
DOI: 10.11817/j.issn.1672-7207.2021.02.003
氨基碳纳米管掺杂铅基阳极材料的析氧行为
杨长江,张旭,赵吕兴
(昆明理工大学 冶金与能源工程学院,云南 昆明,650093)
摘 要:
管制备锌电积用惰性阳极材料,研究氨基碳纳米管掺杂铅基阳极材料在锌电积过程中的析氧行为。研究结果表明:随着复合阳极材料中氨基碳纳米管掺杂量的增加,电极的电化学催化活性逐渐增强,在模拟工业锌电积条件下,氨基碳纳米管掺杂量为0.2%(质量分数)的复合阳极材料的析氧电位比纯铅阳极材料的电位降低了100 mV以上,并比传统铅银合金阳极材料的电位低,其预估服役寿命较纯铅的约低10%。因此,使用碳纳米管制备的铅碳复合材料有望替代传统铅银合金阳极材料,从而为铅合金阳极材料的发展开辟新的途径。
关键词:
中图分类号:TF813 文献标志码:A
文章编号:1672-7207(2021)02-0350-08
Oxygen evolution of amino carbon nanotubes doped lead-based anode
YANG Changjiang, ZHANG Xu, ZHAO Lüxing
(Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology,Kunming 650093, China)
Abstract: The inert anode for zinc electrowinning was prepared using amino carbon nanotube for replacing silver in traditional lead-silver anode. The oxygen evolution of amino carbon nanotubes(CNTs) doped lead-based anode was investigated. The results show that the electrochemical catalytical performance increases with the increase of amino carbon nanotubes in the composite anode. Under the conditions of simulated industrial zinc electrowinning, the overpotential of 0.2%(mass fraction) amino carbon nanotubes doped lead-based anode is decreased by 100 mV compared to that of pure Pb anode, and a little less than that of traditional lead-silver anode. The predicated service life of amino carbon nanotubes doped lead-based anode is about 10% less than that of pure Pb anode. Therefore, it is possible to replace the traditional lead-silver anode by the carbon/lead composite based on CNTs and a new approach is provided for the development of Pb alloy anode.
Key words: amino carbon nanotubes; composite anode; oxygen evolution; zinc electrowinning
目前,全球约85%的锌是由湿法冶炼工艺生产的,其中,作为湿法炼锌的重要工序之一,锌电积的能耗约占整个湿法流程能耗的80%[1-2],而阳极材料的组成和性质是影响锌电积能耗的重要因素。为降低能耗,研究者们从阳极的元素掺杂和基体改性等方面研究了铅基、钛基和铝基等高电化学催化活性的阳极材料,以此来降低析氧电位[3-5]。
钛基阳极材料具有形状尺寸稳定、强度高、对阴极锌的污染小、析氧电位低、抗腐蚀性好的优点,但制作工艺繁琐、涂层易脱落等不足限制了其工业应用[6-8]。铝基阳极材料以铝作为基体[9-10],具有生产本较低、良好的导电性和析氧电位低的特点,但铝在与其他金属结合时容易产生界面缺陷,影响阳极材料的性能。铅基阳极材料在酸性硫酸盐体系中具有高耐蚀性和稳定性,并易加工成型,因而被广泛应用于工业生产中,但其存在析氧电位高、能耗大、机械性能差和导电性不足的缺点。为解决这些问题,研究者通过元素的掺杂来改善阳极材料性能,研究制备了铅基多元合金[11-12]。研究表明:贵金属和稀土金属的加入能够有效提高铅基阳极材料的电化学催化活性,降低析氧电位。与加入金属元素相比,在铅基中加入合适的非金属增强体对提高阳极材料的性能、降低析氧电位和生产成本具有更广阔的前景[9, 13]。
碳纳米管具有良好的导电性和电化学催化活性[14-16],但碳纳米管的比表面积大,容易因较大的表面能和分子之间的范德华力而发生团聚,有效的表面修饰能够降低其团聚效应,提高其在复合材料中的分散性[17]。通过在碳纳米管表面引入氨基,能够很好地提高碳纳米管的亲水性和分散性[16, 18]。本文作者将氨基碳纳米管与金属铅采用机械复合得到氨基碳纳米管分散均匀的复合粉体,利用粉末冶金法制备不同含量氨基碳纳米管的铅基复合阳极材料,采用电化学和加速腐蚀的方法研究氨基碳纳米管含量的影响,并与纯铅阳极材料和传统工业用铅合金阳极材料进行对比,以探索氨基碳纳米管复合铅基阳极材料应用于锌电积过程。
1 试验
1.1 试样制备
采用粉末冶金的方法制备复合阳极材料:称取一定量氨基碳纳米管(纯度>95%,内径为3~5 nm,外径为8~15 nm,长度约50 nm,阿拉丁试剂),置于10 mL的乙醇溶液中超声分散1 h,得到氨基碳纳米管的悬浮液;然后,称取一定量的铅粉(纯度>99.999%,粒度<74 μm,国药试剂),加入上述悬浮液中,超声分散10 min,得到悬浊液;随后将悬浊液过滤、洗涤得到滤饼,并将其放入真空干燥箱中干燥;再将干燥的粉末置于行星式球磨机中(BXQM2L,南京特伦新),充入氩气作为保护气,以球料比30:1、转速260 r/min球磨90 min,得到氨基酸纳米管质量分数分别为0.2%,0.3%和0.5%的复合粉末;最后,将复合粉末以413 MPa的压力冷压成型,在250 ℃真空条件下回火2 h,冷却至室温后,复压得到铅/氨基碳纳米管复合阳极材料。
采用氨基碳纳米管的质量分数分别为0.2%,0.3%和0.5%制得的铅/氨基碳纳米管复合阳极材料分别记为Pb0.2CNT-NH,Pb0.3CNT-NH和Pb0.5CNT-NH。选用高纯铅、铅银钙和铅银阳极材料等进行对比,其中高纯铅由Alfa Aesar公司提供,铅银钙阳极材料(Pb-0.35%Ag-1% Ca(质量分数))和铅银阳极材料(Pb-0.75% Ag(质量分数))由云南某冶炼厂提供,分别记为Pb0.35Ag1Ca和Pb0.75Ag。
1.2 物理表征测试
采用Raman光谱仪(inVia,Renishaw),激发光源为514.5 nm分析氨基碳纳米管的拉曼散射光谱;采用X射线光电子能谱(XPS,K-Alpha+, Thermo Fisher Scientific)分析氨基碳纳米管的元素含量;采用扫描电镜(SEM,EM-30 PLUS,COXEM)对复合粉末和阳极材料表面进行形貌表征。
1.3 电化学测试
电化学测试采用VersaSTAT 3电化学工作站和三电极测试体系。铅及其合金作为工作电极,采用聚四氟乙烯电极夹套以保持工作电极的面积为1 cm2,以4 cm2铂片作为辅助电极,参比电极为Hg/Hg2SO4/饱和硫酸钾(MSE,0.652 V vs SHE)。本文所述电位均参照该参比电极(特殊说明除外)。在25 ℃下,60 g/L Zn2++160 g/L H2SO4的模拟锌电积电解液中进行电化学测试。
测试条件:1) 以扫描速度为30 mV/s,在电位为0.35~1.55 V之间进行循环伏安测试;2) 在电流密度50 mA/cm2的条件下,极化20 h,测试电极耐腐蚀性和析氧电位;3) 在电位0.35~1.75 V之间,以扫描速度1 mV/s进行阳极极化曲线测试;4) 在1.5 V条件下,频率为0.1~100×103 Hz的范围内进行电化学阻抗测试。
2 结果与讨论
2.1 物理表征
氨基碳纳米管的拉曼光谱如图1所示。由图1可见,D峰(1 351 cm-1)为C原子的A1g震动模,表征C原子的无序和sp3杂化。G峰(1 593 cm-1)为石墨E2g拉伸模,为C—C键的sp2面内震动,表征了石墨化程度,当表面附有不规则的石墨微粒时,G峰通常发生分裂,产生肩峰,图中G峰较为完整,表明表面较为纯净。二次谐波G′峰(2 698 cm-1)对应于D峰存在,2 937 cm-1处为G+D峰[19-20]。D峰和G峰的积分面积比(ID/IG)通常用于表示CNT的石墨化均匀程度,氨基化碳纳米管的ID/IG为1.4,表明,由于CNT表面引入氨基等功能性基团,表面产生了较多的缺陷。由于氨基含量较低,吸收弱,且部分特征峰与CNTs接近,因此图中未发现N—H和C—N键的特征峰。
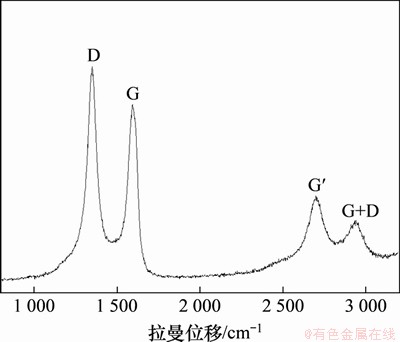
图1 氨基碳纳米管的拉曼光谱
Fig. 1 Raman spectra of as-received amino carbon nanotubes
图2所示为氨基碳纳米管的XPS能谱。可见:在284.96,399.30和532.31 eV分别为C 1s,O 1s和N 1s的特征峰,与拉曼光谱的结果一致,元素分析结果如表1所示。可见:C 1s,O 1s和N 1s的摩尔分数分别为96.81%,2.50%和0.70%。氨基碳纳米管中氧含量较高,表明碳纳米管在氨基化后表面仍存在大量的羟基和羧基。进一步对N 1s进行高分辨光谱分析,可见:仅在399.30 eV处发现主峰,没有出现明显的肩峰,为典型的N—H峰(NIST)。
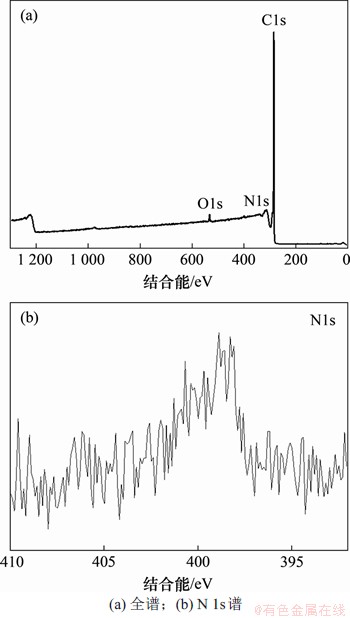
图2 氨基碳纳米管的XPS能谱
Fig. 2 XPS survey spectra of as-received amino carbon nanotubes
表1 氨基碳纳米管元素分析
Table 1 Elemental analysis of as-received amino carbon nanotubes

图3所示为球磨后的铅和氨基碳纳米管复合粉体的扫描电镜照片。从图3可以看出:未出现碳纳米管的团聚现象,铅粉与氨基碳纳米管均匀地复合在一起。
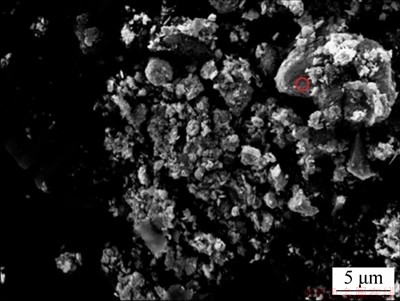
图3 铅/氨基碳纳米管复合粉体的扫描电镜照片
Fig. 3 SEM image of lead/amino carbon nanotubes composite powder
2.2 循环伏安曲线
图4所示为不同铅阳极材料的循环伏安曲线。可见:图中有2个氧化峰(A和B峰)和1个还原峰(C峰),A峰发生反应Pb→α-PbO,B峰发生反应PbSO4→β-PbO2,C峰发生反应PbO2→PbSO4[21-22]。C峰面积与电极表面PbO2生成量有关,图4(a)中显示,随着氨基碳纳米管含量的增加,循环伏安曲线的还原峰面积逐渐减小,说明氨基碳纳米管具有良好的电化学稳定性,在测试过程中未发生氧化还原反应。图4(b)中复合阳极材料与铅及其合金的循环伏安测试对比表明,其阳极极化电流远比铅及其合金的高,表明复合阳极材料表面更容易形成具有催化活性的PbO2。
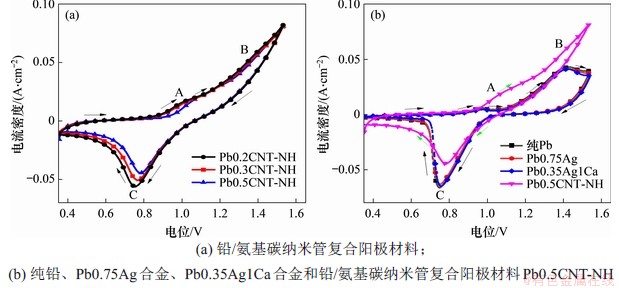
图4 不同铅阳极材料的循环伏安曲线对比
Fig. 4 Comparison of cyclic voltammograms for different Pb and its composite anodes
2.3 极化曲线
图5所示为不同铅阳极材料的极化曲线及Tafel曲线的拟合结果。从图5(a)可知,增加氨基碳纳米管掺杂量可以显著降低析氧过电位。Pb0.2CNT-NH,Pb0.3CNT-NH和Pb0.5CNT-NH复合阳极材料的起始电位(onset potential)分别为1.32,1.26和1.21 V;在1.50 V的析氧电位下,其电流密度分别为39.5,53.8和72.1 mA/cm2。从图5(b)可知,铅及其合金的起始电位(onset potential)在1.44 V以上,在1.50 V的析氧电位下,纯铅、铅银和铅银钙阳极材料的电流密度分别为11.8,11.9和15.1 mA/cm2。
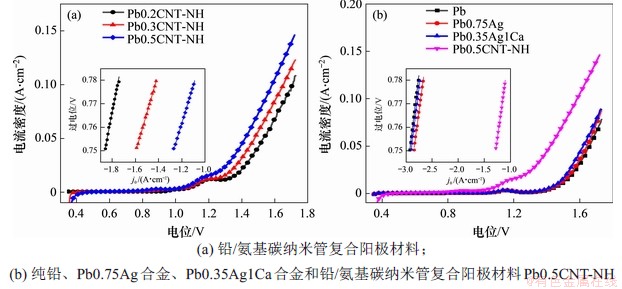
图5 不同铅阳极材料的极化曲线和析氧反应的Tafel曲线
Fig. 5 Linear sweeping voltammograms and Tafel plots of oxygen evolution(insets) for different Pb and its composite anodes
对析氧电位区间进行Tafel拟合,根据Tafel方程:
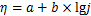
式中:η为析氧过电位;j为电流密度;a和b分别表示塔菲尔曲线的截距和斜率。
参比电极Hg/HgSO4(饱和硫酸钾,MSE)的电极电位为0.65 V,析氧平衡电位为1.206 V,复合阳极材料的η可由以下公式[13]计算:
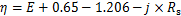
式中:E为复合阳极材料的析氧电位;Rs为溶液电阻,通过电化学阻抗方法测得。
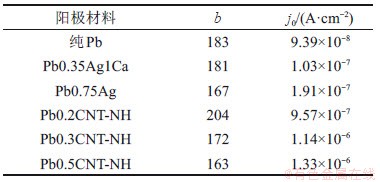
Tafel参数拟合结果见表2。从图5和表2可见:Pb0.35Ag1Ca的Tafel斜率和交换电流密度与纯铅的接近,Pb0.75Ag的交换电流密度为纯铅的一半,而其Tafel斜率为167,显著低于纯铅的Tafel斜率。铅/氨基碳纳米管复合阳极材料的交换电流密度比纯铅的大10倍以上,说明氨基碳纳米管增加了电极表面的电化学催化活性点数。随着氨基碳纳米管掺杂量的增加,铅/氨基碳纳米管复合阳极材料的Tafel斜率降低,表明氨基碳纳米管能够加速析氧去极化,从而有效降低其在工作电流下的过电位。
表2 不同铅阳极材料的线性极化Tafel参数
Table 2 Tafel parameters evaluated from linear sweeping voltammograms for different Pb and its composite anodes
2.4 电化学交流阻抗
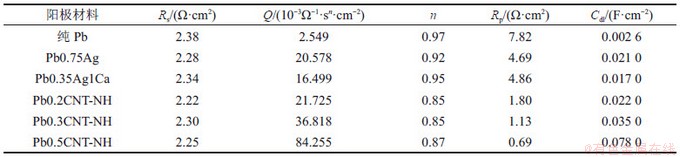
在恒电压1.50 V的析氧条件下,不同铅/氨基碳纳米管复合阳极材料的电化学交流阻抗图谱如图6所示,图中,Rp为阳极电化学过程中电荷传质电阻;Q为等效电容。从图6(a)可以看出:阻抗谱为一个压扁的半圆,含有一个时间常数,因此用Randles等效电路对电化学阻抗谱进行分析,拟合结果见表3。双电层电容Cdl可由式(3)推算得到[23]:
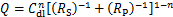
式中:n为与理想电容器的偏差,当n=1时,为理想电容器。
计算结果表明,随着复合阳极材料中氨基碳纳米管掺杂量的增加,极化电阻由1.80 Ω·cm2降到0.69 Ω·cm2,分别约为纯铅的1/4和铅合金的1/3,说明氨基碳纳米管能够显著增加电极表面的析氧活性,与线性极化和循环伏安测试结果一致。双电层电容常用电化学活性面积表示。从表3可以看出:随着氨基碳纳米管的含量增加,复合阳极材料的电化学活性面积增大,约为纯铅的10倍,与铅银合金和铅银钙合金接近,这进一步说明氨基碳纳米管增加了电化学反应面积,从而凸显电化学催化析氧性能。
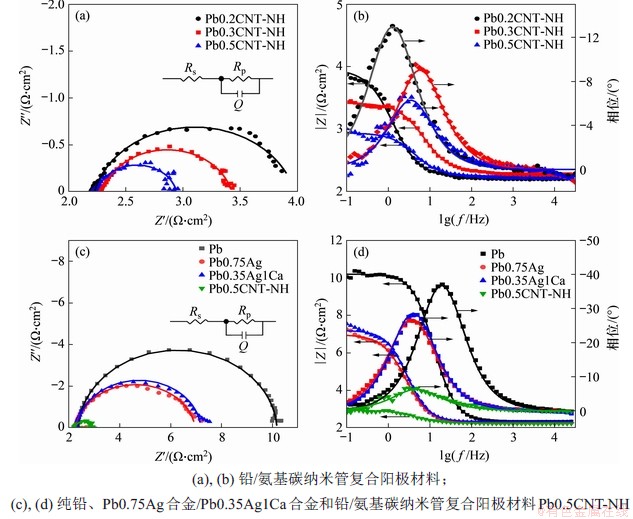
图6 不同铅阳极材料的电化学阻抗谱Nyquist图/Bode图
Fig. 6 Nyquist/ Bode plots of different Pb and its composite anodes
表3 不同铅阳极材料的电化学阻抗计算结果比较
Table 3 Comparison of parameters of equivalent circuit for Pb and its composite anodes
2.5 恒电流极化和腐蚀速率测试
在模拟工业锌电积条件下,对得到的复合阳极材料进行20 h阳极析氧测试,并与纯铅和铅银合金阳极材料进行对比,结果如图7所示。从图7可以看出:在电解液中极化10 h后,复合阳极材料的电位趋于稳定,Pb0.2CNT-NH,Pb0.3CNT-NH和Pb0.5CNT-NH的电极电位分别为1.516,1.486和1.461 V,而对应纯铅阳极材料和Pb0.75Ag阳极材料的电极电位分别为1.621 V和1.582 V。由此可知,添加氨基碳纳米管,复合阳极材料的析氧电位可以降低100 mV以上。
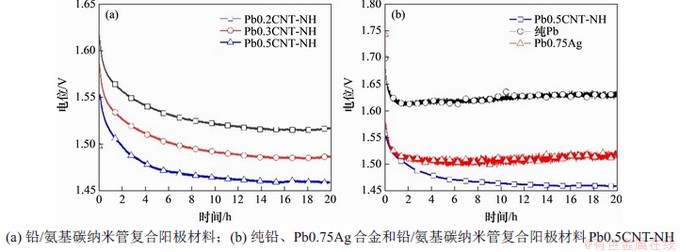
图7 不同铅阳极材料的恒电流极化曲线
Fig.7 Chronopotentiometric curves of different Pb and its composite anodes
阳极材料的腐蚀测试依据文献[2]进行分析,在模拟锌工业电积条件下进行电化学加速腐蚀试验测试,测试电流密度为500 A·m2,时间为20 h,结果如表4所示。由表4可知:复合阳极材料的预估服役寿命较纯铅的约低10%,约为铅银合金阳极材料的一半,原因可能是通过粉末冶金工艺得到的复合阳极材料的致密度比纯金属或金属合金的更低。电化学加速腐蚀试验后的铅/氨基碳纳米管复合阳极材料和纯铅阳极材料的表面扫面电镜照片如图8所示。从图8可以看出:在腐蚀实验以后,铅基复合阳极材料表面更为粗糙,呈沟壑状,而纯铅阳极材料的表面较为平整,局部呈现少量的坑点,说明复合阳极材料的腐蚀主要是局部腐蚀,而纯铅阳极材料主要是均匀腐蚀,其中的原因主要归结于复合材料的致密性和晶界腐蚀。
表4 不同铅合金阳极材料的电化学腐蚀速率
Table 4 Corrosion rates for different Pb and its composite anodes 腐蚀速率/(mg·h-1·cm-2)

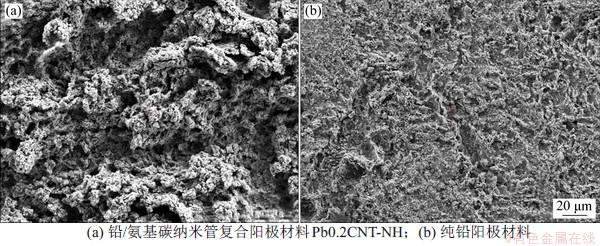
图8 不同阳极材料电化学加速腐蚀后的扫描电镜图片
Fig. 8 SEM images of anodes after electrochemical accelerated corrosion tests
3 结论
1) 铅/氨基碳纳米管复合阳极材料具有优异的电化学析氧性能,其析氧反应交换电流密度和电化学反应活性面积为纯铅阳极材料的10倍以上,极化电阻降低为纯铅阳极材料的1/4,在工业锌电积条件下,其析氧电位比传统的铅银阳极材料的低,其预估服役寿命比纯铅材料的约低10%。
2) 采用氨基碳纳米管替代贵金属银制备新型碳铅复合阳极材料,不但可以降低析氧电位,还可以减少贵金属的使用,在锌冶金电积中具有潜在的应用的价值。
参考文献:
[1] YANG Haitai, GUO Zhongcheng, CHEN Buming, et al. Electrochemical behavior of rolled Pb-0.8%Ag anodes in an acidic zinc sulfate electrolyte solution containing Cl- ions[J]. Hydrometallurgy, 2014, 147/148: 148-156.
[2] YANG Changjiang, ZHAO Lüxing, ZHANG Xu. Service life assessment of lead and its alloy anodes during zinc electrowinning[J]. International Journal of Electrochemical Science, 2019, 14(9): 8720-8732.
[3] WANG Shuai, ZHOU Xiangyang, MA C Y, et al. Electrochemical properties of Pb-0.6 wt% Ag powder-pressed alloy in sulfuric acid electrolyte containing Cl-/Mn2+ ions[J]. Hydrometallurgy, 2018, 177: 218-226.
[4] IVANOV I, STEFANOV Y, NONCHEVA Z, et al. Insoluble anodes used in hydrometallurgy[J]. Hydrometallurgy, 2000, 57(2): 125-139.
[5] JIN Lei, HUANG Hui, FEI Yang, et al. Polymer anode used in hydrometallurgy: anodic behaviour of PANI/CeO2 /WC anode from sulfate electrolytes[J]. Hydrometallurgy, 2018, 176: 201-207.
[6] ZHANG Cheng, LIU Jianhua, CHEN Buming. Effect of Ce(NO3)4 on the electrochemical properties of Ti/PbO2- TiO2-Ce(NO3)4 electrode for zinc electrowinning[J]. Applied Physics A, 2019, 125(2): 150-156.
[7] XU Wenting, HAARBERG G M, SUNDE S, et al. Sandblasting effect on performance and durability of Ti based IrO2-Ta2O5 anode in acidic solutions[J]. Electrochimica Acta, 2019, 295: 204-214.
[8] ZHANG Cheng, LIU Jianhua, CHEN Buming. Effect of CeO2 and graphite powder on the electrochemical performance of Ti/PbO2 anode for zinc electrowinning[J]. Ceramics International, 2018, 44(16): 19735-19742.
[9] CHEN Buming, YAN Wenkai, HE Yapeng, et al. Influence of F-doped β-PbO2 conductive ceramic layer on the anodic behavior of 3D Al/Sn rod Pb-0.75%Ag for zinc electrowinning[J]. Journal of the Electrochemical Society, 2019, 166(4): E119-E128.
[10] HAN Zhaohui, XU L, KANNAN C S, et al. Preparation and electrochemical properties of Al/TiB2/β-PbO2 layered composite electrode materials for electrowinning of nonferrous metals[J]. Ceramics International, 2018, 44(15): 18420-18428.
[11] CLANCY M, BETTLES C J, STUART A, et al. The influence of alloying elements on the electrochemistry of lead anodes for electrowinning of metals: a review[J]. Hydrometallurgy, 2013, 131/132: 144-157.
[12] HRUSSANOVA A, MIRKOVA L, DOBREV T. Influence of additives on the corrosion rate and oxygen overpotential of Pb-Co3O4, Pb-Ca-Sn and Pb-Sb anodes for copper electrowinning: part II[J]. Hydrometallurgy, 2004, 72(3/4): 215-224.
[13] YANG Changjiang, SHEN Qingfeng, ZHAI Dacheng, et al. Carbon nanotubes sheathed in lead for the oxygen evolution in zinc electrowinning[J]. Journal of Applied Electrochemistry, 2019, 49(1): 67-77.
[14] YADAV R M, WU Jingjie, KOCHANDRA R, et al. Carbon nitrogen nanotubes as efficient bifunctional electrocatalysts for oxygen reduction and evolution reactions[J]. ACS Applied Materials & Interfaces, 2015, 7(22): 11991-12000.
[15] EVTUSHOK V Y, SUBOCH A N, PODYACHEVA O Y, et al. Highly efficient catalysts based on divanadium-substituted polyoxometalate and N-doped carbon nanotubes for selective oxidation of alkylphenols[J]. ACS Catalysis, 2018, 8(2): 1297-1307.
[16] ZHU Xinyang, ZHANG Xueping, HUANG Liang, et al. Cobalt doped β-molybdenum carbide nanoparticles encapsulated within nitrogen-doped carbon for oxygen evolution[J]. Chemical Communications, 2019, 55(67): 9995-9998.
[17] CHA S, KIM K, ARSHAD S, et al. Extraordinary strengthening effect of carbon nanotubes in metal-matrix nanocomposites processed by molecular-level mixing[J]. Advanced Materials, 2005, 17(11): 1377-1381.
[18] ZHANG Huabin, LIU Yanyu, CHEN T, et al. Unveiling the activity origin of electrocatalytic oxygen evolution over isolated Ni atoms supported on a N-doped carbon matrix[J]. Advanced Materials, 2019, 31(48): e1904548.
[19] DRESSELHAUS M S, DRESSELHAUS G, SAITO R, et al. Raman spectroscopy of carbon nanotubes[J]. Physics Reports, 2005, 409(2): 47-99.
[20] OSSWALD S, HAVEL M, GOGOTSI Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy[J]. Journal of Raman Spectroscopy, 2007, 38(6): 728-736.
[21] YANG Changjiang, KO Y, PARK S M. Fourier transform electrochemical impedance spectroscopic studies on anodic reaction of lead[J]. Electrochimica Acta, 2012, 78: 615-622.
[22] TUNNICLIFFE M, MOHAMMADI F, ALFANTAZI A. Polarization behavior of lead-silver anodes in zinc electrowinning electrolytes[J]. Journal of the Electrochemical Society, 2012, 159(4): C170-C180.
[23] YANG Changjiang, PARK S M. Electrochemical behavior of PbO2 nanowires array anodes in a zinc electrowinning solution[J]. Electrochimica Acta, 2013, 108: 86-94.
(编辑 赵俊)
收稿日期: 2020 -03 -05; 修回日期: 2020 -05 -20
基金项目(Foundation item):国家自然科学基金资助项目(51664040);昆明理工大学分析测试基金资助项目(2019T20080042,2019M20172202010) (Project(51664040) supported by the National Natural Science Foundation of China; Projects(2019T20080042, 2019M20172202010) supported by the Analysis and Testing Foundation of Kunming University of Science and Technology)
通信作者:杨长江,博士,副教授,从事冶金电化学研究;E-mail: yangc@kust.edu.cn
引用格式:杨长江, 张旭, 赵吕兴. 氨基碳纳米管掺杂铅基阳极材料的析氧行为[J]. 中南大学学报(自然科学版), 2021, 52(2): 350-357.
Citation:YANG Changjiang, ZHANG Xu, ZHAO Lüxing. Oxygen evolution of amino carbon nanotubes doped lead-based anode[J]. Journal of Central South University(Science and Technology), 2021, 52(2): 350-357.
摘要:采用氨基碳纳米管制备锌电积用惰性阳极材料,研究氨基碳纳米管掺杂铅基阳极材料在锌电积过程中的析氧行为。研究结果表明:随着复合阳极材料中氨基碳纳米管掺杂量的增加,电极的电化学催化活性逐渐增强,在模拟工业锌电积条件下,氨基碳纳米管掺杂量为0.2%(质量分数)的复合阳极材料的析氧电位比纯铅阳极材料的电位降低了100 mV以上,并比传统铅银合金阳极材料的电位低,其预估服役寿命较纯铅的约低10%。因此,使用碳纳米管制备的铅碳复合材料有望替代传统铅银合金阳极材料,从而为铅合金阳极材料的发展开辟新的途径。


