Trans. Nonferrous Met. Soc. China 30(2020) 3133-3146
Effect of salt-assisted reduction method on morphologies and size of metallic tungsten particles
Ze-peng Lü1,2, Kai-liang JIAN1,2, Jie DANG1,2
1. College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China;
2. Chongqing Key Laboratory of Vanadium-Titanium Metallurgy and Advanced Materials, Chongqing University, Chongqing 400044, China
Received 8 January 2020; accepted 31 July 2020
Abstract:
A simple method was proposed to produce tungsten (W) particles with controllable shape and size by employing the salt-assisted hydrogen reduction. W particles with controlled shape and size were prepared by adjusting the amount of chlorine salts and the temperature. After adding salt additives, the dispersibility of final particles was obviously improved and more adequate growth of particles was obtained. It was found that the effect of NaCl and LiCl is particularly significant. The average sizes of the obtained W particles at 1038 K after adding 0.1 wt.% NaCl and 0.1 wt.% LiCl were 0.924 and 1.128 μm, respectively. With the increase of temperature and amount of chlorine salts, the dispersity of the produced W particles became much better, the size of W sub-particles was increased,and the shape of W sub-particles was changed from spherical to polyhedral. At 1349 K, the addition of chlorine salts even multiplied the particle size, and the average sizes of W particles with 1 wt.% NaCl and 1 wt.% LiCl were raised up to 21.367 and 29.665 μm, respectively. Based on the conventional pseudomorphic transformation and chemical vapor transport mechanisms, the effects of adding salts on the reaction mechanism were investigated in detail as well.
Key words:
tungsten particles; salt-assisted hydrogen reduction; chlorine salt; morphology; size; controllable synthesis;
1 Introduction
Tungsten (W) is a silvery metal and exhibits numbers of outstanding properties: high melting point (the highest of all metals, 3695 K), low thermal expansion ((4.32-4.68)×10-6 K-1), high density, excellent electrical and thermal conductivity, high strength and yield point at elevated temperature, etc. [1] These advantages make tungsten and tungsten-base materials very attractive for a broad range of applications, such as tungsten-base films [2], filaments [3], electrodes [4], and electrical contacts [5]. Special performance also makes it compatible with glass and ceramics in the field of high temperature applications [6,7].
Over the past several decades, the powder production technology has been developing rapidly, and higher requirements for the quality of powders were also put forward, including surface property, fluidity of powders and particle size [8]. Thus, controllable physical properties of metallic tungsten powders can ensure the optimal properties of the product [9] and make them applicable for a series of high-end products [10,11]. The production of tungsten powders has been widely studied and has caught much attention of numerous researchers with the development of many production methods [12-14]. However, hydrogen reduction is still the dominant production method widely used in industrial production [15,16]. Due to the refractory characteristic of tungsten, it is still a difficult problem to synthesize metallic tungsten with desired morphologies and sizes in industrial production. Plenty of mixed oxide materials have been synthesized by the assisted molten salt synthesis (MSS) method, such as LaAlO3 (formed after 3 h at 903 K in KCl-KF liquid mixtures), MgAl2O4 (formed after 3 h at 1373 K in mixed chloride melts) and other examples including CaZrO3, KSr2Nb5O15, and TiC coatings [17,18]. In addition, various salts were used as additives in powder production [19-21]. For example, SUN et al [20] reported that the ultrafine and uniformed Mo crystals were obtained by adding few chlorine salts. ZHANG et al [21] investigated the effect of adding carbonate on the reduction of MoO2 to Mo by hydrogen. Particularly, lithium and natrium salts were often used as additives for the preparation of coarse-grained tungsten powders [22,23]. However, the previous studies mainly focused on the production of coarse-grained tungsten powders, and the effects of salts on morphology and dispersity of produced particles, and reaction rate were rarely reported.
Therefore, the present study aimed to prepare tungsten with the regulable and desired size and shape through the salt-assisted hydrogen reduction method, and further to reveal changes of dispersity of produced particles and reaction rate after adding salts (NaCl, LiCl and MgCl2). The effects of amount and kind of additives and temperature on the morphologies and size were also studied. The reduction mechanism after adding chlorine salts was investigated as well.
2 Experimental
2.1 Materials
The tungsten sources of WO3 powders (99.9%) were purchased from Shanghai Aladdin Bio- chemical Technology Co., Ltd and the assisted additives of NaCl (99.5%), LiCl (99%) and MgCl2 (99%) were purchased from Shanghai Titan Scientific Co., Ltd. In the present study, additives were first dissolved completely into deionized water, and then mixed with WO3 powders for 30 min under sonication. Whereafter, the mixture was dried at 373 K until turning into dry powders.
2.2 Experimental procedure
The experimental apparatus used in this study was presented in Ref. [19]. An Al2O3 crucible (8 mm × 8 mm) with around 100 mg samples was put into the system first, and then high-purity argon as the protective gas was injected into the furnace to eliminate air. Then, the furnace was raised to desired temperatures with a ramping rate of 10 K/min. After stabilizing the temperature, reaction atmosphere was switched from argon to hydrogen for a certain reaction time, and then pure argon would replace hydrogen and be introduced into the furnace again to protect samples during the cooling process. The gas flow rate was hold at a constant of 60 mL/min in all experimental runs. The phase compositions of all samples were analyzed by X-ray diffraction (XRD, PANalytical X’Pert, Panalytical B.V.) with Cu Kα radiation as the X-ray diffraction. Morphologies of these samples were characterized by SEM (TESCAN VEGA 3 LMH, Czech Republic) technique.
3 Results and discussion
3.1 Isothermal reduction
A thermo-gravimetric analyzer (TGA) (HCT-3, Beijing Hengjiu Instrument Ltd., China) was used to monitor the continuous change of samples during the process of isothermal reduction (1038-1349 K). Under all experimental conditions, the mass change increases smoothly to around -20.71% (Fig. 1) and XRD results confirm that the final products are pure W (Table 1). Because of only adding a small amount of salts, the diffraction peaks of additives are undetectable. It is worth noting that two slope discontinuities are observed in the TG (thermo- gravimetry) curves: the mass loss of 1.9% is for the reduction from WO3 to W18O49 and 6.9% is for the formation of WO2 from WO3, and thus the reduction pathway is expected as WO3→W18O49→ WO2→W. With the influence of the reaction temperature and additive, the visible change occurs in the reaction process. As shown in Fig. 1, the completion time of the reduction of pure WO3 by H2 at 1038, 1130, 1245 and 1349 K, corresponds to around 40, 17, 10 and 6 min, respectively. The reaction is noticeably accelerated with the temperature increasing. Changes in the kind of additives affect the reaction time in different ways based on analyzing DTG (differential thermo- gravimetry) curves and the reaction rate varies with different rules with changing the kind of additives. At the low temperature of 1038 K, the initial reaction rate (WO3→W18O49→WO2) of samples with 0.1 wt.% MgCl2 is very close to that of sample without additives, while other additives (LiCl, NaCl) more or less extend the reaction time, but in the later reduction stage (WO2→W) the reduction rates of samples with adding LiCl and NaCl increase apparently. With temperature increasing to 1130 K, the reaction rate of sample with 0.1 wt.% NaCl is accelerated significantly compared with that at low temperature. However, the reaction of sample with adding 0.1 wt.% LiCl still occurs slowly. While at 1245 and 1349 K, the reaction rates of samples with 0.1 wt.% NaCl and 0.1 wt.% LiCl are very close and higher than that of samples without additives, respectively. By contrast, the initial reaction rate for sample with 0.1 wt.% MgCl2 is the lowest at 1349 K.
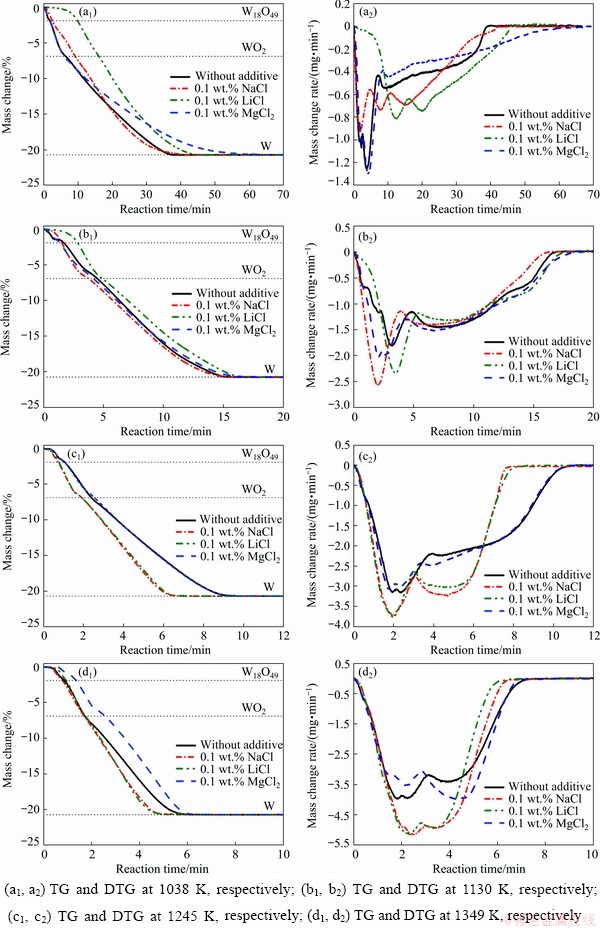
Fig. 1 Reduction curves of pure WO3 and mixed powders (by adding salts) with H2 at different temperatures
Table 1 Phases in samples with or without additives at different temperatures (based on XRD results, W PDF No. 4-806)
3.2 Effect of additives on morphologies and size distributions
Figures 2-5 show the morphologies and size distributions of the as-prepared metallic W particles obtained under different conditions. In order to figure out the effect of additives on the particle size, the statistical particle size is estimated from SEM images as well. As shown in Fig. 2, W powders obtained at 1038 K for reducing pure WO3 remain the raw shape of WO3, except forming some fissures and cracks because of the deoxidation. It should be noted that few sub-particles (around 0.486 μm) exist in the cracks, which was caused by the humidity (tiny amount of water vapor was formed and not carried out by the gas flow timely). With the increase of temperature, the surface of particles becomes more and more rough, and these dominant particles degrade gradually. Eventually, W powders degrade to a number of aggregated, irregular sub-particles (1.257 μm). The dispersity and size of W sub-particles are obviously affected by the reaction temperature.
3.2.1 Addition of 0.1 wt.% NaCl
Figure 3 shows the morphologies and size distributions of obtained metallic W by reduction of WO3 (with 0.1 wt.% NaCl) at different temperatures. The addition of 0.1 wt.% NaCl makes radical changes in the shape of sub-particles. At 1038 K, although the large particles also maintain the parallelepiped-shaped and irregular blocky-shaped morphology, a great number of small spherical sub-particles (0.924 μm) are formed in the large particles. This is similar with the products obtained by reducing pure WO3 at 1130 K; however, the final product for samples with 0.1 wt.% NaCl has a more uniform size and a more spherical shape. With the temperature increasing and under the action of NaCl, the large particles degrade dramatically and become more and more dispersed, the size of sub-particles increases and the shape changes from spherical to polyhedral. Especially at 1245 and 1349 K, the sizes of obtained sub-particles are 6.696 and 7.961 μm respectively, being over two times larger than those obtained at 1130 K.
3.2.2 Addition of 0.1 wt.% LiCl
Figure 4 shows the effect of addition of 0.1 wt.% LiCl on the morphologies and sizes of as-prepared W sub-particles. It can be clearly seen that the addition of LiCl has a similar effect on the final products compared with the addition of NaCl as a whole: the obtained W sub-particles are more dispersed and become larger with increasing temperature. However, the obtained sub-particles are around 1.5 times as large as those of adding NaCl at 1245 and 1349 K, respectively. Moreover, the shape of sub-particles is more regular, and at 1130 K, even regular polyhedrons appear.
3.2.3 Addition of 0.1 wt.% MgCl2
The morphologies and size distributions of samples with 0.1 wt.% MgCl2 completely reduced at different temperatures are shown in Fig. 5. At 1038 K, it seems that the addition of MgCl2 has little effect on the products, but it hiders the dispersion of final sub-particles at 1130 K. However, when the temperature increases to 1245 K, large particles begin to degrade into a large number of irregular small sub-particles (1.126 μm). With further increasing temperature to 1349 K (Fig. 5(d)) the obtained sub-particles have a similar shape with smaller and more uniform size (3.624 μm), which is larger than those prepared by the reduction of pure tungsten oxides (1.257 μm) but smaller than those obtained by reducing samples with adding NaCl and LiCl. What’s more, the shape is more regular at 1349 K.

Fig. 2 Morphologies and size distributions of samples without additive completely reduced at different temperatures
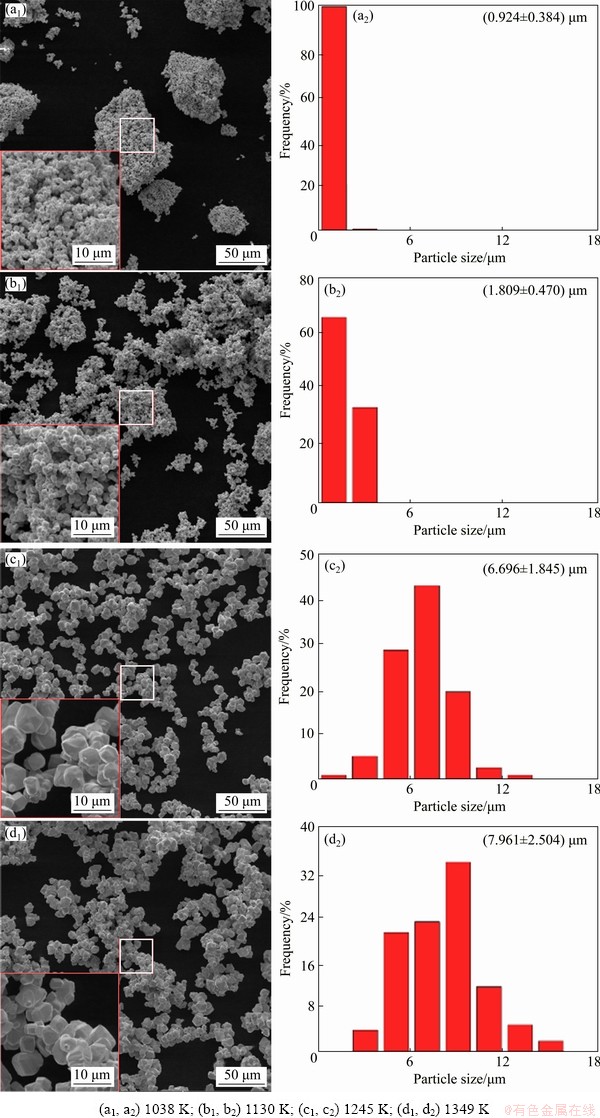
Fig. 3 Morphologies and size distributions of samples with 0.1 wt.% NaCl completely reduced at different temperatures

Fig. 4 Morphologies and size distributions of samples with 0.1 wt.% LiCl completely reduced at different temperatures
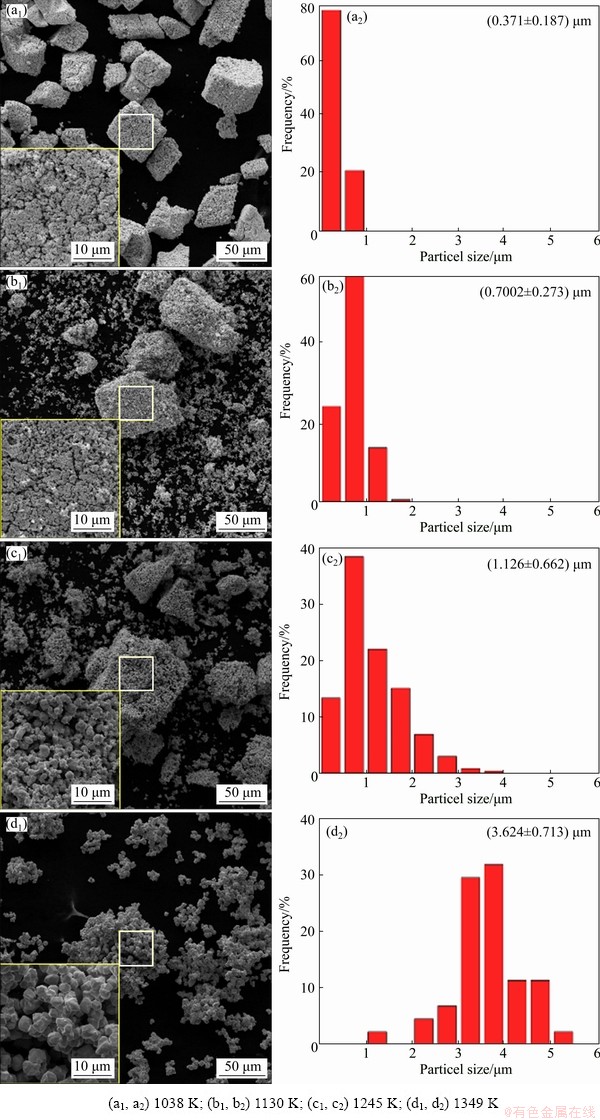
Fig. 5 Morphologies and size distributions of samples with 0.1 wt.% MgCl2 completely reduced at different temperatures
By summarizing the above morphological analyses, the schematic diagrams of final products obtained under different conditions are shown in Fig. 6. Both additive and temperature affect the evolution tendency obviously: (1) addition of salts and the increase of temperature cause the shape of sub-particles changing from spherical to polyhedral; (2) besides, additives can also promote particles to degrade to different degrees, making them more dispersed; (3) at higher temperature (especially 1349 K), chlorine salts are visible to promote the growth of sub-particles. Here, the effect of amount of additives on the morphologies and size distributions of the final product was studied with NaCl and LiCl as representatives, and SEM images of samples (with 1 wt.% NaCl and 1 wt.% LiCl) completely reduced are shown in Figs. 7 and 8, respectively. It can be seen that the size of final products by adding 1 wt.% NaCl (Fig. 7) is larger than that obtained by adding 0.1 wt.% NaCl (Fig. 3) apparently, and the amplification increases rapidly with the increase of temperature. Furthermore, at 1349 K (Fig. 7(d)), the molten matter appears on the surface of sub-particles and the sub-particles have a fairly obvious polyhedral shape with edges and corners. The changes of morphology and size of final products for sample with 1 wt.% LiCl have similar rules with those of the sample added with 1 wt.% NaCl, but the particle size is much larger for sample added with 1 wt.% LiCl (Fig. 8).
4 Reduction mechanism

Fig. 6 Schematic diagram of as-prepared metallic tungsten

Fig. 7 Morphologies and size distributions of samples with 1 wt.% NaCl completely reduced at different temperatures
In industrial production process of tungsten by hydrogen reduction [15,16], pseudomorphic transformation and chemical vapor transport (CVT) are two different reaction mechanisms which have significant effects on the properties of the product. These are also verified in the production of Mo [24]. The volatile phase (WO2(OH)2), formed via the following Reaction (1), makes the morphologies of the products different through its formation, transference and reduction (Eq. (2)). The change of reaction mechanism with temperature increasing should be the main reason resulting in different shapes and sizes of final products for the precursor without any additives. And visible results obtained from Fig. 2 support the hypothesis directly.
WO3(s)+H2O(g)=WO2(OH)2(g) (1)
WO2(OH)2(g)+3H2(g)=W(s)+4H2O(g) (2)
However, the morphologies and sizes of W particles change visually after adding additives. The effects of additives on the reaction mechanism can be analyzed as follows.

Fig. 8 Morphologies and size distributions of samples with 1 wt.% LiCl completely reduced at different temperatures
(1) The melting points of chloride salts NaCl, LiCl and MgCl2 are 1074, 878 and 987 K respectively. When chloride salts are used as additives, during the reduction (1038-1349 K), the reaction temperature will exceed all the salts’ melting points except NaCl at 1038 K, and the added salts will melt and act as high-temperature solvents. WO3 or W can then dissolve and undergo reactions in the molten salts. When the solubility of WO3 or W exceeds the limit, precipitation will occur in molten salts. The liquid salts promote the rapid diffusion of reactant species at low temperatures and can give a product with better quality in a shorter time and at much lower temperature. Oxides and salts can disperse spontaneously on the surface of particles with diminishing free energy G (ΔG<0) [25], and molten salts can adhere to the surface of sub-particles and promote the dispersion of sub-particles in the solvent. XIA et al [26] found that the molten salt was capable of accelerating the material formation kinetics and improving the product crystallinity. Therefore, for large parallelepiped-shaped and irregular blocky-shaped particles, the dissolution- precipitation cycle will make some sub-particles dissolve and others will crystallize and then grow up. This may break up the original WO3 particle and make it degrade, which has been observed in our experiments. It is worth nothing that the relative molecular masses of NaCl and LiCl are less than that of MgCl2, which means that the additives of NaCl and LiCl have larger mole numbers with the same mass fraction of 0.1%, which can improve mass transfer much more effectively.
(2) According to SEM analysis, with adding salts, the large particle degrades and transforms to a great number of small sub-particles at lower temperatures. This phenomenon strongly suggests that the CVT mechanism also plays an important role to some extent during the reduction. Investigation has proved that the formation of the volatile oxide hydrate can be accelerated by the catalytic action of liquid alkali compounds in the reaction [27]. Therefore, the CVT mechanism will be enhanced. In addition, Li and Na, as the first main group elements, have the similar chemical properties with H, and their compounds can combine with water vapor to form volatile substances (WO3·nX2O, X represents Na and Li elements) [23], which can also intensify CVT process and the dispersity of sub-particles although water vapor is difficult to form at a relatively low temperature. That is why sub-particles with adding NaCl and LiCl are much more dispersed than those with adding MgCl2. At higher temperature, a large amount of W atoms will be produced in a short time to attach to the surface of pre-nucleated W and molten salt particles. Thus, W sub-particles become more dispersed but larger than those without additives. Therefore, the separated sub-particles are formed and the dispersity of products is greatly improved.
(3) At lower temperatures, the morphology of the particles presents an irregular shape which is approximately spherical (Figs. 2-5), and the inherent agglomeration of sub-particles is very remarkable. Through spontaneous agglomeration, the surface energy will be decreased [28]. The aggregation is formed by the solid phase bonding between the particles, which is very hard to separate, instead of the electrostatic interaction or the VDW (van der Waals force) between the particles. In order to obtain stable and dispersed particles, the surface modification of particles using additives is especially important. Thus, the chlorine salts (NaCl, LiCl and MgCl2) can work as dispersants to weaken the force between particles, and the surface energy is also decreased.
(4) It is well known that crystals can spontaneously present closed, regular polyhedral shapes under suitable conditions, and the formation of W crystal is also related to this law. However, the experimental results show that W sub-particles present different shapes under different production conditions caused by different reaction mechanisms. The sublimation of WO3 appears when the temperature is over 1173 K, and higher temperature increases reaction and sublimation rates, and sintering behavior. At higher temperature, these effects will work together and promote the growth of W particles during the reduction process. When the temperature is lower, low reaction rate will lead to incomplete development of W particles, resulting in the formation of spherical particles. After adding salts, the sublimation of WO3 and volatile substances (WO3·nX2O, X represents Na and Li elements) is facilitated. Hence, more adequate growth of particles will be obtained with the addition of additives.
In addition, with temperature increasing, the above mechanisms and the sintering of W particles will promote the growth of product particles. Finally, in order to improve the purity of the product, the chlorine salts (NaCl, LiCl and MgCl2) can be removed by water washing or acid leaching without losing W because of the chemical stability of W [1]. The inductively coupled plasma optical emission spectrometry (ICPOES) (iCAP 6300 Duo, Thermo Scientific) analysis results of the obtained W after hydrochloric acid leaching and water washing are given in Table 2.
Table 2 Element contents of produced metallic tungsten with 0.1 wt.% LiCl (wt.%)

5 Prospect of application
As shown in Fig. 6, spherical sub-particles with improved dispersibility and uniformed particle size (~2 μm) can be obtained at lower temperature, which will significantly improve the formability, physical and mechanical properties of tungsten- based products [29]. Furthermore, additives multiply the particle size of polyhedral particles significantly at higher temperature, and coarse- grained tungsten powders are the important precursor for the production of coarse-grained tungsten carbide. Compared with fine or medium grain tungsten carbide, coarse grain tungsten carbide can be widely used in geological mining tools, petroleum drill bit, hard wear-resistant materials due to the advantages of less high- temperature defects, less micro-strain and higher micro-hardness, etc [30]. The present study provides a method of producing both large and small tungsten particles.
6 Conclusions
(1) The salt-assisted method to prepare different morphologies and sizes of metallic tungsten particles was investigated, and the reduction and affecting mechanisms of the additives were proposed as well.
(2) Without additive, W sub-particles with poor dispersion were produced and the large particles almost maintained the shape and size of raw WO3 at low temperature; while uniform, regular and dispersive W sub-particles were obtained by adding 0.1 wt.% additives (especially NaCl and LiCl).
(3) With the increase of temperature and amount of salts, the produced particles become more dispersed and the size of sub-particles is increased. Furthermore, the shape of sub-particles is changed from spherical to polyhedral.
References
[1] LASSNER E, SCHUBERT W, LUDERITZ E, WOLF H U. Tungsten, tungsten alloys, and tungsten compounds [M]. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 2000.
[2] DAI Ming-jiang, WEI Chun-bei, ZHOU Ke-song, ZHU Min, HOU Hui-jun, LIN Song-sheng, TONG Xin. Properties of W/DLC/W-S-C composite films fabricated by magnetron sputtering [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 3002-3011.
[3] BLOOMER R N. Tungsten filaments [J]. Journal of the Institution of Electrical Engineers, 1957, 3: 160-161.
[4] BOONE J M, SEIBERT J A. An accurate method for computer-generating tungsten anode X-ray spectra from 30 to 140 kV [J]. Medical Physics, 1997, 24: 1661-1670.
[5] MESSIER D R. Tungsten deposition from tungsten mesh heating element in vacuum above 2450 K [J]. Review of Scientific Instruments, 1966, 37: 1610-1611.
[6] ZHANG Jia-jia, LIU Wen-sheng, MA Yun-zhu, YE Xiao-shan, WU Ya-yu, HUANG Bao-yun. Preparation and properties of Ni68.6W17.9B13.5 metallic glass [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 1575-1579.
[7] HAN Yin-ben, XUE Xiang-yi, ZHANG Tie-bang, HU Rui, LI Jin-shan. Effects of hot compression on carbide precipitation behavior of Ni-20Cr-18W-1Mo superalloy [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 2883-2891.
[8] GAI Guo-sheng, YANG Yu-fen, JIN Lan, ZOU Xin, WU Yun-xin. Particle shape modification and related property improvements [J]. Powder Technology, 2008, 183: 115-121.
[9] SKACHKOV O A, BASHUROV Y P, MAKAREVICH O N, KASPAROVA T V, CHECHUROV M V. Influence of the production technology on the morphology and physicochemical properties of tungsten powder [J]. Steel in Translation, 2012, 42: 276-280.
[10] HAN C, NA H, KIM Y, CHOI H. In-situ synthesis of tungsten nanoparticle attached spherical tungsten micro- powder by inductively coupled thermal plasma process [J]. International Journal of Refractory Metals and Hard Materials, 2015, 53: 7-12.
[11] PUSAVEC F. Porous tungsten machining under cryogenic conditions [J]. International Journal of Refractory Metals and Hard Materials, 2012, 35: 84-89.
[12] ERDOGAN M, KARAKAYA O. Electrochemical reduction of tungsten compounds to produce tungsten powder [J]. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science, 2010, 41: 798-804.
[13] LEE J H, SEO D H, WON C W, BOROVINSKAYA I P, VERSHINNIKOV V I. Combustion characteristics of WO3/Zn reaction system in SHS process [J]. Journal of Materials Science, 2001, 36: 5311-5314.
[14] RICCERI R, MATTEAZZI P. A study of formation of nanometric W by room temperature mechanosynthesis [J]. Journal of Alloys and Compounds, 2003, 358: 71-75.
[15] CHARLTON M G. Hydrogen reduction of tungsten oxides [J]. Nature, 1954, 174: 703-703.
[16] KIM G S, LEE Y J, KIM D G, SUNG T O, KIM D S, KIM Y D. The behavior of tungsten oxides in the presence of copper during hydrogen reduction [J]. Journal of Alloys and Compounds, 2006, 419: 262-266.
[17] LIU Liang-liang, GAO Feng, HU Guo-xin, LIU Jiang-nan. Fabrication of KSr2Nb5O15 particles with high aspect ratio by two-step molten salt synthesis [J]. Advanced Powder Technology, 2014, 25: 219-225.
[18] WEST A R. Solid state chemistry and its application [M]. Chichester: John Wiley & Sons, 1984.
[19] LV Ze-peng, LIU Dong, WU Yi-jie, ZHANG Run, SUN Hai-bo, DANG Jie, HU Li-wen. Effect of Y(NO3)3 additive on morphologies and size of metallic W particles produced by hydrogen reduction [J]. Advanced Powder Technology, 2019, 30: 2768-2778.
[20] SUN Guo-dong, ZHANG Guo-hua, JIAO Shu-qiang, CHOU Kuo-chih. Shape-controlled synthesis of ultrafine Mo crystals via salt-assisted reduction of MoO2 with H2 [J]. Journal of Physical Chemistry C, 2018, 122: 10231-10239.
[21] ZHANG Guo-hua, LI Jing-jing, WANG Lu, CHOU Kuo-chih. Effects of R2CO3 (R=Li, Na and K) on the reduction of MoO2 to Mo by hydrogen [J]. International Journal of Refractory Metals and Hard Materials, 2017, 69: 180-188.
[22] XIN Yan-jun, GUO Zhi-meng, LI Yan-jun, WU Cheng-yi. The coarse grain tungsten powder reduced by ammonium paratungstate at medium temperature [J]. Superhard Material Engineering, 2011, 23: 31-35. (in Chinese)
[23] ZHENG Feng. Effect of some metal elements on particle size of tungsten powder [J]. Cemented Carbide, 1995, 3: 143-145. (in Chinese)
[24] LV Ze-peng, DANG Jie, WU Yi-jie, LV Xue-wei, ZHANG Sheng-fu. Preparation of Mo2C by reduction and carbonization of MoO2 with CH3OH [J]. Journal of Materials Science, 2018, 53: 10059-10070.
[25] XIE You-chang, TANG You-qi. Spontaneous monolayer dispersion of oxides and salts onto surfaces of supports: Applications to heterogeneous catalysis [J]. Advances in Catalysis, 1990, 37: 1-43.
[26] XIA B, LENGGORO W, OKUYAMA K. Novel route to nanoparticle synthesis by salt-assisted aerosol decomposition [J]. Advanced Materials, 2001, 13: 1579-1582.
[27] ZIMMERL T, SCHUBERT W D, BICHERL A, BOCK A. Hydrogen reduction of tungsten oxides: Alkali additions, their effect on the metal nucleation process and potassium bronzes under equilibrium conditions [J]. International Journal of Refractory Metals and Hard Materials, 2016, 62: 87-96.
[28] LIU D M, TSENG W. Influence of powder agglomerate on the structure and rheological behavior of injection-molded zirconia-wax suspensions [J]. Journal of the American Ceramic Society, 1999, 10: 2647-2652.
[29] WANG W S, HWANG K S. The effect of tungsten particle size on the processing and properties of infiltrated W-Cu compacts [J]. Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science, 1998, 29: 1509-1516.
[30] PAVLOVSKII V A, REZNICHENKO V A. An electrolytic method of manufacture of a coarse tungsten powder [J]. Soviet Powder Metallurgy Metal Ceramics, 1986, 25: 863-865.
盐助还原法对金属钨颗粒形貌和尺寸的影响
吕泽鹏1,2,简开亮1,2,党 杰1,2
1. 重庆大学 材料科学与工程学院,重庆 400044;
2. 重庆大学 钒钛冶金及新材料重庆市重点实验室,重庆 400044
摘 要:提出一种利用盐助氢气还原制备形状和尺寸可控钨颗粒的简单方法,并通过调节氯盐添加量和温度制备形状和尺寸可控的钨颗粒。加入盐添加剂后,最终产物的分散性得到明显改善,颗粒生长更加充分。其中,NaCl与LiCl的作用尤为显著。在1038 K时添加0.1% NaCl (质量分数)和0.1% LiCl (质量分数)的钨颗粒平均粒径分别为0.924和1.128 μm。随着温度和氯盐添加量的增加,生成的钨颗粒的分散性变好,小颗粒的粒径增大,形状由球形变为多面体。特别是在1349 K下,氯盐的加入使钨颗粒的粒径显著增大,添加1% NaCl (质量分数)和1% LiCl (质量分数)钨颗粒的平均粒径分别达到21.367和29.665 μm。基于传统假晶转变和化学气相传输机理,还详细探讨添加盐对反应机理的影响。
关键词:钨粉;盐助氢气还原;氯盐;形貌;尺寸;可控合成
(Edited by Wei-ping CHEN)
Foundation item: Project (171111) supported by Fok Ying Tung Education Foundation, China; Projects (cx2018055, cx2019041) supported by the Venture & Innovation Support Program for Chongqing Overseas Returnees, China
Corresponding author: Jie DANG; Tel: +86-23-65112631; Fax: +86-23-65112631; E-mail: jiedang@cqu.edu.cn
DOI: 10.1016/S1003-6326(20)65449-8
Abstract: A simple method was proposed to produce tungsten (W) particles with controllable shape and size by employing the salt-assisted hydrogen reduction. W particles with controlled shape and size were prepared by adjusting the amount of chlorine salts and the temperature. After adding salt additives, the dispersibility of final particles was obviously improved and more adequate growth of particles was obtained. It was found that the effect of NaCl and LiCl is particularly significant. The average sizes of the obtained W particles at 1038 K after adding 0.1 wt.% NaCl and 0.1 wt.% LiCl were 0.924 and 1.128 μm, respectively. With the increase of temperature and amount of chlorine salts, the dispersity of the produced W particles became much better, the size of W sub-particles was increased,and the shape of W sub-particles was changed from spherical to polyhedral. At 1349 K, the addition of chlorine salts even multiplied the particle size, and the average sizes of W particles with 1 wt.% NaCl and 1 wt.% LiCl were raised up to 21.367 and 29.665 μm, respectively. Based on the conventional pseudomorphic transformation and chemical vapor transport mechanisms, the effects of adding salts on the reaction mechanism were investigated in detail as well.


