L A, MARONI V A, SWAINE J W Jr, PLUMB R C, Raman and infrared spectra and structures of the aluminate ions [J]. The Journal of Chemical Physics, 1966, 45(6): 2216-2220.
[18] LI H X, ADDAI-MENSAH J, THOMAS J C, GERSON A. The influence of Al (III) supersaturation and NaOH concentration on the rate of crystallization of Al(OH)3 precursor particles from sodium aluminate solutions [J]. Journal of Colloid and Interface Science, 2005, 286(2): 511-519.
[19] SIPOS P, HEFTER G, MAY P M. 27Al NMR and Raman spectroscopic studies of alkaline aluminate solutions with extremely high caustic content—Does the octahedral species Al(OH)63- exist in solution? [J]. Talanta, 2006, 70(4): 761-765.
[20] BRADLEY S M, HANNA J V. 27Al and 23Na MAS NMR and powder X-ray diffraction studies of sodium aluminate speciation and the mechanistics of aluminum hydroxide precipitation upon acid hydrolysis [J]. Journal of the American Chemical Society, 1994, 116(17): 7771-7783.
[21] RADNAI T, MAY P M, HEFTER G T, SIPOS P. Structure of aqueous sodium aluminate solutions: A solution X-ray diffraction study [J]. The Journal of Physical Chemistry A, 1998, 102(40): 7841-7850.
[22] SWEEGERS C, MEEKES H, ENCKEVORT V W J P. Growth rate analysis of gibbsite single crystals growing from aqueous sodium aluminate solutions [J]. Crystal Growth & Design, 2004, 4(1): 185-198.
[23] GUTOWSKY H S, SAIKA A, Dissociation, chemical exchange, and the proton magnetic resonance in some aqueous electrolytes [J]. The Journal of Chemical Physics, 1953, 21(10): 1688-1694.
[24] LIPPINCOTT E R, PSELLOS J A, TOBIN M C. The Raman spectra and structures of aluminate and zincate ions [J]. The Journal of Chemical Physics, 1952, 20(3): 536.
[25] MOOLENAAR R J, EVANS J C, MCKEEVER L D. Structure of the aluminate ion in solutions at high pH [J]. The Journal of Physical Chemistry, 1970, 74(20): 3629-3636.
[26] SIPOS P. The structure of Al(III) in strong alkaline aluminate solutions—A review [J]. Journal of Molecular Liquids, 2009, 146(1-2): 1-14.
[27] LI Yun-feng. Study on surface modification and flame retardant of super-fine aluminium trihydroxide powder [D]. Changsha: Central South University, 2012. (in Chinese).
铝酸钠溶液种分过程中电导率的连续变化规律
刘桂华,李 铮,齐天贵,周秋生,彭志宏,李小斌
中南大学 冶金与环境学院,长沙 410083
摘 要:通过在线测定种分过程铝酸钠溶液的电导率,计算种分过程中组分的活度和缔合度,研究铝酸钠溶液的种分机理。结果表明:浆液电导率随着氢氧化铝含量增加呈线性降低;种分初期60 min内浆液电导率和铝酸钠溶液和浆液电导率的差值均显著降低,然后随种分的进行而显著升高,但在10 h后电导率增大幅度变小。低浓度溶液中NaOH和 NaAl(OH)4活度较高,有利于种分,使溶液和浆液间电导率差值增大;且种分初期溶液中离子对多,有利于Na+Al(OH)4- 吸附在晶种表面,导致溶液中生成离子对的平衡被打破,从而使电导率差值减小。
关键词:铝酸钠溶液;种分;电导率;活度因子;离子对
(Edited by Wei-ping CHEN)
Foundation item: Project (51274242) supported by the National Natural Science Foundation of China
Corresponding author: Zheng LI; Tel: +86-731-88877830; Fax: +86-731-88830453; E-mail: lizhengtctc@163.com
DOI: 10.1016/S1003-6326(15)64066-3
Abstract: The mechanism of seeded precipitation of sodium aluminate solution was studied by measuring the seeded-precipitation rate and electrical conductivity online, as well as calculating the activity and fraction of ion pair. The results show that the electrical conductivity of sodium aluminate slurry linearly decreases with increasing aluminum hydroxide addition. Moreover, both the electrical conductivity of slurry and the difference in electrical conductivity between sodium aluminate solution and slurry remarkably decline in the first 60 min before gradually increasing in the preliminary 10 h and finally reaching almost the same level after 10 h. In low Na2O concentration solution the activities of NaOH and NaAl(OH)4 in seeded precipitation are high, which can enlarge the difference in conductivity between slurry and solution. Additionally, more ion pairs exist in solution in preliminary seeded precipitation, and the adsorption of Na+Al(OH)4- on seed surface is likely to break the equilibrium of ion pair formation and to decrease the difference in conductivity in preliminary seeded precipitation.


