
ARTICLE
J. Cent. South Univ. (2019) 26: 323-330
DOI: https://doi.org/10.1007/s11771-019-4004-z
Salt ions accumulation and distribution characteristics of pioneer plant species at a bauxite residue disposal area, China
HUANG Nan(黄楠)1, TANG Lu(唐璐)1, ZHU Feng(朱锋)1, WU Chuan(吴川)1,HARTLEY William2, ZHOU Jing-ju(周晶菊)1, XUE Sheng-guo(薛生国)1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Crop and Environment Sciences Department, Harper Adams University, Newport, Shropshire,TF10 8NB, United Kingdom
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
Bauxite residue disposal areas (BRDAs) are physically degraded and hostile to plant growth. Nevertheless, natural plant colonization was observed in an abandoned BRDA in Central China. The pioneer plant species at the disposal area were identified, whilst distribution characteristics of salt ions such as Na+, K+, and Ca2+ in plant tissues and rhizosphere residues were investigated. The mean concentration of exchangeable Na+ in the rhizosphere soils was 19.5 cmol/kg, which suggested that these pioneer plants had relatively high salinity resistance. Sodium content varied from 0.84 cmol/kg (Digitaria sanguinalis) to 39.7 cmol/kg (Kochia scoparia), whilst K to Na ratio varied from 0.71 (Myricaria bracteata) to 32.39 (Digitaria sanguinalis) in the shoots, which demonstrated that the salinity tolerance mechanisms of these pioneer species differed significantly. Accumulation factors of Na+ in local plant species ranged from 0.04 (D. sanguinalis) to 3.29 (M. bracteata), whilst the translocation factor varied from 0.13 (D. sanguinalis) to 2.92 (M. bracteata). The results suggested that four pioneer plant species including K. scoparia, M. bracteate, Cynodon dactylon and D. sanguinalis could be suitable for revegetation at other disposal areas.
Key words:
Cite this article as:
HUANG Nan, TANG Lu, ZHU Feng, WU Chuan, HARTLEY William, ZHOU Jing-ju, XUE Sheng-guo. Salt ions accumulation and distribution characteristics of pioneer plant species at a bauxite residue disposal area, China [J]. Journal of Central South University, 2019, 26(2): 323–330.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4004-z1 Introduction
Management of mine tailing disposal sites is a global environmental problem [1, 2]. Bauxite residue, an alkaline-sodic waste, is generated from the process of alumina production [3, 4]. The global inventory of bauxite residue reached 4.4 billion tons in 2017, and is increasing at a rate of 1.2 million tons per year [5–7]. Large volumes of bauxite residue are stacked in BRDAs, which may cause air, water and soil pollution to the surrounding environment [8]. Research has suggested that establishing plant communities on BRDAs is a promising strategy to regenerate the hostile physicochemical properties of the residues, as plants exude organic acids and CO2 from their roots improving the surrounding area [9–13].
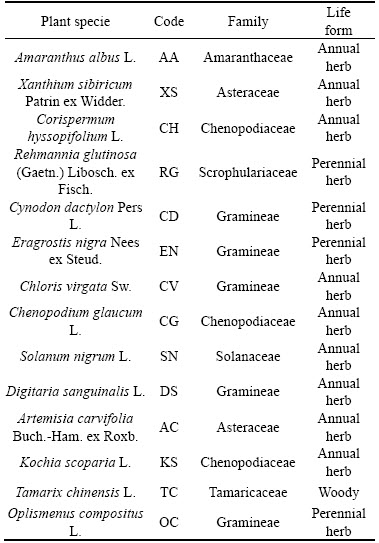
A variety of halophyte plant species have been tested on BRDAs, but most of the experiments failed [14–17]. There are two limitations to plant growth on BRDAs. Firstly, the hostile properties of bauxite residue, including high alkalinity and salinity, low water permeability, nutrient deficiencies and trace metal toxicity are immediate major limitations [18, 19]. ZHU et al [20] found that natural weathering processing could improve residue microaggregates in bauxite residue disposal areas. SANTINI et al [10] discovered that natural weathering processes could improve bauxite residue properties without amendment additions, with spontaneous vegetation encroaching on the disposal area after 30 years. Although gypsum and organic materials are effective ameliorants to improve bauxite residue [21–25], the application of amendments may require a certain amount of expenditure [26]. Screening of suitable plant species is essential as they should be resistant to salinity and alkalinity and adapted to the local climate. An ecological investigation at a BRDA in Henan province, China, discovered that spontaneous vegetation has encroached onto an abandoned BRDA in the Henan province. Plants have been used in the past for in-situ remediation of mine tailings and saline-alkali soils [27–29], and the presence of these pioneer plants presents a great opportunity and the potential for revegetation of bauxite residue. The aim of the current investigation was firstly to identify these local pioneer plant species, secondly to determine the salt distribution in their tissues and rhizosphere soils and finally to evaluate their salt tolerance characteristics.
2 Materials and methods
2.1 Study area
The BRDA is located in the Henan province, China, which has a temperate continental monsoon climate. Average annual rainfall ranges from 600 to 1200 mm. The average annual daily temperature is approximately 12.8–14.8 °C. The annual frostless period is approximately 226 d with approximately 2353 h of sunlight a year.
2.2 Sample preparation and analytical methods
The plants and bauxite residue in the study area were sampled in August 2014. Plant samples were collected and stored in polyethylene bags. Soil which adhered to the roots was removed by gentle shaking and stored in polyethylene bags as rhizosphere residues. Plant samples were thoroughly washed with deionized water and divided into plant roots and shoots. The samples were dried separately to a constant weight at 40 °C for 48 h, and then crushed and sieved (<2 mm). The rhizosphere residues were air-dried and sieved (<2 mm) to remove gravel and large particles prior to chemical analysis.
The cation contents (Ca2+, Na+, K+) in plants and the rhizosphere residues were dissolved using acid (65% HNO3, 35% H2O2) and detected by inductively coupled plasma atomic emission spectroscopy (ICP-AES). All analyses were performed in triplicate.
2.3 Translocation factor (TF) and bioaccumulation factor (BF)
Two factors, translocation factor (TF) and bioaccumulation factor (BF) were applied to evaluating salt ion distribution and accumulation in plant tissues. TF can be used to show the translocation of salt ions from roots to shoots. A TF>1 indicated that salt ions were more labile and could be readily translocated to the shoots, whilst a TF <1 indicated that salt ions were lass labile and remained in the roots. BF can be used to exhibit the accumulation ability of salt ions in plants. A BF>1 indicates that plants tend to accumulate salt ions in the shoots.
The two factors were calculated using the following equations [2]:
 (1)
(1)
 (2)
(2)
2.4 Statistical analyses
The data were recorded with Microsoft Excel 2010, and analyzed statistically with SPSS version 20.0. Figures were constructed with Origin 8.0. Plant samples of different species were determined using one-way analysis of variance (ANOVA) with homogeneity of variance tests. Duncan’s post hoc test was applied if the factor was homogenous, and Dunnett’s T3 test was employed if no homogeneity existed.
3 Results and discussion
3.1 Plant species at BRDA
Fourteen local plant species were found and identified at the BRDA (Table 1). These naturally colonized plants belong to seven families: Asteraceae, Chenopodiaceae, Amaranthaceae, Scrophulariaceae, Solanaceae, Tamaricaceae and Gramineae. Nine species were dicotyledonous with the remaining species being monocots. Four species were perennial herbs, C. dactylon, O. compositus, Eragrostis nigra, and Rehmannia glutinosa; the others were annual herbs. The mean concentrations of exchangeable Na and pH of bauxite residue in this area were 19.5 cmol/kg (dry weight; DW) and 9.45 respectively. Plant species that can survive in such high sodium and alkali environments suggest that they have the potential for future BRDA bioremediation projects.
Table 1 Naturally colonized plant species at BRDA
3.2 Na+, K+ and Ca2+ contents in bauxite residue and plant tissues
Sodic soil can cause osmotic stress and ionic toxicity to plant species and encumber plant growth [30]. When the salt concentration is more than 20 cmol/kg, 99% of plant species are unable to survive [31]. Bauxite residue contained high concentration of Na+, which limited vegetation establishment [4, 5]. In this study, the mean value of Na+ in the rhizosphere residues was 19.5 cmol/kg (Table 2). Among them, Na+ in 8 residue samples was greater than 20 cmol/kg. The rhizosphere residues from C. dactylon and A. carvifolia had the highest value of Na+ (28.5 and 25.9 cmol/kg, respectively).
Table 2 Selected elemental concentrations of rhizosphere residue from plant species
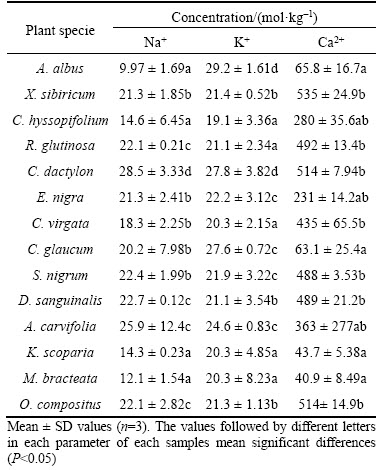
The salt tolerance mechanism of the plant species varied greatly. Several plant species excluded the excess salt ions from entering their shoot tissues, and several species accumulated salt ions in their vacuoles [32, 33]. There were low Na+ contents in both shoot and root parts of C. dactylon and C. virgata, which showed that these plant species could prevent Na+ from entering their tissues. Furthermore, there were high Na+ contents in both shoot and root parts of A. albus and K. scoparia, which revealed that these plants had a high tolerance of Na+ in their tissues.
The concentrations of Na+ in plant tissues ranged from 2.75 to 51.8 cmol/kg (DW), which were close to the results of WOODARD et al [34]. The highest Na concentrations in roots were found in AA (A. albus), KS (K. scoparia) and CG (C. glaucum), whilst the lowest Na concentrations in roots were found in CD (C. dactylon), CV (C. virgata) and EN (E. nigra). The highest Na concentrations in shoots were found in MB (M. bracteata), KS (K. scoparia) and CG (C. glaucum), whilst the lowest Na concentrations in shoots were found in DS (D. sanguinalis), CD (C dactylon) and OC (O. compositus).
Related studies on salt-tolerant mechanisms of plants showed that plants can reduce salt stress by limiting salt ions into their shoots [30]. In this study, Na concentrations in the roots of most local plant species were higher than those in the shoots, and the average value of Na+ content in the roots was twice as high as that in the shoots (Figure 1). The contents of Na+ in roots of three of the plant species, EN (E. nigra), SN (S. nigrum) and MB (M. bracteata), showed the opposite trend, which indicated that excess Na+ may remain in the roots or be secreted through salt glands to reduce sodium toxicity to these plant species.
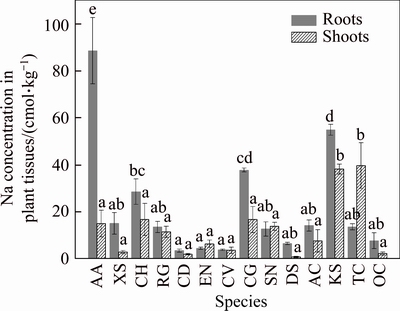
Figure 1 Sodium concentration in roots and shoots of 14 plant species growing naturally at BRDA (Data are means ± SD of three individual replicates. Mean values followed by different letters (a-e) are significantly different (P<0.05))
Although the physicochemical properties of Na and K were similar, K was more essential for plant growth, which means that plants in saline environments may selectively absorb K to relieve salt stress [30]. The K+ contents in the roots of local plant species were lower than those in the shoots (Figure 2), and the average concentration of K+ in plant shoots was more than two times that in the roots. The highest K+ content in roots was found in AA (A. albus) and KS (K. scoparia), whilst the lowest K+ content in roots was found in CV (C. virgata) and DS (D. sanguinalis). The K+ contents in the shoots of five plant species were more than 100 cmol/kg. The highest K+ content in shoots was found in KS (K. scoparia), CG (C. glaucum) and AA (A. albus), whilst the lowest K+ content in shoots was found in DS (D. sanguinalis), CD (C. dactylon) and OC (O. compositus).
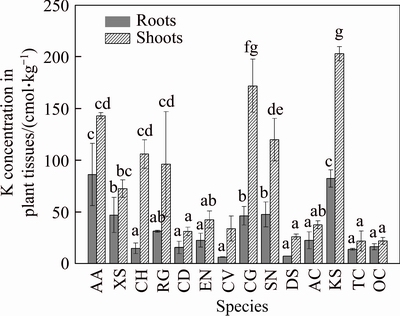
Figure 2 Potassium concentrations in roots and shoots of 14 plant species growing naturally at BRDA (Data are means ± SD of three individual replicates. Mean values followed by different letters (a-e) are significantly different (P<0.05))
The average Ca2+ content in shoots and roots was 13.4 and 12.4 cmol/kg, respectively (Figure 3). There was no significant difference between Ca2+ contents in roots and shoots of these plant species, although there was a significant difference (P<0.05) in Ca2+ content between the plant species. The highest Ca2+ content in roots was found in XS (X. sibiricum) and AA (A. albus), whilst the lowest Ca2+ content in roots was found in KS (K. scoparia) and RG (R. glutinosa). The content of Ca2+ in plant shoots varied from 6.78 cmol/kg (in KS, K.Scoparia) to 17.1 cmol/kg (in XS, X. Sibiricum). Except for DS, the content of Ca2+ in shoots of the other plant species was higher than 10 cmol/kg.
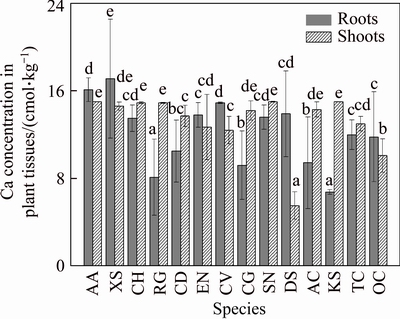
Figure 3 Calcium concentrations in roots and shoots of 14 plant species growing naturally at BRDA (Data are means ± SD of three individual replicates. Mean values followed by different letters (a-e) are significantly different (P<0.05))
Correlation analysis revealed that Na+ content in the rhizosphere residues had a significant negative correlation in plant shoots and roots (P<0.01). This suggested that plants could absorb Na+ and reduce the salt concentration from the bauxite residue. KONG et al [35] found that Ca2+ could induce the transformation of Na+ from its free state to a chemically bonded state, which may decrease the effective Na+ for plant uptake or accumulation. In this study, it was observed that Ca2+ in the rhizosphere residues was significantly negatively correlated with that in the plant tissues, which was consistent with the results of KONG et al [35].
3.3 K+/Na+ and Ca2+/Na+ of local plant species
Salt-tolerant plant species maintain greater K+/Na+ and Ca2+/Na+ ratios in their roots to improve growth under saline conditions [36, 37]. Tolerant plant species regulate intracellular ions (Na+, K+ and Ca2+) to resist external water stress [30]. WEI et al [38] found that, higher K+/Na+ and Ca2+/Na+ ratios in plant tissues could be a crucial mechanism to improve their salt tolerance. CHERIAN et al [39] suggested that the K+/Na+ ratio increased in Suaeda nudiflora with increasing levels of salinity in the growth medium.
In this study, the K+/Na+ ratio varied from 0.71 (M. bracteata) to 32.39 (D. sanguinalis). A maximum K+/Na+ ratio was observed in C. dactylon and D. sanguinalis, whilst a minimum K+/Na+ ratio was observed in K. scoparia and M. bracteata. The Ca2+/Na+ ratio varied from 0.39 (K. scoparia) to 7.06 (C. dactylon). A maximum Ca2+/Na+ ratio was observed in C. dactylon, whilst a minimum Ca2+/Na+ ratio was observed in K. scoparia. The contents of related salt ions were not found in plants including D. sanguinalis, C. dactylon, O. compositus and X. sibiricum, which indicated that these species may reduce Na+ uptake making it easier to achieve osmotic balance and ion homeostasis. In contrast, plant species such as K. scoparia, C. hyssopifolium, and C. glaucum could maintain cell penetration balance by absorbing and accumulating inorganic ions.
3.4 BF and TF of Na+ and K+
The average values for BF and TF of Na+ and K+ in the 14 species are presented in Table 3. There were significant differences in Na+ and K+ contents in the roots, shoots and rhizosphere residues, which indicated that BF and TF varied greatly.
The highest Na+ BF was observed in M. bracteata, K. scoparia, A. albus and C. hyssopifolium, indicating that these plant species have a habit of accumulating Na+ in their shoots; whilst the lowest Na+ BF was observed in D. sanguinalis, C. dactylon, and O. compositus, indicating that their root systems had the ability to immobilize Na+.
Table 3 Bioaccumulation factor (BF) and translocation factor (TF) of pioneer plant species
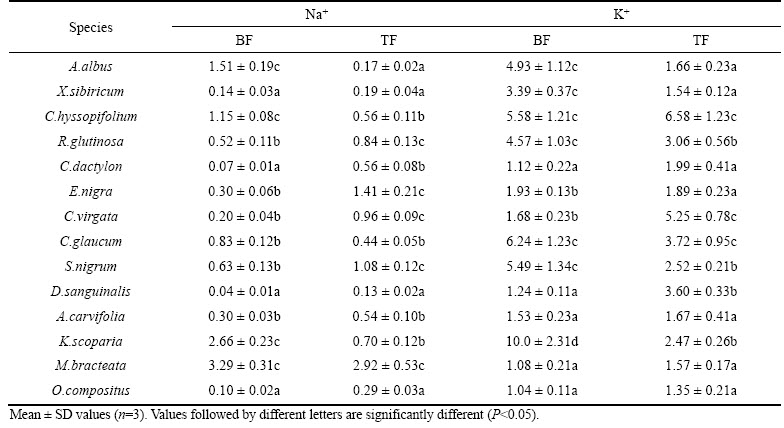
M. bracteata, E. nigra, and S. nigrum showed the highest TF for Na+, which was more than 1, indicating that Na+ was effectively translocated to the shoots of these plant species; whilst the lowest TF for Na was found in D. sanguinalis, A. albus, and X. sibiricum. O. compositus showed that Na+ was more likely to remain in the roots.
X. sibiricum and M. bracteata revealed the lowest TF for K, indicating that these plant species translocate and accumulate K+ in their shoots; whilst the lowest BF for K+ was found in M.bracteata, C. dactylon, and O. compositus, indicating that their root systems had the ability to immobilize K+.
C. hyssopifolium, and C. virgata showed the highest TF for K+, showing that K+ was more likely to be translocated to the shoots; the lowest TF for K+ was observed in M. bracteata and O. compositus, indicating that K+ would remain in the roots.
Due to the high alkalinity and salinity of bauxite residue, re-entrainment of dust particulates from the surface of BRDA’s may present environmental risks to surrounding farmland and residents. Plant establishment on BRDA’s is an economic and feasible strategy to alleviate these risks [2]. It was demonstrated that C. dactylon and D. sanguinal could stabilize Na+ in their roots, which shows greater potential for phytostabilization. Phytostabilization could prevent wind erosion, and the root systems could immobilize harmful elements, thereby reducing the exposure risk to humans and animals. C. dactylon and D. sanguinalis had relatively low TF values for salt ions, and may be regarded as suitable plant species for revegetation on BRDA’s.
4 Conclusions
Native plants had encroached upon the abandoned BRDA in Henan province. Fourteen pioneer plant species were identified and salt ion absorption and distribution of these plant species were investigated. Rhizosphere residues of the plants had high pH (9.45) and Na+ contents (19.5 cmol/kg). These plant species could survive in the high sodium and alkali environment of the BRDA, which suggested that their salinity tolerance was relatively high. Sodium, K+ and Ca2+ contents in shoots and roots of all 14 species varied greatly, which demonstrated different salt tolerant characteristics in the local plant species. There were low concentrations of salt ions in plants including D. sanguinalis, C. dactylon, O. compositus, X. sibiricum, which indicated that these plants could reduce the translocation of salt ions and alleviate the salt stress. Furthermore, the plants including K. scoparia, C. hyssopifolium and C. glaucum could absorb and accumulate salt ions to maintain cell penetration balance. The BF and TF differed significantly in between plant species. C. dactylon and D. sanguinalis revealed the least salt-accumulation in their tissues, which showed great potential for revegetation on other BRDA sites.
References
[1] SMART D, CALLERY S, COURTNEY R. The potential for waste-derived materials to form soil covers for the restoration of mine tailings in Ireland [J]. Land Degradation & Development, 2016, 27: 542–549. DOI:10.1002/ldr.2465.
[2] MENDEZ M O, MAIER R M. Phytostabilization of mine tailings in arid and semiarid environments— An emerging remediation technology [J]. Environmental Health Perspectives, 2008, 116: 278–283. DOI:10.1289/ehp.10608.
[3] KONG Xiang-feng, GUO Ying, XUE Sheng-guo, HARTLEY W, WU Chuan, YE Yu-zhen, CHENG Qin-yu. Natural evolution of alkaline characteristics in bauxite residue [J]. Journal of Cleaner Production, 2017, 143: 224–230. DOI: 10.1016/j.jclepro. 2016. 12. 125.
[4] GR F M, KLAUBER C. Bauxite residue issues: IV. Old obstacles and new pathways for in situ residue bioremediation [J]. Hydrometallurgy, 2011, 108: 46–59. DOI: 10.1016/j.hydromet. 2011. 02. 005.
[5] XUE Sheng-guo, ZHU Feng, KONG Xiang-feng, WU Chuan, HUANG Ling, HUANG Nan, WILLIAM H. A review of the characterization and revegetation of bauxite residues (red mud) [J]. Environmental Science and Pollution Research, 2016, 23: 1120–1132. DOI:10.1007/s11356-015 -4558-8.
[6] POWER G., GR FE M, KLAUBER C. Bauxite residue issues: I. Current management, disposal and storage practices [J]. Hydrometallurgy, 2011, 108: 33–45. DOI: 10.1016/ j.hydromet.2011.02.006.
[7] XUE Sheng-guo, KONG Xiang-feng, ZHU Feng, HARTLEY W, LI Xiao-fei, LI Yi-wei. Proposal for management and alkalinity transformation of bauxite residue in China [J]. Environmental Science and Pollution Research, 2016, 23(13): 12822–12834. DOI: 10.1007/s11356-016- 6478-7.
[8] JONES B E H, HAYNES R J. Bauxite processing residue: A critical review of its formation, properties, storage, and revegetation [J]. Critical Reviews in Environmental Science and Technology, 2011, 41: 271–315. DOI:10.1080/ 10643380902800000.
[9] XUE Sheng-guo, YE Yu-zhen, ZHU Feng, WANG Qiong-li, JIANG Jun, HARTLEY W. Changes in distribution and microstructure of bauxite residue aggregates following amendments addition [J]. Journal of Environmental Sciences, 2019, 78: 276–286, DOI: 10.1016/j.jes.2018.10.010.
[10] SANTINI T C, FEY M V. Spontaneous vegetation encroachment upon bauxite residue (Red Mud) as an indicator and facilitator of in situ remediation processes [J]. Environmental Science & Technology, 2013, 47: 12089– 12096. DOI: 10.1021/es402924g.
[11] ZHU Feng, XUE Sheng-guo, HARTLEY W, HUANG Ling, WU Chuan, LI Xiao-fei. Novel predictors of soil genesis following natural weathering processes of bauxite residues [J]. Environmental Science & Pollution Research, 2015, 23: 1–8. DOI: 10.1007/s11356-015-5537-9.
[12] PILON-SMITS E. Phytoremediation [J]. Annu Rev Plant Biol, 2005, 56: 15–39. DOI: 10.1146/annurev.arplant.56. 032604.144214.
[13] ZHU Feng, LI Yu-bing, XUE Sheng-guo, HARTLEY W, WU Hao. Effects of iron-aluminium oxides and organic carbon on aggregate stability of bauxite residues [J]. Environmental Science and Pollution Research, 2016, 23: 9073–9081. DOI:10. 1007/ s11356-016-6172-9.
[14] XIAO Guang-li, LI Ting-xuan, ZHANG Xi-zhou, YU Hai-ying, HUANG Hua-gang, GUPTA D K. Uptake and accumulation of phosphorus by dominant plant species growing in a phosphorus mining area [J]. Journal of Hazardous Materials, 2009, 171: 542–550. DOI: 10.1016/ j.jhazmat.2009. 06. 034.
[15] RABHI M, FERCHICHI S, JOUINI J, HAMROUNI M H, KOYRO H W, RANIERI A, ABDELLY C, SMAOUI A. Phytodesalination of a salt-affected soil with the halophyte Sesuvium portulacastrum L. to arrange in advance the requirements for the successful growth of a glycophytic crop [J]. Bioresource Technology, 2010, 101: 6822–6828. DOI: 10.1016/j. biortech. 2010.03.097.
[16] ZHANG Xing-feng, XIA Han-ping, LI Zhi-an, ZHUANG Ping, GAO Bo. Potential of four forage grasses in remediation of Cd and Zn contaminated soils [J]. Bioresource Technology, 2010, 101: 2063–2066. DOI: 10.1016/j.biortech.2009.11.065.
[17] RAVINDRAN K C, VENKATESAN K, BALAKRISHNAN V, CHELLAPPAN K P, BALASUBRAMANIAN T. Restoration of saline land by halophytes for Indian soils [J]. Soil Biology and Biochemistry, 2007, 39: 2661–2664. DOI: 10.1016 /j.soilbio.2007.02.005.
[18] GR FE M, POWER G, KLAUBER C. Bauxite residue issues: III. Alkalinity and associated chemistry [J]. Hydrometallurgy, 2011, 108: 60–79. DOI: 10.1016 /j.hydromet.2011.02.004.
[19] ZHU Feng, LIAO Jia-xin, XUE Sheng-guo, HARTLEY W, ZOU Qi, WU Hao. Evaluation of aggregate microstructures following natural regeneration in bauxite residue as characterized by synchrotron-based X-ray micro-computed tomography [J]. Science of the Total Environment, 2016, 573: 155–163. DOI: 10.1016 /j.scitotenv .2016.08.108.
[20] ZHU Feng, CHENG Qing-yu, XUE Sheng-guo, LI Chu-xuan, HARTLEY W, WU Chuan. TIAN Tao. Influence of natural regeneration on fractal features of residue microaggregates in bauxite residue disposal areas [J]. Land Degradation and Development, 2018, 29(1): 138–149. DOI: 10.1002/ ldr.2848.
[21] BANNING N C, SAWADA Y, PHILLIPS I R, MURPHY D V. Amendment of bauxite residue sand can alleviate constraints to plant establishment and nutrient cycling capacity in a water-limited environment [J]. Ecological Engineering, 2014, 62: 179–187. DOI: 10.1016/j.ecoleng. 2013. 10. 034.
[22] ZHU Feng, HOU Jing-tao, XUE Sheng-guo, WU Chuan, WANG Qiong-li, HARTLEY W. Vermicompost and gypsum amendments improve aggregate formation in bauxite residue [J]. Land Degradation & Development, 2017, 28: 2109–2120. DOI: 10.1002/ ldr.2737.
[23] COURTNEY R, MULLEN G, HARRINGTON T. An evaluation of revegetation success on bauxite residue [J]. Restoration Ecology, 2009, 17: 350–358. DOI: 10.1111/ j.1526-100x. 2008. 00375.x.
[24] XUE Sheng-guo, LI Meng, JIANG Jun, MILLAR G J, LI Chu-xuan, KONG Xiang-feng. Phosphogypsum stabilization of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Environmental Sciences, 2019, 77: 1–10. DOI: 10.1016/j.jes.2018.05.016.
[25] KONG Xiang-feng, TIAN Tao, XUE Sheng-guo, HARTLEY W, HUANG Long-bin, WU Chuan, LI Chu-xuan. Development of alkaline electrochemical characteristics demonstrates soil formation in bauxite residue undergoing natural rehabilitation [J]. Land Degradation and Development, 2018, 29(1): 58–67. DOI: 10.1002/ldr.2836.
[26] COURTNEY R G., JORDAN S N., HARRINGTON T. Physico-chemical changes in bauxite residue following application of spent mushroom compost and gypsum [J]. Land Degradation & Development, 2009, 20: 572–581. DOI: 10.1002/ldr.926.
[27] FELLET G, MARMIROLI M, MARCHIOL L. Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar [J]. The Science of the Total Environment, 2014, 468–469: 598–608. DOI: 10.1016/ j.scitotenv. 2013.08.072.
[28] CHANDRA R, YADAV S, YADAV S. Phytoextraction potential of heavy metals by native wetland plants growing on chlorolignin containing sludge of pulp and paper industry [J]. Ecological Engineering, 2017, 98: 134–145.
[29] LIU Yue-hong, YAN Xiao-zhen, JIAO zhen-fa. Effects of climate and eco-environment conditions on four-major- huai-medicine growth in Jiaozuo, Henan [J]. Meteorological, 2007, 33: 105–110.
[30] MUNNS R, TESTER M. Mechanisms of salinity tolerance [J]. Annu Rev Plant Biol, 2008, 59: 651–681. DOI: 10.1146/ annurev.arplant.59.032607.092911.
[31] ZHU Feng, LI Xiao-fei, XUE Sheng-guo, HARTLEY W, WU Chuan, HAN Fu-song. Natural plant colonization improves the physical condition of bauxite residue over time [J]. Environmental Science and Pollution Research, 2016, 23(22): 22897–22905. DOI: 10.1007/s11356-016-7508-1.
[32] FLOWERS T J, COLMER T D. Salinity tolerance in halophytes [J]. New Phytologist, 2008, 179: 945–963. DOI: 10.1111/j.1469-8137.2008.02531.x.
[33] DEINLEIN U, STEPHAN A B, HORIE T, LUO W, XU G, SCHROEDER J I. Plant salt-tolerance mechanisms [J]. Trends Plant Sci., 2014, 19: 371–379. DOI: 10.1016/ j.tplants.2014.02. 001.
[34] WOODARD H J, HOSSNER L, BUSH J. Ameliorating caustic properties of aluminum extraction residue to establish a vegetative cover [J]. Journal of Environmental Science and Health Part A-Toxic/Hazardous Substances & Environmental Engineering, 2008, 43: 1157–1166. DOI: 10.1080/ 10934520802171659.
[35] KONG Xiang-feng, LI Meng, XUE Sheng-guo, HARTLEY W, CHEN Cheng-rong, WU Chuan, LI Xiao-fei, LI Yi-wei. Acid transformation of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Hazardous Materials, 2017, 324: 382–390. DOI: 10.1016/j.jhazmat. 2016.10.073.
[36] CHEN Z, NEWMAN I M, MENDHAM N, ZHANG G, SHABALA S. Screening plants for salt tolerance by measuring K+ flux: A case study for barley [J]. Plant Cell & Environment, 2005, 28: 1230–1246. DOI: 10.1111/j.1365- 3040.2005.01364.x.
[37] SHABALA S, CUIN T A. Potassium transport and plant salt tolerance [J]. Physiologia Plantarum, 2008, 133: 651–669. DOI: 10.1111/ j.1399-3054. 2007. 01008.x.
[38] WEI Wen-xue, BILSBORROW P E, HOOLEY P, FINCHAM DARON A, LOMBI E. Salinity induced differences in growth, ion distribution and partitioning in barley between the cultivar Maythorpe and its derived mutant Golden Promise [J]. Plant and Soil, 2003, 250: 183–191. DOI: 10.1023/A:1022832107999.
[39] CHERIAN S, REDDY M P. Evaluation of NaCl tolerance in the callus cultures of suaeda nudiflora Moq [J]. Biologia Plantarum, 2003, 46: 193–198. DOI: 10.1023/A: 1022838224429.
(Edited by YANG Hua)
中文导读
赤泥堆场先锋植物的盐分积累及分布特性
摘要:盐分含量高是影响赤泥堆场植物生长的主要限制因子之一。通过对一个20多年的赤泥堆场开展生态调查,研究了先锋植物盐分积累及分布特性。结果发现:赤泥堆场出现14 种先锋植物;根系土Na+ 平均含量为19.5 cmol/kg,这表明先锋植物具有较高的盐耐性;植物地上部分Na+含量差异明显,马唐地上部Na+ 含量仅0.84 cmol/kg,扫帚苗地上部Na+ 含量高达39.7cmol/kg;马唐Na+迁移系数为0.13,水柏枝的Na+迁移系数高达2.92;扫帚苗和水柏枝的Na富集系数为2.66和3.29;狗牙根和马唐的Na+ 积累系数分别为0.07和0.04;扫帚苗、水柏枝、狗牙根和马唐均可作为赤泥堆场植被重建的先锋植物。研究结果为赤泥土壤化和堆场生态重建提供了科学参考。
关键词:赤泥堆场;先锋植物;盐分;赤泥土壤化;植被重建
Foundation item: Project(41877511) supported by the National Natural Science Foundation of China
Received date: 2018-10-29; Accepted date: 2018-11-28
Corresponding author: XUE Sheng-guo, PhD, Professor; Tel: +86-13787148441; E-mail: sgxue70@hotmail.com, sgxue@csu.edu.cn; ORCID: 0000-0002-4163-9383
Abstract: Bauxite residue disposal areas (BRDAs) are physically degraded and hostile to plant growth. Nevertheless, natural plant colonization was observed in an abandoned BRDA in Central China. The pioneer plant species at the disposal area were identified, whilst distribution characteristics of salt ions such as Na+, K+, and Ca2+ in plant tissues and rhizosphere residues were investigated. The mean concentration of exchangeable Na+ in the rhizosphere soils was 19.5 cmol/kg, which suggested that these pioneer plants had relatively high salinity resistance. Sodium content varied from 0.84 cmol/kg (Digitaria sanguinalis) to 39.7 cmol/kg (Kochia scoparia), whilst K to Na ratio varied from 0.71 (Myricaria bracteata) to 32.39 (Digitaria sanguinalis) in the shoots, which demonstrated that the salinity tolerance mechanisms of these pioneer species differed significantly. Accumulation factors of Na+ in local plant species ranged from 0.04 (D. sanguinalis) to 3.29 (M. bracteata), whilst the translocation factor varied from 0.13 (D. sanguinalis) to 2.92 (M. bracteata). The results suggested that four pioneer plant species including K. scoparia, M. bracteate, Cynodon dactylon and D. sanguinalis could be suitable for revegetation at other disposal areas.


