
Spectra of sodium aluminate solutions
MA Shu-hua(马淑花)1, 2, ZHENG Shi-li(郑诗礼)1, XU Hong-bin(徐红彬)1, ZHANG Yi(张 懿)1
1. Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100080, China;
2. Graduate University of Chinese Academy of Sciences, Beijing 100039, China
Received 28 September 2006; accepted 16 April 2007
Abstract:
The UV spectra of sodium aluminate solutions were obtained in the sodium oxide concentration range from 59 to 409 g/L and the caustic ratio range from 1.5 to 4.0 to reveal the structure characteristics of them. It is found that a new peak appears at about 370 nm besides peaks at about 220 and 266 nm in all solutions. The new peak is strongly favored by high hydroxide concentration and high caustic ratio. And it only appears when the solutions are prepared by dissolving sodium hydroxide and aluminum hydroxide. In addition, the IR and Raman spectra of sodium aluminate solutions with high alkali concentration and high caustic ratio were measured, and the UV spectra of aqueous solutions of ![]() and
and ![]() were measured as well. According to the crystal field theory in coordination chemistry as well as the above spectra characteristics, this new peak at about 370 nm is determined as the evidence of a new species of aluminate ion with a coordination number of 6.
were measured as well. According to the crystal field theory in coordination chemistry as well as the above spectra characteristics, this new peak at about 370 nm is determined as the evidence of a new species of aluminate ion with a coordination number of 6.
Key words:
sodium aluminate solution; UV spectrum; IR absorption; Raman spectrum; aluminate ion;
1 Introduction
The alkali processing methods, especially the Bayer process, have played a very important role in the production of alumina for more than 100 years[1-3]. But the key problem of slow crystal growth from supersaturated sodium aluminate solutions has not been solved because of the complexity in the structure of all aluminate species present in aqueous sodium aluminate solution[4]. Although extensive work on identifying the solution structure has been carried out since last century, no agreeable result has so far been achieved. Nowadays, the existence of four-coordinate aluminum species is not doubtful, but it continues to be a subject of considerable controversy for other species.
LIPPINCOTT et al[5] measured the Raman spectra of the aluminate ions in alkaline solutions and took the observed spectra and agreement in force constant calculations as evidence for the existence of a Td ![]() structure. Later, CARREIRA et al[6] pointed out that two aluminate species,
structure. Later, CARREIRA et al[6] pointed out that two aluminate species, ![]() or a polymeric 6-coordinate aluminate ion and
or a polymeric 6-coordinate aluminate ion and![]() , predominantly existed in the pH range of 8-12 and above 12.5, respectively, by using Raman and infrared data. In a subsequent detailed study[7], on the basis of the Raman and infrared spectra of sodium aluminate dissolved in H2O and D2O as well as the 23Na and 27Al NMR spectra of sodium aluminate dissolved in H2O, it was reported that a structure of tetrahedral
, predominantly existed in the pH range of 8-12 and above 12.5, respectively, by using Raman and infrared data. In a subsequent detailed study[7], on the basis of the Raman and infrared spectra of sodium aluminate dissolved in H2O and D2O as well as the 23Na and 27Al NMR spectra of sodium aluminate dissolved in H2O, it was reported that a structure of tetrahedral ![]() was formed when the aluminum concentration is below 1.5 mol/L, followed by a process of gradual dehydration to
was formed when the aluminum concentration is below 1.5 mol/L, followed by a process of gradual dehydration to ![]() as the concentration is increased to 6 mol/L. According to the similar results in Refs.[8-9] based on the infrared and Raman spectra data as well as the MD simulation results, more than one aluminate ion species were in equilibrium within concentrated aluminate solutions besides
as the concentration is increased to 6 mol/L. According to the similar results in Refs.[8-9] based on the infrared and Raman spectra data as well as the MD simulation results, more than one aluminate ion species were in equilibrium within concentrated aluminate solutions besides![]() .
.
Another spectra data presumed that several other aluminate species, including ![]() or
or![]() , were present in the sodium aluminate solutions except
, were present in the sodium aluminate solutions except ![]() and
and ![]() [10-13]. But no direct evidence was ever given.
[10-13]. But no direct evidence was ever given.
In a recent study about the UV spectra of sodium aluminate solutions by using MNDO and DV-Xα methods, LIU et al[14] reasoned that the UV absorption peaks at 266.6 and 234.4 nm were due to the electronic transition between the HOMO and LUMO of Al2O-![]() and
and ![]() respectively. Comparatively, in a former study by SATO[15], these peaks were presumed to be related to the formation of
respectively. Comparatively, in a former study by SATO[15], these peaks were presumed to be related to the formation of ![]() and
and ![]() respectively. However, such result can hardly be accepted due to the strong caustic environment.
respectively. However, such result can hardly be accepted due to the strong caustic environment.
In contrast to the evidence of different aluminate species present in alkaline solution, there seems no controversy about the aluminum-bearing ion octahedrally coordinated by 6?H2O moleculars in acidic solutions. During the investigation on the 17O-enriched acidified aqueous solutions of AlCl3 via the enriched water and sideband detection technique to increase the signal-to- noise ratio of NMR, it was reported that the aluminate species has a coordination number of 6 when the Al3+ concentration is about 1.5 mol/L[16-17].
Due to its high accuracy and sensitivity, UV spectra analysis is widely used in various fields including chemical industry, biological engineering, and medicine industry. In order to obtain further information on the aluminate species in sodium aluminate solutions, the UV spectra of sodium aluminate solutions with different concentrations and alkaline ratios were systematically investigated with additional infrared and Raman spectra in this work.
2 Experimental
Concentrated sodium aluminate solutions with different caustic ratios (i.e. molar ratio of Na2O to Al2O3, αk) were prepared by dissolving appropriate amount of sodium hydroxide(AR) into super-purified water in a polytetrafluoroethylene reactor, dissolving aluminum trihydroxide(AR) in the obtained hot caustic solution, and then rapidly filtering the hot aqueous solution system through a membrane with pore size of 0.22 ?m. The other sodium aluminate solutions were obtained by diluting the corresponding concentrated sodium aluminate solutions with the same caustic ratio.
Aluminum chloride was prepared by adding hydrochloric acid with a mass fraction of 10% to fresh sodium aluminate solution prepared according to the method mentioned above. Sodium hexafluoroaluminate solution was prepared by adding excess sodium fluoride to aluminum chloride.
The UV spectra were recorded on a UV2000 spectrophotometer (manufactured by LabTech Co., USA) which was fitted with the usual accessories for the ultra-violet range by using matched 1.00 cm fused silica cells.
The IR spectra of thin films of solution between KBr plates were collected using an IMPCT-400 spectrophotometer (manufactured by Nicolet Co., USA).
The Ramam spectrum of sodium aluminate solution was obtained with an HR800 spectrometer at a wavelength of 532 nm (manufactured by JY Co., France).
The concentration of sodium aluminate solution was determined by an Optima 5300DV ICP-AES (manufactured by Perlin-Elmer, USA).
3 Results
The UV absorption spectra of the sodium aluminate solutions, obtained by varying the concentration of sodium hydroxide at fixed molar ratios of Na2O to Al2O3 of 1.5, 2.0 and 4.0, are shown in Fig.1. In addition, the UV spectra of the sodium hexafluoroaluminate solution and aluminum chloride solution, the FT-IR and Raman spectra of the aqueous solution with a caustic concentration of 353 g/L Na2O and a caustic ratio of 4.0 are also obtained, as shown in Figs.2-4, respectively.
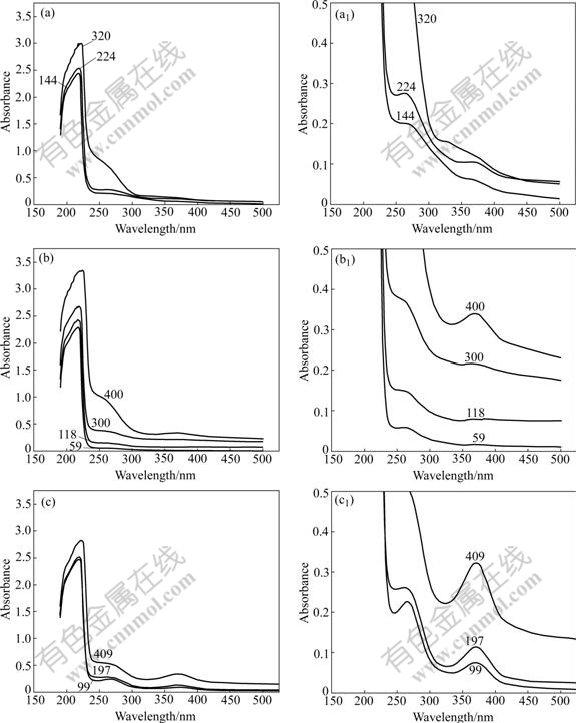
Fig.1 UV absorption spectra of sodium aluminate solutions containing alkalies of various concentrations at molar ratios of Na2O to Al2O3 of 1.5 (a, a1), 2.0 (b, b1) and 4.0 (c, c1) (Numerals on curves denote Na2O concentration in g/L. Figs.1(a1), (b1) and (c1) are partial zooms in Figs.1(a), (b) and (c), respectively)
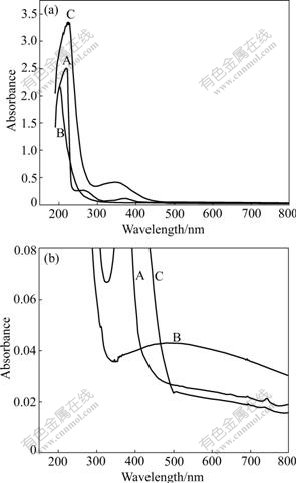
Fig.2 UV absorption spectra of sodium aluminate solution (curve A), sodium hexafluoroaluminate solution (curve B) and aluminum chloride solution (curve C) (Fig.2(b) is partial zoom in Fig.2(a))

Fig.3 FT-IR spectrum of sodium aluminate solution with caustic concentration of 353 g/L Na2O and caustic ratio of 4.0
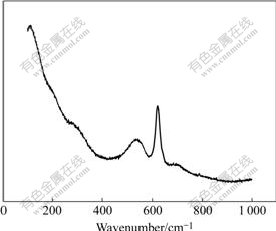
Fig.4 Raman spectrum of sodium aluminate solution with caustic concentration of 353 g/L Na2O and caustic ratio of 4.0
Three bands, centered at about 220, 260 and 370 nm, respectively, were observed on the UV spectra of each solution. Because of the caustic conditions, the observed bands of the intense vibrations at about 220 and 266 nm should be attributed to the electronic transition between the HOMO and LUMO of the aluminate ions of ![]() and
and ![]() instead of
instead of ![]() and
and ![]() ·2H2O, in solutions, respectively[14]. The broad and low-intensity band centered at about 370 nm has not been reported previously. It exists only in the solutions prepared by aluminum trihydroxide.
·2H2O, in solutions, respectively[14]. The broad and low-intensity band centered at about 370 nm has not been reported previously. It exists only in the solutions prepared by aluminum trihydroxide.
It is found that the bands associated with aluminate ion vibrations increase in intensity as the alkaline concentration or caustic ratio increases, as shown in Fig.1. The increase in intensity seems to be proportional to the increase in caustic ratio, but not directly proportional to the increase in alkaline concentration in the tested concentration range. Furthermore, the changes in band frequency at the minimum intensity near 370 nm are too little to be detected.
The comparative spectra of aluminum-contained solutions with different ligand systems including OH- (0.8 mol/L of Al), H2O (0.1 mol/L of Al), and F-(0.1 mol/L of Al), are shown in Fig.2. Two new low-intensity bands are found at about 340 nm in the spectra for aluminum chloride solution and about 500 nm for sodium hexafluoroaluminate solution.
Besides the above spectra, the FT-IR and Raman spectra of a sodium aluminate solution with caustic concentration of 353 g/L Na2O and caustic ratio of 4.0 are also shown in Figs.3 and 4, respectively. Three bands are found at about 890, 725 and 630 cm-1 on the infrared spectrum. And four peaks appear at about 285, 540, 621and 702 cm-1 on the Raman spectrum. Bands observed at about 725 cm-1 (infrared) and 621 cm-1 (Raman) are attributed to asymmetric and symmetric Al—O stretching vibrations, respectively, of the aluminate ion ![]() in solution. Other low-intensity bands at frequencies of about 890 and 630 cm-1 (infrared) and 702 and 540 cm-1 (Raman) indicate the formation of a second aluminate ion species, the dimmer
in solution. Other low-intensity bands at frequencies of about 890 and 630 cm-1 (infrared) and 702 and 540 cm-1 (Raman) indicate the formation of a second aluminate ion species, the dimmer ![]() [7]. Obviously, the band at about 285 cm-1 suggests that a new ion really exists in such aluminate solutions.
[7]. Obviously, the band at about 285 cm-1 suggests that a new ion really exists in such aluminate solutions.
4 Discussion
For sodium aluminate solutions, it is generally agreed that the most abundant aluminate ion in solutions is of a Td ![]() or dimmer
or dimmer ![]() structure[7], but other polyvalent ions such as
structure[7], but other polyvalent ions such as ![]()
![]()
![]()
![]() and
and![]() are also suggested[11]. The observed frequencies in infrared and Raman spectra provide the evidence of the existence of
are also suggested[11]. The observed frequencies in infrared and Raman spectra provide the evidence of the existence of ![]() or
or ![]() UV spectra studies demonstrate that the bands at about 234 and 266.6 nm are due to existence of
UV spectra studies demonstrate that the bands at about 234 and 266.6 nm are due to existence of ![]() and Al2O
and Al2O![]() respectively. Accordingly, the discussion here has excluded these two bands in UV spectra, and mainly focuses on the low-intensity band near 370 nm.
respectively. Accordingly, the discussion here has excluded these two bands in UV spectra, and mainly focuses on the low-intensity band near 370 nm.
Compared with data obtained by LIU et al[14] and SATO et al[15], a new band has been found at about 370 nm, as illustrated in Figs.1 and 2. Although the new band is significantly weak in intensity, it must be assigned to a new species favored by high hydroxide concentration and especially by high caustic ratio.
A possible process, for the new ion-pair formation between ![]() and OH-, occurs according to Eqns.(1) and (2) due to the fact that the band near 370 nm is strongly favored by caustic ratio as above analysis:
and OH-, occurs according to Eqns.(1) and (2) due to the fact that the band near 370 nm is strongly favored by caustic ratio as above analysis:
![]() →
→![]() (1)
(1)
![]() →
→![]() (2)
(2)
In order to seek the evidence of the above hypothesis, a series of UV spectra of other 6-coordinate compounds, including aluminum chloride and sodium hexafluoroaluminate, are obtained. According to the crystal field theory in coordination chemistry, the spectrum chemistry order is: F-<OH-<H2O. That is, for the same central ion-aluminum, the intensity of coordination field for H2O-contained ligand is the strongest in the three compounds, that for OH--containing ligand is medium, and that for F--containing complex is the weakest. Therefore, the UV spectra band frequencies (in nm-1) should be in the order of F->OH->H2O. In fact, the spectra measured are exactly consistent with this interpretation. On the basis of this comparison, it can be concluded that the band at about 370 nm is due to the existence of 6-coordinate aluminum, in spite of its weak intensity.
To further study the aluminate ions in the caustic solution, the FT-IR and Raman spectra of sodium aluminate with caustic concentration of 353g/L Na2O at caustic ratio of 4 were collected. Except the bands at about 630, 725 and 890 cm-1 (infrared) and the bands at 540, 621 and 702 cm-1 (Raman), which are interpreted as the formation of the aluminate ion ![]() or
or ![]() in solution, a new weaker band appears at about 285 cm-1 (Raman). Undoubtedly, this new band suggests that a new ion really exists in such aluminate solutions, although it cannot provide sufficient evidence to explain the presence of 6-coordinate aluminum.
in solution, a new weaker band appears at about 285 cm-1 (Raman). Undoubtedly, this new band suggests that a new ion really exists in such aluminate solutions, although it cannot provide sufficient evidence to explain the presence of 6-coordinate aluminum.
An additional interesting phenomenon observed in this study is that the band at about 370 nm disappears when the solution temperature is increased up to 135 ℃, demonstrating that the equilibrium between different aluminate ions is highly temperature-dependent.
5 Conclusions
On the basis of the UV spectroscopic evidence as well as some FT-IR and Raman data obtained about aluminates solutions over a wide range of sodium concentration and caustic ratio, it is concluded that, a new species of aluminate ion with a coordination number of 6 exists in the equilibrium with![]() and
and ![]() although the presence of other less concentrated polymeric aluminate species cannot be excluded. Additional evidence demonstrates that the equilibrium between these new ions is highly temperature-dependent.
although the presence of other less concentrated polymeric aluminate species cannot be excluded. Additional evidence demonstrates that the equilibrium between these new ions is highly temperature-dependent.
Acknowledgments
The authors are thankful to Professor KE Jia-jun for his useful suggestions on the presentation of these results. Financial supports from the National Natural Science Foundation of China (50234040) and the National High- Tech Research and Development Program of China (863 Project) (2005AA647010) are gratefully acknowledged.
References
[1] WHITTINGTON B I, CARDILE C M. The chemistry of tricalcium aluminate hexahydrate relating to the Bayer industry [J]. Mineral Processing, 1996, 48: 21-38.
[2] COUNTER J A, ADDAI-MENSAH J, RALSTON J. The formation of Al(OH)3 crystals from supersaturated sodium aluminate solutions revealed by cryovitrification-transmission electron microscopy [J]. Colloids and Surfaces, 1999, 154: 389-398.
[3] CHEN G H, CHEN Q Y, YIN Z L, YIN Z M. Characterization of irregular seeds on gibbsites precipitated from caustic aluminate solutions [J]. Trans Nonferrous Met Soc China, 2006, 16(2): 483-487.
[4] LI X B, FENG G T, ZHOU Q S, PENG Z H, LIU G H. Phenomena in late period of seeded precipitation of sodium aluminate solution [J]. Trans Nonferrous Met Soc China, 2006, 16(4): 947-950.
[5] LIPPINCOTT E R, PSELLOS J E, TOBIN M C. The Raman spectra and structures of aluminate and zincate ions [J]. J Chem Phys, 1952, 20: 536.
[6] CARREIRA L A, MARONI V A, SWAINE J W, PLUMB R C. Raman and infared spectra and structures of the aluminate ions [J]. J Chem Phys, 1966, 45(6): 2216-2220.
[7] MOOLENAAR R J, EVANS J C, MCKEEVER L D. The structure of the aluminate ion in solutions at high pH [J]. J Phys Chem, 1969, 74(20): 3629-3636.
[8] HELEN W. Spectroscopy of concentrated sodium aluminate solutions [J]. Applied Spectroscopy, 1998, 52(2): 250-258.
[9] CHEN Y, FENG Q M, LIU K, CHEN Y D, ZHANG G F. Study on the structure of Bayer liquor with spectroscopy and MD simulation [J]. Chemical Physics Letters, 2006, 422: 406-411.
[10] QIU G F, Chen N Y, YAN L C, Li Y. Spectroscopic studies on highly concentrated sodium aluminate solutions [J]. The Chinese Journal of Nonferrous Metals, 1996, 6(3): 53-56. (in Chinese)
[11] WANG Y J, ZHAI Y C, TIAN Y W, LIU L L. Effect of caustic ratio on structure and characteristic of sodium aluminate solution [J]. Journal of Northeastern University: Nature Science, 2003, 24(8): 774-776. (in Chinese)
[12] WANG Y J, ZHAI Y C, TIAN Y W, HAN Y X. Infrared and raman spectra of aluminate and SiO2-containing sodium aluminate solutions [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(1): 271-275. (in Chinese)
[13] SIPOS P, HEFTER G, MAY P M. 27Al NMR spectroscopic studies of alkaline aluminate solutions with extremely high caustic content— Does the octahedral species Al(OH)63- exist in solution? [J]. Talanta, 2006, 70(4): 761-765.
[14] LIU H L, CAO Y L, CHEN N Y. The absorption peaks of sodium aluminate solutions [J]. Acta Physico-chemica Sinica, 1992, 8(4): 441-444. (in Chinese)
[15] SATO T. Absorption spectra of sodium aluminate solutions [J]. Anorg All Chem, 1970, 376: 205-209.
[16] GRUNWALD E, FONG D W. Acidity and association of aluminum ion in dilute aqueous acid [J]. J Phys Chem, 1969, 73(3): 650-653.
[17] CONNICK R E, FIAT D N. Coordination numbers of beryllium and aluminum ions in aqueous solutions [J]. Ibid, 1963, 39: 1349-1351.
Foundation item: Project(50234040) supported by the Key Project of the National Natural Science Foundation of China; and Project(2005AA647010) supported by the National High-Tech Research and Development Program of China
Corresponding author: ZHENG Shi-li; Tel: +86-10-62610244; Fax: +86-10-62561822; E-mail: slzheng@home.ipe.ac.cn


