J. Cent. South Univ. Technol. (2011) 18: 1833-1837
DOI: 10.1007/s11771-011-0910-4![]()
Dissolution behavior of calcium-magnesium-silicate glass fiber
LIU Hao(刘浩), WANG Xi-tang(王玺堂), ZHANG Bao-guo(张保国), WANG Zhou-fu(王周福)
Key State Laboratory Breeding Base of Refractories and Ceramics,Wuhan University of Science and Technology, Wuhan 430081, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract:
The dissolution behavior of CaO-MgO-SiO2 glass fiber was investigated by scanning electron microscopy (SEM), Fourier-transform infrared spectrometer (FTIR) and inductively coupled plasma atomic emission spectroscopy (ICP-AES) using in-vitro tests. The results show that the soaked fiber is surrounded by an outer calcium-magnesium silicate hydrated layer, and there exists a balancing function between the formation and abscission of the hydrated layer during the dissolution process. The concentrations of leached ions increase constantly, and the mass loss of the fibers and pH changes of the solution are found to rise rapidly during the initial dissolution process, then their increasing rates are controlled by the balancing function of the hydrated layer at the subsequent dissolution stages. The dissolution rate constant and time for complete dissolution are estimated to be 274 ng/(cm2·h) and 15.2 d, respectively, presenting preferable biosolubilities.
Key words:
calcium-magnesium-silicate glass; glass fiber; dissolution; hydrated layer;
1 Introduction
The conventional aluminosilicate refractory ceramic fiber is classified as agents that are possibly carcinogenic to humans, that is, the dust particles of the conventional fiber may cause serious health effects such as cancer or fibrosis if inhaled in human respiratory system [1]. Thus, there has been increasing researches on the development of ceramic/glass fibers with increased biosolubility, on the premise of assuring the relevant fiber qualities for the different application areas [2].
Numerous studies have been conducted to estimate the dissolution properties of various inorganic fibers by using in-vivo and in-vitro tests [3-5]. In-vivo tests are usually carried out by three ways: long-term inhalation, abdominal and tracheal injection. These tests are fairly expensive and time-consuming, thus, most of the determinations are carried out by in-vitro by using physiological saline solutions [6-7]. In the fibers that have been investigated for dissolution tests, some additives (Al2O3, ZrO2, B2O3, and so on) in favor of fiber preparation could adjust the dissolution behavior and affect the surface morphology of the fibers [8]. However, as reported by WANG et al [8-9], the existence of Al2O3 and ZrO2 in the fibers could decrease the solubility, and B2O3 could decrease the serving temperature and increase the degree of shrinkage under high temperatures (>900 °C). Hitherto, a large number of researches have been investigated on the biosoluble ceramic/glass fibers. STRNADOVA et al [10] studied the dissolution rate of glass fiber in simulated lung fluid and indicated that the rate increased with increasing the flow rate. WANG et al [8] researched the effects of several additives on the change of ion (leached from fibers) concentrations in the solution. The simulated body fluid results by YOGANAND et al [11] showed the formation of carbonated hydroxyapatite like layer on the surface of CaO-MgO-SiO2 glass. In all the above studies, the additives and the immersing duration affected the dissolution behavior greatly. However, the dissoluition mechanisms of the fibers in simulated lung fluid had not been elucidated clearly, such as the relations between leaching of the ions and dissolution behavior and effects of outer layer on the degradation of fibers.
In order to investigate the dissolution mechanisms of CaO-MgO-SiO2 glass fiber in simulated lung fluid, the fibers have been prepared by melt-blowing method without the addition of Al2O3, ZrO2 or B2O3. The dissolution mechanisms (in-vitro flow-through test) of CaO-MgO-SiO2 glass fiber, especially the effects of its immersing time on the changes of ion concentrations, surface morphology and structure in simulated lung fluid, and relations between the outer hydrated layers and dissolution behavior were investigated. The dissolution rate of the as-prepared glass fibers was also evaluated based on the experimental data.
2 Experimental
The main chemical compositions of the studied CaO-MgO-SiO2 glass fiber are listed in Table 1. The dissolution of the fiber was investigated at 37 °C in simulated lung fluid with the initial pH of 7.4. Compositions of the fluid are shown in Table 2. To prevent the growth of algae or bacteria, 0.5 mL/L formaldehyde was added. Investigations of dissolution behavior for the mentioned fiber were carried out as shown in Fig.1. After being ground, about 2 g fragments of the fibers 50-100 μm in length were laid in the teflon reactor with 1 000 mL solution. The runs were performed by 0-72 h. After each run, the fibers were removed from the solution, then rinsed with deionized water and dried at 37 °C for 12 h in an oven before being weighed. After the solution was extracted from collection flask, the changes of the concentration of ions leached from the fibers in the solution were analyzed by an inductively coupled plasma atomic emission spectroscopic (ICP-AES) analysis.
Table 1 Main compositions of glass fiber (mass fraction, %)
![]()
Table 2 Compositions of simulated lung fluid
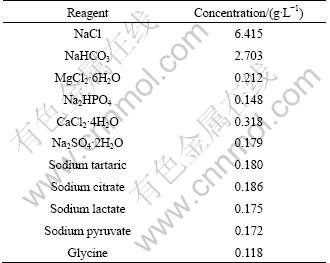
The surfaces of the exposed fibers were observed by a PHILIPS XL30 TMP scanning electron microscopy (SEM-EDS). Infrared spectra of the fibers were measured with a Fourier-transform infrared spectrometer (Vertex 70). The samples were in the form of KBr pellets containing approximately 5% (mass fraction) of fine fiber powder that was obtained by hand-milling. The spectra were collected in the wave-number range of 1 400-400 cm-1, and the data-points were averaged over 16 scans and obtained at an interval of 4 cm-1.
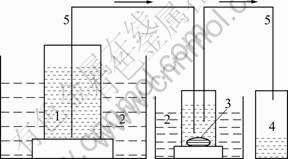
Fig.1 Experimental device for dissolution process: 1— Simulated lung fluid; 2—Water bath; 3—Fiber sample; 4— Collection flask; 5—Delivery conduit
3 Results
Figure 2 shows the changes of pH value of the simulated lung fluid for immersing sample for different durations. At the initial dissolution stage (0-12 h), the pH of the fluid increases rapidly from the original value of 7.4 to 8.3, then tends to increase slightly. Similar phenomenon is observed for the mass loss, the rate of mass loss increases rapidly during the initial 24 h to 7.5%, then tends to increase slowly, as shown in Fig.3.

Fig.2 Changes of pH value of simulated lung fluid versus immersing durations
The changes of main ion (Ca2+, Mg2+ and Si4+) concentrations in the simulated lung fluid are presented in Fig.4. The ion concentrations increase with increasing the immersing duration. The changing rates of the concentrations for the three ions are not the same, meaning the leaching of the ions in fibers is incongruent.
Figure 5 shows the SEM photographs of the fiber samples immersed in the fluid for 24 h (Fig.5(a)) and 72 h (Fig.5(b)), respectively. It can be seen that the immersed fiber is surrounded by an even hydrated layer at the initial dissolution stage (Fig.5(a)). As the dissolution proceeds, the outer layer becomes rough and uneven (Fig.5(b)), presenting the intensified erosion with long time soaking in the fluid.
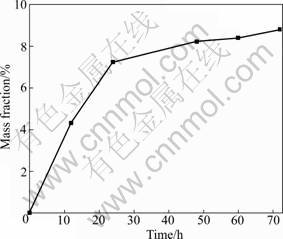
Fig.3 Mass loss of fibers versus immersing duration
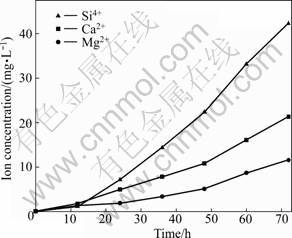
Fig.4 Changes of ion concentrations in simulated lung fluid versus immersing durations
Table 3 shows the results of the EDS analysis on the main compositions of the hydrated layer, which indicate that the residual hydrated layer is essentially siliceous. On the other hand, the EDS shows negligible K and Na concentrations. Samples (immersing time 24 h and 72 h) exhibit decreasing molar ratios of x(Si)/x(Ca+Mg) compared with the original sample (immersing time 0 h).
4 Discussion
4.1 Dissolution behavior
The studied fiber belongs to silicate glass system, which is susceptible to the corrosion of alkaline solution [12]. During the dissolution process, the alkaline earth cations (Ca2+ and Mg2+) were firstly leached out (Fig.4) from the surface of the fibers into solution with the following chemical reaction:
Si—O—R+H2O=Si—OH+R2++OH- (1)
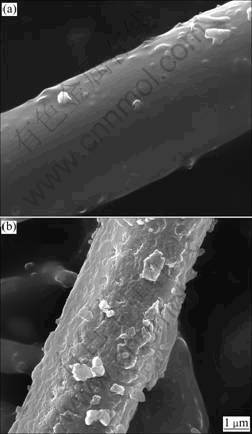
Fig.5 SEM photographs of fiber samples immersed in simulated lung fluid for 24 h (a) and 72 h (b)
Table 3 Composition determined on fiber surface by EDS

Thus, the pH value increased rapidly at the initial dissolution process (~12 h) with the increasing concentration of OH- (Fig.2). Besides, the OH- concentration and ions in the solution (Ca2+, Mg2+ and Na+) affect the dissolution of the fibers greatly. In neutral or slightly alkaline solution, OH- is not only adsorbed on the fiber surface but also coordinated by cations (Ca2+, Mg2+ and Na+) [13]. The adsorption and enhancement of concentration for OH- around the fiber surface increase the attack for silicate network. The leaching of alkaline earth cations also results in the loose and subsequent breakage of silicate network, which transforms into silicate ions.
As the dissolution proceeds, the leached and adsorbed alkaline cations could react with silicon ions, generating alkaline earth silicate, part of which might temporarily deposit on the fiber surface, forming an even hydrated layer around the fibers in the initial process (Fig.5(a)), and the layer becomes uneven and rough with prolonging the immersing duration (Fig.5(b)). The rest of the leached Ca2+, Mg2+ and Si4+ enters the solution, resulting in the continuous increasing concentration of the ions in the solution (Fig.4). According to the results of EDS analysis (Table 3), even if hydration variations exist from inside to outside, the composition of the hydrated layer might be assumed to be calcium- magnesium silicate hydrate.
The encapsulated layer around the soaked fiber decreases the dissolution rate. However, as shown in Fig.6, the hydrated layer could be washed away by the effect of fluid flow and the diameter of the residual fiber decreases compared with the original fibers. Then the etched surface of the individual fibers serves as a new site for restarting dissolution after the layer has been washed away, presenting a repeated state between the formation and abscission of the hydrated layer and a continual dissolution process for the fibers. Thus, there existed a balancing function between the formation and abscission of hydrated layer with the development of dissolution. Therefore, the increasing rate of pH tends to be slow after its rapid increase during the initial 12 h (Fig.2). Similar phenomenon is observed for the mass loss, which is an important parameter to evaluate the solubility of biosoluble materials [14]. That is, the rate of mass loss increases rapidly during the initial 24 h to 7.5%, and then tends to a slow increase controlled by the balancing function between the formation and abscission of hydrated layer (Fig.3).
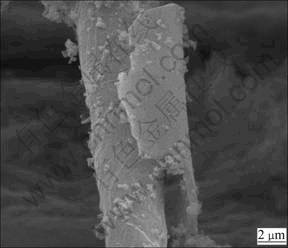
Fig.6 SEM photograph of fiber immersed for 72 h (Part of hydrated larger has been washed away by effect of fluid flow)
In order to obtain a further study on the surface state of the fibers, the FTIR transmittance spectra of two fiber samples (immersed for 0 and 72 h, respectively) were investigated (Fig.7). It has been known that the band at about 900 cm-1 is due to the stretching vibration of ≡Si—O—R, characterizing the alkali ions concentration. The bands at about 1 050 cm-1 and 465 cm-1 are assigned to anti-symmetric vibration and bending vibration of Si—O—Si within [SiO4], respectively, characterizing the concentration of SiO2 near the fiber surface [15-16]. After the fiber sample was immersed for 72 h, the band at about 910 cm-1 nearly disappears, and the intensity of the bands at about 1 050 and 465 cm-1 is weakened compared with the original fiber (0 h). All these can be attributed to the uniform leaching of alkali ions (Ca2+ and Mg2+) and corrosion on the fiber surface by the simulated lung fluid.
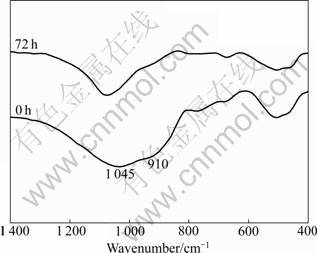
Fig.7 FTIR spectra of fiber samples (immersing time of 0 and 72 h)
4.2 Estimation of dissolution rate
Dissolution rate is an important parameter to evaluate the biosolubility of inorganic fiber, and it is typically characterized in terms of a dissolution rate constant, Kdis, as measured in in-vitro tests using simulated lung fluid [17]. In principle, Kdis can be used as a surrogate for in-vivo measurements. Usually larger value of Kdis means the shorter time a fiber resides in the body and less damage it will do. The dissolution rate constant of the studied fiber can be calculated by the following equation [18]:
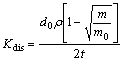 (2)
(2)
where Kdis is measured in units of nanograms per square centimeter per hour (ng/(cm2·h)); d0 is the initial fiber diameter (3-4 μm); ρ represents the initial density of the fiber (2.5-2.7 g/cm3), estimated from the quenching melt obtained during fiber preparation; m0 and m represent the initial mass and the mass of residual fiber after dissolution procedure (g), respectively; and t represents the test time.
Kdis can be used to calculate the estimated time, which is relevant for risk estimation purposes, for complete dissolution of fibers in lung tissue based on the densities and assumed fiber diameter. If the fibers are assumed to be cylinders with diameter that decreases as dissolution proceeds and the surface area of the fiber ends is neglected, the time for complete dissolution can be estimated from the following equation [7]:
t=2 083.3ρd0/Kdis (3)
With the test time of 72 h, Kdis of the studied fiber calculated from Eq.(2) is 274 ng/(cm2·h), which is higher by a factor of approximately 36 relative to the conventional aluminosilicate ceramic fiber. Assuming a fiber with diameter of 0.8 μm (the approximate average diameter of fibers found in the rat lung in several studies [19]), with the fiber density ρ and the calculated Kdis, the estimated time for complete dissolution of the studied fiber estimated from Eq.(3) is 15.2 d. It has been reported that the fiber with Kdis of 100 ng/(cm2·h) or larger, does not cause fibrosis or tumor in animal inhalation test [20]. However, with the existence of deviation for Kdis value tested using simulated lung fluid, the threshold of Kdis value for biosoluble ceramic/glass fiber should be at least 150 ng/(cm2·h). Based on that, the studied glass fiber with the designed composition possesses preferable biosolubilities.
5 Conclusions
1) During the dissolution process, Ca2+ and Mg2+ are firstly leached out from the fiber surface into the simulated lung fluid, resulting in the breakage of silicate network and subsequent leaching of Si4+. As the dissolution proceeds, the leached Ca2+, Mg2+ and Si4+ form a calcium-magnesium silicate hydrated layer on the fiber surface and there is a decrease of the residual fiber diameter after the leached layer is washed away.
2) The degradation rate of soaked fiber is rapid at the initial stage (~20 h), then it slows down by the balancing function between the formation and abscission of the hydrated layer.
3) The dissolution rate constant of the studied fiber with the designed composition is estimated to be 274 ng/(cm2·h), and the estimated time for complete dissolution of the fibers is 15.2 d, presenting preferable biosolubilities.
References
[1] WANG Xi-tang, LUO Chen-ze, ZHANG Bao-guo. Solution behavior of CaO-MgO-SiO2 system bio-soluble refractory ceramic fibers [J]. Key Eng Mater, 2007, 336/337/338: 1556-1558.
[2] EASTES W, BARON P A, BAIER R E, GULDBERG M, POTTER R. Do vitreous fibers break in the lung [J]. Inhal Toxicol, 2007, 19(4): 311-315.
[3] CAVALLO D, CAMPOPIANO A, CARDINALI G, CASCIARDI S, SIMONE P D, KOVACS D, PERNICONI B, SPAGNOLI G, URSINI C L, FANIZZA C. Cytotoxic and oxidative effects induced by man-made vitreous fibers(MMVFs) in a human mesothelial cell line [J]. Toxicol, 2004, 201(1/2/3): 219-229.
[4] BERNSTEIN D M. Special-purpose fiber type 475-toxicological assessment [J]. Inhal Toxicol, 2007, 19(2): 149-159.
[5] SZ?KE R, ALF?LDY B, BAL?SH?ZY I, HOFMANN W, SZIKLAI-L?SZL? I. Size distribution, pulmonary deposition and chemical composition of Hungarian biosoluble glass fibers [J]. Inhal Toxicol, 2007, 19(4): 325-332.
[6] SEBASTIAN K, FELLMAN J, POTTER R, BAUER J, SEARL A, MERINGO A D, MAQUIN B, REYDELLET A D, JUBB G, MOORE M, PREININGER R, ZOITOS B, BOYMEL P, STEENBERG T. EURIMA test guideline: In-vitro acellular dissolution of man-made vitreous silicate fibres [J]. Glass Sci Technol, 2002, 75(5): 263-270.
[7] MAXIM L D, HADLEY J G, POTTER R M, NIEBO R. The role of fiber durability/biopersistence of silica-based synthetic vitreous fibers and their influence on toxicology [J]. Regul Toxicol Pharm, 2006, 46(1): 42-62.
[8] WANG Xi-tang, ZHANG Bao-guo, WANG Zhou-fu, LIU Hao, ZHANG Shao-wei. Bio-soluble CaO-MgO-SiO2 system ceramic fibers [J]. Journal of Wuhan University of Science and Technology, 2008, 31(3): 238-241. (in Chinese)
[9] LIU Hao, WANG Xi-tang, WANG Zhou-fu, ZHANG Bao-guo. Effect of TiO2-coating on the crystallization behavior of CaO-MgO-SiO2 system ceramic fibers [J]. Journal of Wuhan University of Science and Technology, 2009, 32(1): 170-172. (in Chinese)
[10] STRNADOVA M, PODLOUCKA D, HAMACEK J, HELEBRANT A. The effect of heat treatment of glass fibers on their phase composition and solubility in simulated lung fluid [J]. Ceram Forum Int, 2005, 82(5): 40-43.
[11] YOGANAND C P, SELVARAJAN V, LUSVARGHI L, GOUDOURI O M, PARASKEVOPOULOS K M, ROUABHIA M. Bioactivity of CaO-MgO-SiO2 glass ceramics synthesized using transferred arc plasma (TAP) process [J]. Mater Sci Eng C, 2009, 29(5): 1759-1764.
[12] ZHANG Rui, XU Hong-liang, WANG Hai-long. Glass technology [M]. Beijing: Chemical Industry Press, 2008: 8-9. (in Chinese)
[13] OTTAVIANI M F, TOMATIS M, FUBINI B. Surface properties of vitreous fibers [J]. J Colloid Interface Sci, 2000, 224(1): 169-178
[14] MANUPRIYA, THIND K S, SINGH K, KUMAR V, SHARMA G, SINGH D P, SINGH D. Compositional dependence of in-vitro bioactivity in sodium calcium borate glasses [J]. J Phys Chem Solids, 2009, 70(8): 1137-1141.
[15] DUAN Ji-an, CAI Guo-hua, SHUAI Ci-jun. The relationship between IR characteristic peak and microstructure of the glass used as optical fiber [J]. Journal of Central South University of Technology, 2006, 13(3): 238-241.
[16] LI Yu-li, CHEN Xiao-feng, WANG Ying-jun, ZHAO Na-ru. Preparation and bio-mineralization in vitro of the sol-gel derived bioactive glass fiber [J]. J Inorg Mater, 2007, 22(4): 617-621. (in Chinese)
[17] UTELL M J, DANIEL MAXIM L. Refractory ceramic fiber (RCF) toxicity and epidemiology: A review [J]. Inhal Toxicol, 2010, 22(6): 500-521.
[18] LEINEWEBER J P. Solubility of fibers in vitro and in vivo. In: biological effects of man-made mineral fibers [R]. Copenhagen: World Health Organization, 1984.
[19] MAXIM L D, MAST R W, UTELL M J, YU C P, BOYMEL P M, ZOITOS B K, CASON J E. Hazard assessment and risk analysis of two new synthetic vitreous fibers [J]. Regul Toxicol Pharm, 1999, 30(1): 54-74.
[20] EASTES W, POTTER R M, HADLEY J G. Estimating in vitro glass fiber dissolution rate from composition [J]. Inhal Toxicol, 2000, 12(4): 269-280.
(Edited by HE Yun-bin)
Foundation item: Projects(50872098, 51004080) supported by the National Natural Science Foundation of China; Project(B0903) supported by the Opening Fund of Research Center of Green Manufacturing and Energy-saving & Emission Reduction Technology of Wuhan University of Science and Technology, China
Received date: 2010-10-22; Accepted date: 2011-02-05
Corresponding author: LIU Hao, PhD; Tel: +86-27-68862933; E-mail: wustlh@163.com
Abstract: The dissolution behavior of CaO-MgO-SiO2 glass fiber was investigated by scanning electron microscopy (SEM), Fourier-transform infrared spectrometer (FTIR) and inductively coupled plasma atomic emission spectroscopy (ICP-AES) using in-vitro tests. The results show that the soaked fiber is surrounded by an outer calcium-magnesium silicate hydrated layer, and there exists a balancing function between the formation and abscission of the hydrated layer during the dissolution process. The concentrations of leached ions increase constantly, and the mass loss of the fibers and pH changes of the solution are found to rise rapidly during the initial dissolution process, then their increasing rates are controlled by the balancing function of the hydrated layer at the subsequent dissolution stages. The dissolution rate constant and time for complete dissolution are estimated to be 274 ng/(cm2·h) and 15.2 d, respectively, presenting preferable biosolubilities.


