
J. Cent. South Univ. (2018) 25: 1928-1937
DOI: https://doi.org/10.1007/s11771-018-3883-8
Flocculation of flotation tailings in presence of silicate gel and polymer
YIN Zhi-gang(殷志刚)1, 2, Sultan Ahmed KHOSO1, 3, SUN Wei(孙伟)1, HU Yue-hua(胡岳华)1,ZHAI Ji-hua(翟计划)1, GAO Yue-sheng(高跃升)1, ZHANG Chen-hu(张谌虎)1, LIU Run-qing(刘润清)1
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Sichuan Non-ferrous Technology Group Co., Ltd, Chengdu 610037, China;
3. Department of Mining Engineering, Mehran University of Engineering & Technology,Jamshoro 76062, Pakistan
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
Flotation tailings were successfully flocculated in the presence of cationic polyacrylamide and silica gel. The effects of various parameters such as polymer weight, charge density, and pH on the rate of flocculation were also investigated in the current study. The flocculation mechanism of the flocculant on tailings was investigated using zeta potential and Fourier transform infrared (FTIR) measurements. The results obtained reveal that 1) sodium silicate gel, used as a binder for the consolidation of tailings form primary flocs, acts as an anchor and the adsorption of polymer flocculant on these anchors results in the formation of larger flocs and, consequently, enhanced settling rate; 2) flocculation in the presence of silica gel and polymer has a faster settling rate than single-polymer flocculation owing to the mechanisms of charge neutralization and bridging as identified using zeta potential and FTIR measurements. A pilot level study was conducted to investigate the influence of processed water on the flotation of scheelite. The results show that the proposed tailing disposal method could improve scheelite recovery by 2% (approximately) and could reduce the daily operation costs of the plant by approximately 108.57 USD.
Key words:
silica gel; polymer; flocculation; tailings disposal;
Cite this article as:
YIN Zhi-gang, Sultan Ahmed KHOSO, SUN Wei, HU Yue-hua, ZHAI Ji-hua, GAO Yue-sheng, ZHANG Chen-hu, LIU Run-qing. Flocculation of flotation tailings in presence of silicate gel and polymer [J]. Journal of Central South University, 2018, 25(8):.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3883-81 Introduction
China Molybdenum Co., Ltd., located in Henan province, currently processes more than 30000 t of ores per day to recover molybdenum and tungsten via the sequential selective flotation technique. Owing to the availability of land space, there are three molybdenum extraction plants distributed in the narrow valley. After molybdenum extraction, the rougher scheelite concentrate is recovered via flotation and thereafter transported to a tungsten upgrading plant via truck or pipeline depending on the distance between the upgrading and rougher flotation plants. In the upgrading plant (1200 t per day of rougher concentrate), a large amount of sodium silicate (10–20 kg/t rougher concentrate) is added as a dispersant to depress gangue minerals in order to separate scheelite from slurry. The discharged tailings are pumped into a buffer pond and thereafter mixed with the tailings from a nearby scheelite rougher flotation plant (3000 t/d raw ore). Approximately 3–5 kg/t of lime, used as a flocculant, is added to the buffer ponds to form a tailing stream in the tailing ponds where the coarse fraction of solids quickly settles. In contrast, fine particles settle very slowly and even remain suspended owing to the dispersion of sodium silicate. Owing to the increased tailing deposits, reduced water area, and reduced settling time, China Molybdenum Co., Ltd., is currently facing new challenges such as recycling of poor-quality supernatant from tailing ponds, which affects the recovery of valuable minerals. Furthermore, the addition of a large amount of lime increased the pH of the supernatant, resulting in a decrease in the recovery of molybdenite. Therefore, high-alkalinity recycled water is acidified before being pumped into the grinding and flotation system, which will further increase the capital cost. Considering all the aforementioned aspects, particularly the control of operation costs, China Molybdenum Co., Ltd., has undertaken considerable efforts to determine a replacement for the tailing disposal method.
Flocculation is an effective method for the treatment of tailings. Flocculation of minerals can be achieved via physical, chemical, or a combination of both mechanisms [1, 2]. The aggregation of fine particles can be achieved using organic polymers, inorganic polymers, or a combination of organic and inorganic compounds and is applied in many fields, such as waste-water treatment, mineral processing, and dewatering of oil sand [3–5]. Factors that influence the flocculation/ sedimentation of fine or ultra-fine solids in suspensions include the type of flocculant, charge density, molecular weight and ionization degree of the flocculant, mineralogical composition and particle size distribution of solid particles, and pH and chemical composition of the solutions [2, 6]. The evaluation of flocculation of fine particles usually depends on the measurements of the settling rate [7–9], turbidity [10–13], floc size [14, 15], and kinetics [16, 17].
The utilization of organic polymers, bioflocculant, inorganic polymers, or a combination of organic and inorganic compounds (polymer or metal ions) in waste-water treatment, mineral processing, and oil sand treatment can result in rapid flocculation, which in turn results in better settling or dewatering behavior, higher removal efficiency of hazardous compounds, and low costs [18]. Moreover, flotation separation efficiencies of ultrafine minerals can be improved via selective flocculation [19–21]. Various flocculation mechanisms have been reported to be responsible for different floc characteristics [22, 23]. Flocculation mechanisms usually include charge neutralization, electrostatic patch, bridging, depletion flocculation, or a combination of these mechanisms [13, 15]. A previous study reported that ideal flocculants are those that not only neutralize the charge but also form a bridge for particles to form larger flocs [24].
To achieve satisfactory liquid–solid separation efficiency, the optimization of different parameters, such as type, weight, charge density, and ionization of polymers, should be studied. In the present study, the settling rate, supernatant turbidity, zeta potential, and Fourier transform infrared (FTIR) spectra have been measured to investigate the settling behaviors of real mineral tailings. The aim of this work is to systematically examine the physical/chemical characteristics of real mineral tailing flocs coagulated using polyacrylamide (PAM) with silica gel used as a flocculant aid with different molecular weights and charge densities.
2 Experimental
2.1 Materials
Tailing pulp (which was a mixture of slurry of cleaner and rougher tailings) was used in the flocculation experiments. The representative samples of tailing pulp were collected from discharged tailings at the tungsten upgrading plant of China Molybdenum Co., Ltd., which contained an average solid content of 33 wt.%. The samples were collected during a day shift and stored in a 100-L container in ambient environment to protect from direct sunlight. Before conducting flocculation or characterization study, the slurry was stirred to obtain homogeneous pulp samples. The concentration of pulp solids, density, and water hardness were determined (see Table 1). Three high- molecular-weight polyacrylamide-based polymers were used in the flocculation tests. The characteristics of each polymer are listed in Table 2. Prior to the flocculation tests, a homogeneous stock solution (0.1%) of the polymer was prepared using distilled water. The pH of the slurry was adjusted to the desired value by adding sulfuric acid (AR or analytical reagent grade) or sodium hydroxide (AR) solutions prior to the addition of flocculants.
Table 1 Characterization test results of combined pulp

Table 2 Characteristics of polymers

2.2 Sample characterization
The mineralogical composition of the samples was determined using X-ray diffraction (XRD) with Rigaku D/max 2500, Japan. The particle size distribution of the combined tailings was determined by using a handle sieve. The size distribution of fine particles of the untreated flocculant and treated sample was analyzed by using a Malvern Mastersizer 2000 instrument (MALVERN Instruments Ltd., Worcestershire, UK) with a detection range 0.02–2000 μm. The pH was measured using a pH meter (PHS-3C).
2.3 Flocculation experiments
The flocculation experiments were conducted using the conventional cylinder test procedure recently reported by UCBEYIAY et al [25]. For each test, 1000 mL of homogeneous pulp (33% solids) was stirred first in a beaker provided with two baffles at 500 r/min for 5 min. Before adding the flocculants, the pH of the tailing pulp was adjusted by adding sulfuric acid or sodium hydroxide solutions. The requisite amount of flocculant was thereafter added under continued stirring at 500 r/min for 1 min to attain proper mixing of the flocculant solution in the suspension. Thereafter, the stirring speed was reduced to r/min for 1 min to allow floc growth. Subsequently, the suspension was transferred to a cylinder for sedimentation. After allowing the suspension to settle for 30 min, 100 mL of the supernatant was used to perform turbidity measurement using a turbidimeter (TSS Portable handheld instrument, HACH Company).
2.4 Zeta potential experiments
Zeta potential experiments were conducted using the Malvern Zeta Sizer Nano Series. A powder sample weighing 0.5 g (100% passing,5 μm) was suspended in 100 mL of the solution whose pH was adjusted using potassium hydroxide or sulfuric acid and the coarse particles were allowed to settle for 30 min. Subsequently, the suspension containing fine particles was introduced into the zeta potential analyzer. The potential at various pH values was measured automatically. The results presented herein are the average of three independent measurements.
2.5 FT-IR spectra analysis
FTIR spectra of the samples were recorded using a Bruker Alpha (Thermo, USA) FT-IR spectrometer at room temperature ((25±1) °C) in the range of 400–4000 cm–1 with KBr disk pellets. Prior to the test, the pure minerals were ground to a size of less than 5 μm in an auto agate mortar. Subsequently, 0.5 g of the samples was added to 100 mL of an aqueous solution with or without the flocculant at pH 8.0 and conditioned for 30 min. Subsequently, the samples were filtered, washed three times with distilled water, and dried in a vacuum oven at room temperature for 24 h.
2.6 Flotation experiments
The flotation experiments were conducted in an XFD-type flotation cell with a volume capacity of 1.5 L. The feed was added to the flotation cell with tap water/ recycled water or processed water to achieve the required pulp density (33%). Subsequently, the required amount of reagent was added sequentially, followed by conditioning for several minutes. The flotation time was set to 5 min based on the previous experimental results. The conditions of the flotation tests are illustrated in Figure 1. After successful flotation experiments, the concentrate and tailings were collected, filtered, dried, and weighed. Subsequently, they were examined using chemical analysis via inductively coupled plasma–atomic emission spectrometry (Barid Company, USA).
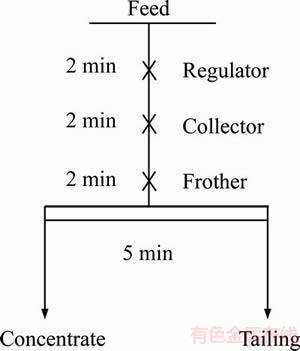
Figure 1 Flotation flowsheet of conditional experiment
3 Results and discussion
3.1 Characterization of tailings
The original and ≤0.025 mm tailings were analyzed using XRD to identify their major mineral compositions. It can be observed from Figure 2that both the samples exhibit similar peaks attributed to quartz, calcite, fluorite, garnet, and some dolomite, chlorite, and amphibole. A typical size distribution of the original tailings demonstrated in Figure 2(a) indicates that d80 and d50 of the tailings are 150 μm and 70 μm, respectively.
3.2 Flocculation tests
3.2.1 Determination of flocculant type
The flocculation experiments were performed in the presence of different types of polymers at 20 r/min. The effect of polymer type on the settling rate of the combined flotation tailings can be observed in Figure 3. The results shown in Figure 3 reveal that the settling rate increased with the increase in polymer molecular weight and charge density. Moreover, it is observed that an increase in the weight of the polymer results in an increase in the collision frequency in the attachment between the polymer and particles and consequently, the possibility of collision between the particles and polymer molecules is enhanced. This effect can be explained by the adsorption of the primary layer of flocculant and neutralization of the surface charge of particles; thus, the hydrogen or hydrophobic bonding force between the free polymer and adsorbed polymer caused the formation of further layers of polymer [15]. Furthermore, the neutralization between the particle and polymer could be improved by the higher charge density caused by the adsorption of a large amount of polymer onto the surface of the particles. However, all the polymers used in this work were long- chained with different charge densities and the high-charge polymer in particular resulted in the highest settling rates. This indicates that the charge neutralization mechanism plays an important role in the flocculation under the test conditions. 530AH, which has a higher molecular weight and density than 650B, was selected as the candidate for further testing, as described in the following section.
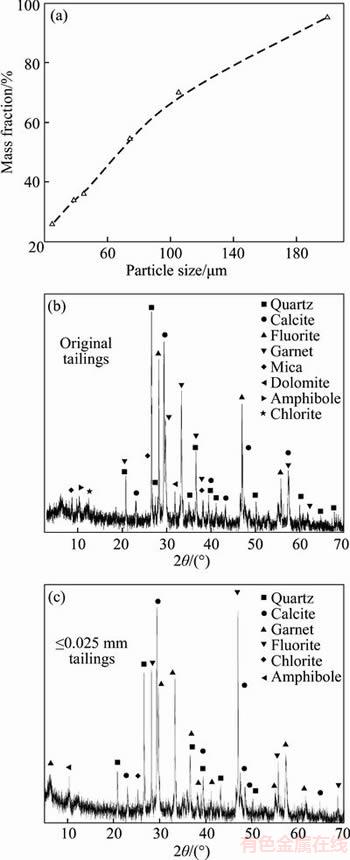
Figure 2 Particle size distribution of tailings (a) and XRD analysis of tailings (b, c)
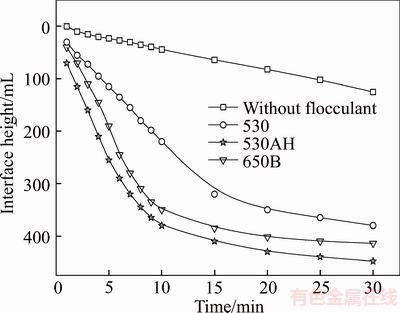
Figure 3 Volume settling curve for flocculants at pH 9.0 and dosage of 20×10–6
3.2.2 Effect of pH on flocculation process
It is widely accepted that pH has a significant influence on flocculation behavior and even determines the distribution of floc size [12, 26]. The optimal pH of the solution of combined slurry of tailings for the flocculation tests was determined by varying the pulp pH at a flocculant dosage of 20×10–6. Figure 4 shows the effect of change in pH on the single flocculant system. Notably, at low pH, the performance is significantly improved as compared with that under higher alkaline experimental conditions. As mentioned above, a large amount of sodium silicate (10–20 kg/t rougher concentrate) is required as a dispersant to depress the gangues in the upgrading plant. Therefore, silica gel could be formed with the addition of acid owing to the higher concentration of SiO32– ions. In an aqueous solution, the major species of sodium silicates are Si(OH)4 (uncharged silica gel) at pH<9.4, SiO(OH)3– at pH>9.4, and SiO2(OH)2– at pH>12.6 [27]. The formation of gel at pH<9.4 could trap the fine particles and form larger flocs, thus improving the settling rate of the tailings. It is widely acknowledged in the literature that the sodium silicate solution used is mainly composed of Si7O18H4Na4-type species [28], which, by adding dilute HCl, reacts through poly-condensation reactions and may form a semisolid network of silica gel by releasing water and NaCl (see Eq. (1)).
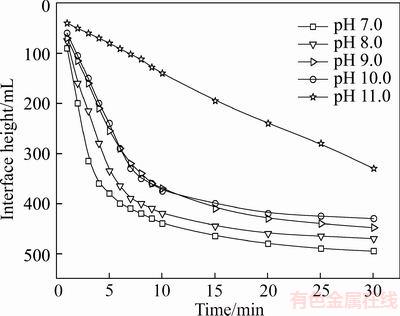
Figure 4 Volume settling curve for 530AH at a dosage of 20×10–6 at different pH values
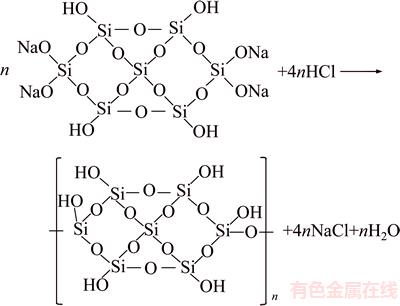 (1)
(1)
The silicate gel consists of a network of polymerized Si—O—Si—OH groups (silicate gel), which could trap mineral particles via solvation and hydrogen bonding forces, thus forming large flocs. The use of sodium silicate gel as a binder for cold consolidation of silica-based aggregates has been investigated. The results suggest that the gel–silica– sand system exhibits the existence domain of materials depending on the size distribution of sand [29]. Conversely, the polymers can flocculate particles via charge neutralization, electrostatic patch, bridging, depletion flocculation, or a combination of these mechanisms. This effect is explained by the theory mentioned previously, whereby the primary flocs (silica-gel floc) acted as an anchor, and the rest of the polymer flocculant was adsorbed on the anchor and formed larger flocs, thus enhancing the settling rate. It is apparent from a practical perspective that the optimal pH of pulp could satisfy both higher settling rate and low cost. Therefore, the pH level of the combined tailings pulp should be adjusted to approximately 8 before the addition of the polymer flocculant.
3.2.3 Effect of flocculant dosage on flocculation process
The effect of polymer dosage on the settling rate of the combined tailings is demonstrated in Figure 5. It can be observed from Figure 5 that the settling rate increases rapidly with the increase in the concentration of 530AH and reaches a maximum with the polymer dosage of 30×10–6. It was reported that the molecule cannot stretch fully once the concentration increases to a certain value as it reduces the contact opportunities between the particles and flocculant molecules [30]. The changes in turbidity as a function of the flocculant dosage are shown in Figure 6. The results indicate that the turbidity decreases sharply with the increase in the polymer dosage and reaches a maximum at a particular polymer dosage. However, flocculant dosage larger than 30×10–6 in the cylinder appears to be ineffective for the reduction of turbidity. At lower dosages, the floc size is very small owing to insufficient polymer adsorption on the particles. In particular, the flocs formed at low flocculant dosages are very fine and thus exhibit high turbidity, which sharply decreases with the addition of a higher flocculant dosage, which forms larger flocs and enhances the settling rate rapidly. The turbidity results and settling rate curves show consistency with the settling rates; the maximum settling rates are observed to correspond to the minimum turbidity of the tested flocculant.
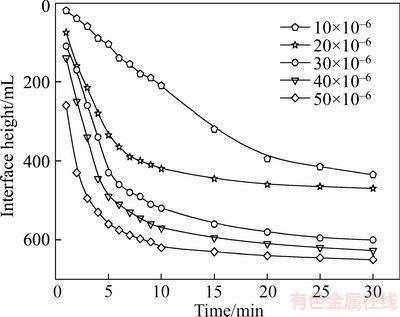
Figure 5 Effect of flocculant dosage on settling
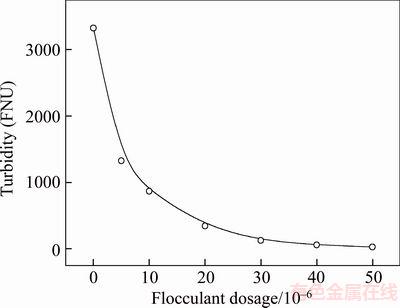
Figure 6 Curve of turbidity versus flocculant dosage (pH 8.0)
3.2.4 Size distribution of silica gel shear flocculation
The size distribution curves of the flocs produced with single-polymer flocculation and with polymer flocculation using silica gel in addition to those of unflocculated particles are shown in Figure 7. It is apparent that polymer flocculation with silica gel exhibits a faster settling rate than single-polymer flocculation. The typical size distributions of the flocs, presented in Table 3, indicate the average size, specific surface area, surface weighted mean, and volume weighted mean of the particles produced with flocculant in addition to those of the unflocculated particles. As compared with single-polymer flocculation, the floc size distribution obtained with polymer shear flocculation using silica gel shows a lower specific surface area and higher surface weighted mean and volume weighted mean. The d50 values for unflocculated particles, and particles produced with single-polymer flocculation and polymer flocculation with silica gel are 3.998, 7.170 and 9.192 μm, respectively. These values indicate that polymer flocculation with silica gel can form larger flocs with the same dosage of flocculant, as compared with single-polymer flocculation.
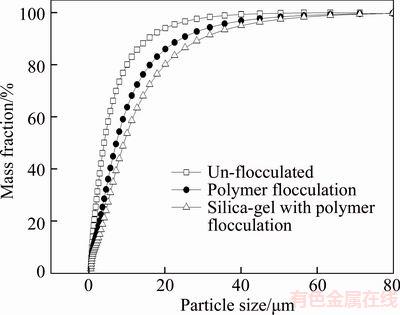
Figure 7 Size distribution of fine particles with different flocculation methods
Table 3 Summary of floc size measurements

3.3 Zeta potential
The zeta potential curves of the combined tailing suspensions plotted as a function of pH in the presence and absence of a flocculant are shown in Figure 8. It is evident that the tailings exhibit negative charge for the tested pH values with no zero point of charge. The absolute value of the charge increases with the increase in the pH and it increases significantly at higher pH values. With the addition of a flocculant, the surface charge of the particles increases, which indicates the adsorption of the flocculant on the particles owing to neutralization of charge. During the experiments, it was observed that 1×10–6 of 530AH did not reverse the surface charge of the fine particles, which indicates that complete charge neutralization of the particle surfaces is not mandatory for this system. The reduction of zeta potential to approximately –10 mV after the introduction of a flocculant (1×10–6) may have led to the charge neutralization through electrostatic attraction between the positively charged polymer and the negative surface sites of the particle. Thus, the adsorption of a large amount of positively charged polymer onto the surface of the combined tailings results in a decrease in the absolute charge value. This confirms the charge neutralization mechanism whereby the high-charge-density polymers neutralize the surface charge of tailings more rapidly than the low-charge- density polymers, which indicates consistency with the effect of polymer type on the settling rate of the combined tailings. Moreover, the bridging flocculation is enhanced as the bridge length is shortened owing to the reduction of repulsive interaction between the particles. Thus, it is concluded that the PAM (530AH) can lead to larger flocs owing to the contribution of both charge neutralization (with higher charge density) and bridging mechanism (higher molecular weight).
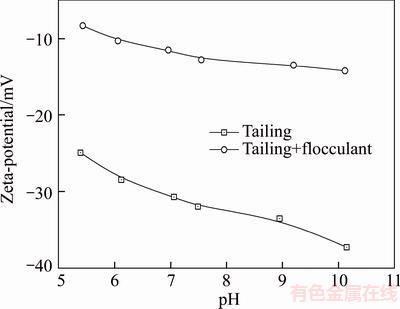
Figure 8 Zeta potential curves of combined tailing in 10–3 NaCl with 0.5 wt% solids as function of pH in presence and absence of flocculant (1×10–6)
3.4 FTIR study
FTIR has been used to identify the mechanism by which organic compounds are adsorbed on the surface of minerals. However, to the best of our knowledge, there are no reported studies regarding the applications of FTIR to describe the mechanism between a flocculant and minerals. Therefore, FTIR measurements were obtained to determine the mechanism by which reagents are adsorbed on mineral surfaces. The spectrum of 530AH only shows the 250–4000 cm–1 region as this area includes most of the main adsorption bands attributed to the flocculant. In the spectrum of the flocculant, the main bands were observed at 3438.23, 2514.84, 1797.23, 1429.21, 878.15, 841.70 and 715.15 cm–1 owing to –NH2 stretching, OH stretching vibration, C=O stretching, CH2 scissors vibration, CH=CH2 stretching, CH out-of-plane vibration, and strong out-of-plane vibration of CH2, respectively. The FT-IR spectra of the tailings before and after the treatment with a flocculant are presented in Figure 9. The FT-IR results, shown in Figure 9, reveal that, with the adsorption of the flocculant onto the tailings after treatment with 530AH, new bands appeared at 3434.14, 2515.15, 1799.49, 1423.88, 881.34, 842.85 and 713.14 cm–1. This indicates that the adsorption of the flocculant on the tailings is mainly physical adsorption because of the lower shift of IR bands on the surface of the tailings compared with the spectrum of the flocculant. It is apparent from the spectrum of the flocculant and the tailings onto which the flocculant is adsorbed that the higher coverage of surface with polymer adsorbed on the surface of tailings is due to the similar spectra.
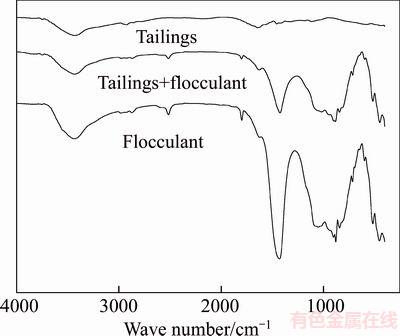
Figure 9 FTIR spectra of flocculant and combined tailings before and after treatment with 530AH at pH 8.0
3.5 Bench scale tests
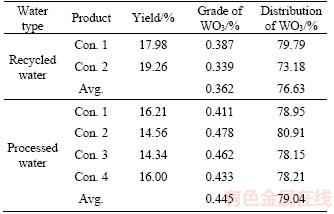
The effect of residual flocculants or their degradation products, which are polymer segments of low molecular weight, on the floatability of scheelite is investigated. The supernatant and dual-system shear flocculation (gel polymer) were collected by placing hoses in the bucket and siphoning the water out. As shown in Figure 10, the supernatant in all the test cases was clear. The principle flowsheet for processing the ore is demonstrated in Figure 1, and the results are summarized in Table 4.
Bench flotation tests using the supernatant from solid–liquid flocculation separation in the present study have shown a slight increase in the flotation performance as compared with that of recycled water. The results showed that water quality has a significant impact on the recovery of scheelite. The average recoveries of scheelite using processed water and recycled water are 76.63% and 79.04%, respectively. In other words, more than 2% of scheelite could be recovered by using the new tailing disposal method. Therefore, it is essential to adopt the newly developed tailing disposal method to replace the original method.

Figure 10 Image of supernatant under optimal flocculation condition
Table 4 Flotation test results of scheelite
3.6 Economic benefit analysis
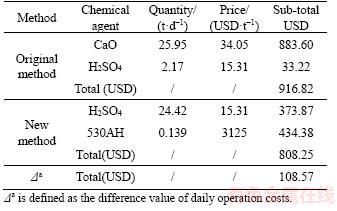
Rapid dewatering and thickening of whole tailings with ultrafine particles is one of the most important processes for the preparation of whole- tailings paste [31], which will benefit the mine operation. Different methods have different operating costs. The comparison of daily operation costs between the two methods (including only reagent cost) is listed in Table 5. The daily operation costs for the original method and the new process are 916.82 USD and 808.25 USD, respectively. Therefore, considering the efficiency and economy, the new process is more efficient, promising, and economical, and can reduce the daily costs of the plant by approximately 108.57 USD.
Table 5 Daily operation cost comparison between two methods
4 Conclusions
1) Characterization studies on the original and ≤0.025 mm flotation tailings revealed that the tailings are mainly composted of quartz, calcite, fluorite, and garnet mineral phases. Moreover, some minor minerals, such as dolomite, chlorite, and amphibole, were present.
2) The effect of molecular weight of PAM on the characteristics of the combined flotation tailing was investigated. The results suggested that, with the increase in the polymer molecular weight and charge density, the settling rate increases. This is mainly due to the long molecular chain, which interacts with the particles at longer distances, thus enhancing the settling rate.
3) Flocculation experiments suggested that the flocculation in the presence of silica gel and polymer exhibits a faster settling rate than the conventional single-polymer flocculation owing to the formation of larger flocs.
4) Zeta potential and FTIR measurements further confirmed that PAM can form larger flocs owing to the contribution of both charge neutralization and bridging.
5) The flotation results indicated that the proposed process is more efficient, promising, and economical, and can lower the cost of tailing treatment.
References
[1] SWORSKA A, LASKOWSKI J S, CYMERMAN G. Flocculation of the Syncrude fine tailings Part I. Effect of pH, polymer dosage and Mg2+, Ca2+ cations [J]. Int J Miner Process, 2000, 60: 85–93.
[2] WANG Chen, HARBOTTLE D, LIU Qing-xia, XU Zheng-he. Current state of fine mineral tailings treatment: A critical review on theory and practice [J]. Miner Eng, 2014, 58: 113–131.
[3] FAN Ai-xing, NICHOLAS J T, SOMASUNDARAN P. A study of dual polymer flocculation [J]. Colloid Surface A, 2000, 162: 141–148.
[4] FARKISH A, FALL M. Rapid dewatering of oil sand mature fine tailings using super absorbent polymer (SAP) [J]. Miner Eng, 2013, 50–51: 38–47.
[5] YANG Zhong-lian, LU Xi-wen, GAO Bao-yu, WANG Yan, YUE Qin-yan, CHEN Ting. Fabrication and characterization of poly (ferric chloride)-polyamine flocculant and its application to the decolorization of reactive dyes [J]. J Mater Sci, 2014, 49: 4962–4972.
[6] JI Ya-guan, LU Qing-ye, LIU Qing-xia, ZENG Hong-bo. Effect of solution salinity on settling of mineral tailings by polymer flocculants [J]. Colloid Surface A, 2013, 430: 29–38.
[7] DASH M, DWARI R K, BISWAL S K, REDDY P S R. CHATTOPADHYAY P, MISHRA B K. Studies on the effect of flocculant adsorption on the dewatering of iron ore tailings [J]. Chem Eng J, 2011, 173: 318–325.
[8] GORAKHKI M H, BAREITHER C A. Salinity effects on sedimentation behavior of kaolin, bentonite, and soda ash mine tailings [J]. Appl Surf Sci, 2015, 114: 593–602.
[9] MCGUIRE M J, ADDAI-MENSAH J, BREMMELL K E. The effect of polymer structure type, pH and shear on the interfacial chemistry, rheology and dewaterability of model iron oxide dispersions [J]. Colloid Surface A, 2006, 275: 153–160.
[10] LI Tao, ZHU Zhe, WANG Dong-sheng, YAO Chong-hua, TANG Hong-xiao. Characterization of floc size, strength and structure under various coagulation mechanisms [J]. Powder Technol, 2006, 168: 104–110.
[11] LIU Ya, LV C C, DING Jian, QIAN Peng, ZHANG Xiao-meng,YU Yang, YE Shu-feng, CHEN Yun-fa. The use of the organic–inorganic hybrid polymer Al(OH)3- polyacrylamide to flocculate particles in the cyanide tailing suspensions [J]. Miner Eng, 2016, 89: 108–117.
[12] SABAH E, CENGIZ I. An evaluation procedure for flocculation of coal preparation plant tailings [J]. Water Res, 2004, 38: 1542–1549.
[13] ZHU Zhe, LI Tao, LU Jia-juan, WANG Dong-sheng, YAO Chong-hua. Characterization of kaolin flocs formed by polyacrylamide as flocculation aids [J]. Int J Miner Process, 2009, 91: 94–99.
[14] ALAM N, OZDEMIR O, HAMPTON M A, NGUYEN A V. Dewatering of coal plant tailings: Flocculation followed by filtration [J]. Fuel, 2011, 90: 26–35.
[15] NASSER M S, JAMES A E. The effect of polyacrylamide charge density and molecular weight on the flocculation and sedimentation behaviour of kaolinite suspensions [J]. Sep Purif Technol, 2006, 52: 241–252.
[16] ADACHI Y, XIAO J. Initial stage of bridging flocculation of PSL particles induced by an addition of polyelectrolyte under high ionic strength [J]. Colloid Surface A, 2013, 435: 127–131.
[17] GRABSCH A F, FAWELLl P D, ADKINS S J, BEVERIDGE A. The impact of achieving a higher aggregate density on polymer-bridging flocculation [J]. Int J Miner Process, 2013, 124: 83–94.
[18] WANG Hui-min, MIN Xiao-bo, CHAI Li-yuan, SHU Yu-de. Biological preparation and application of poly-ferric sulfate flocculant [J]. Trans Nonferrous Met Soc China, 2011, 21: 2542–2547.
[19] ZHANG Ting, QIN Wen-qing, YANG Cong-ren, HUANG Shui-peng. Floc flotation of marmatite fines in aqueous suspensions induced by butyl xanthate and ammonium dibutyl dithiophosphate [J]. Trans Nonferrous Met Soc China, 2014, 24: 1578–1586.
[20] YIN Wan-zhong, YANG Xiao-sheng, ZHOU Da-peng, LI Yan-jun, LU Zhen-fu. Shear hydrophobic flocculation and flotation of ultrafine Anshan hematite using sodium oleate [J]. Trans Nonferrous Met Soc China, 2011, 21: 652–664.
[21] LIU Wen-li, SUN Wei, HU Yue-hua. Effects of water hardness on selective flocculation of diasporic bauxite [J]. Trans Nonferrous Met Soc China, 2012, 22: 2248–2254.
[22] HUANG Xin, GAO Bao-yu, YUE Qin-yan, ZHANG Ying-ying, SUN Sheng-lei. Compound bioflocculant used as a coagulation aid in synthetic dye wastewater treatment: the effect of solution pH [J]. Sep Purif Technol, 2015, 154: 108–114.
[23] ZHENG Huai-li, MA Jiang-ya, ZHU Chuan-jun, ZHANG Zhi, LIU Li-wei, SUN Yong-jun, TANG Xiao-min. Synthesis of anion polyacrylamide under UV initiation and its application in removing dioctyl phthalate from water through flocculation process [J]. Sep Purif Technol, 2014, 123: 35–44.
[24] BESRAA L, SENGUPTA D K, ROY S K, AY P. Polymer adsorption: Its correlation with flocculation and dewatering of kaolin suspension in the presence and absence of surfactants [J]. Int J Miner Process, 2002, 66: 183–202.
[25] UCBEYIAY H, OZKAN A. Two-stage shear flocculation for enrichment of fine boron ore containing colemanite [J]. Sep Purif Technol, 2014, 132: 302–308.
[26] YU Xiang, SOMASUNDARAN P. Role of polymer conformation in interparticle-bridging dominated flocculation [J]. J Colloid Interf Sci, 1996, 177: 283–287.
[27] FENG Bo, LUO Xian-ping, WANG Jin-qing, WANG Peng-cheng. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant [J]. Miner Eng, 2015, 80: 45–49.
[28] MONIQUE T T, DOMINIQUE M, ANDRE L, SYLVIE R. Identification of solvated species present in concentrated and dilute sodium silicate solutions by combined 29Si NMR and SAXS studies [J]. J Colloid Interf Sci, 2010, 352: 309–315.
[29] SEKA S K, MONIQUE T T, JULIEN S, SYLVIE R. Consolidation mechanism of materials obtained from sodium silicate solution and silica-based aggregates [J]. J Non-cryst Solids, 2011, 357: 3013–3021.
[30] LIU Jin-wei, HU Hui-ping, WANG Meng, CHEN Xiang-pan, CHEN Qi-yuan, DING Zhi-ying. Synthesis of modified polyacrylamide with high content of hydroxamate groups and settling performance of red mud [J] Journal of Central South University, 2015, 22(6): 2037–2080.
[31] RUAN Zhu-en, LI Cui-ping, SHI Cong. Numerical simulation of flocculation and settling behavior of whole- tailings particles in deep-cone thickener [J]. Journal of Central South University, 2016, 23(3): 740–749.
(Edited by YANG Hua)
中文导读
高分子有机物与硅胶混凝处理浮选尾矿
摘要:采用阳离子聚丙烯酰胺与硅胶作为絮凝剂成功实现尾矿混合絮凝,考察了有机高分子絮凝剂分子量、电荷密度、溶液pH值等参数对絮凝沉降速度的影响。通过动电位、红外光谱研究了混凝剂絮凝尾矿的作用机理,结果表明:1)硅胶作为尾矿颗粒的粘结剂,先将尾矿形成第一集合颗粒;高分子絮凝剂絮凝第一集合颗粒并形成较大的絮团,同时加速尾矿沉降;2)动电位与红外光谱分析结果表明硅胶与有机高分子组合对尾矿的沉降速度效果明显优于单一絮凝剂,主要原因是混凝剂同时兼具中和电荷和桥联作用。考察了混凝处理尾矿回水对白钨矿浮选指标的影响,试验结果表明:采用回水能够提高白钨矿回收率2%,且每天能够为企业节约108.75美元的成本。
关键词:硅胶;聚合物;絮凝;尾矿处理
Foundation item: Project(2016zzts109) supported by the Innovation Driven Plan of Central South University, China; Project(2015CX005) supported by the Innovation driven Program of National Basic Research Program of China; Project(B14034) supported by the Program of Introdution Talents of Discipline to Universities, China (111 Project)
Received date: 2016-09-06; Accepted date: 2016-10-24
Corresponding author: SUN Wei, PhD, Professor; Tel: +86–731–88830482; E-mail: sunmenghu@csu.edu.cn; ORCID: 0000-0002- 9173-2682; LIU Run-qing, PhD, Associate Professor; Tel: +86–731–88830482; E-mail: liurunqing@126.com; ORCID: 0000-0002-7600-5452
Abstract: Flotation tailings were successfully flocculated in the presence of cationic polyacrylamide and silica gel. The effects of various parameters such as polymer weight, charge density, and pH on the rate of flocculation were also investigated in the current study. The flocculation mechanism of the flocculant on tailings was investigated using zeta potential and Fourier transform infrared (FTIR) measurements. The results obtained reveal that 1) sodium silicate gel, used as a binder for the consolidation of tailings form primary flocs, acts as an anchor and the adsorption of polymer flocculant on these anchors results in the formation of larger flocs and, consequently, enhanced settling rate; 2) flocculation in the presence of silica gel and polymer has a faster settling rate than single-polymer flocculation owing to the mechanisms of charge neutralization and bridging as identified using zeta potential and FTIR measurements. A pilot level study was conducted to investigate the influence of processed water on the flotation of scheelite. The results show that the proposed tailing disposal method could improve scheelite recovery by 2% (approximately) and could reduce the daily operation costs of the plant by approximately 108.57 USD.


