
Preparation of nanometer oxides La1-xSrxMnO3 by absolute alcohol as solvent
HU Jie(胡 婕)1, 2, SHAO Guang-jie(邵光杰)1, GUO Peng(郭 鹏)1,
QIN Xiu-juan(秦秀娟)1, XING Guang-zhong(邢广忠)2
1. Department of Environment and Chemistry, Yanshan University, Qinhuangdao 066004, China;
2. State Key Laboratory of Metastable Materials Science and Technology,
Yanshan University, Qinhuangdao 066004, China
Received 27 April 2007; accepted 13 September 2007
Abstract:
Nanometer oxides La1-xSrxMnO3 were synthesized by absolute alcohol as solvent. The desired metal cations were chelated in a solution using citric acid as the chelating agents. In order to get the optimum preparation condition for La1-xSrxMnO3, the pH of primal commix solution, the molar fraction of citric acid and baking temperature of predecessor block were researched by orthogonal test design method with different x. The thermal decomposition of the metal carboxylate precursor gels was studied by TG/DTA and the products derived from calcinations of the gels were characterized by XRD and TEM. The polarization curves were acquired on an electrochemical work station (LK98) and the discharge curves were acquired on a testing instrument of batteries (DC-5) with a constant current discharge under 120 mA/cm2. The results reveal that the nanometer oxides can be achieved by absolute alcohol as solvent and it has better catalytic activity.
Key words:
nanometer oxides; La1-xSrxMnO3; organic solvent;
1 Introduction
When lanthanum manganite LaMnO3 is partially substituted by Sr2+, the ionic concentration of Mn4+ will increase with increasing of Sr2+. Oxides La1-xSrxMnO3 would have 1+x unconjugated electrons[1], which makes La1-xSrxMnO3 have better electric catalysis than LaMnO3[2]. The La1-xSrxMnO3 powders are usually synthesized by sol-gel method, which has some advantages such as lesser particle, high surface area and special physical chemistry capability[3]. Usually, gels are prepared under water liquor, which may be produced with serious conglomeration. In order to debase the conglomeration, a lots of methods were adopted such as appending surface-active agent and milling[4]. But the effect is not available. When wet gel is prepared in water solvent, a number of freedom watering-molecules react with hydroxyls that are presented on the surface of colloid grains to from hydrogen bonds. Along with the liquor evaporating, there are lots of lacuna in gels and plenty of curved liquid surface, hence the particles are reunited by capillarity which helps the watering- molecules and hydroxyls to become a hydrogen bond [5-6], which come into being hard reunite. When the gels are baked, the reuniting of particles are strengthened. In order to reduce the surface tension of waterish gels and improve the dispersing- ability, the key is to get rid of those bridged watering-molecules and hydroxyls[7]. So the dehydration process of gels is very important. Absolute alcohol has good dehydration character. It can replace moisture that is absorbed on the surface of grains by its surface tension, and decrease the capillarity. Thus, absolute alcohol can get rid of the water of coordination and make alkoxy instead of hydroxyl, which can minish effectively particle size of nanometer powders and improve their dispersing. In this work, water solvent was replaced by absolute alcohol when sols were prepared with different x. The pH of primal commix solution, complexing agent citric acid and bake temperature were studied by orthogonal test design method in order to obtain the optimum preparation condition for La1-xSrxMnO3.
2 Experimental
2.1 Sample preparation
In our preparation process, the La(NO3)3?6H2O, Sr(NO3)2 and Mn(NO3)2 in appropriate quantity were dissolved in absolute alcohol, and citric acid was added in proper molar ratio, churning up for 2 h with plusator in constant temperature water tank. Water was evaporated at 298 K until the gels appear. Then the precursor was calcined in air for 5 h at 873 K[8] to obtain the oxides.
2.2 Orthogonal test design
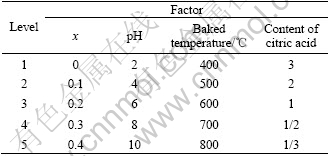
To sol-gel method, the particle size and crystal structure are different if experimental factors or levels are changed, and the catalysis is subsequently changed [9]. The pH, ratio of citric acid, different x values for primal commix solution and baking temperature of precursor are all the major factors to influence the particle size and crystal structure of La1-xSrxMnO3. In this work, orthogonal test was designed by four factors and five levels, which is listed in Table 1.
Table 1 Factor and level of orthogonal test for synthesis of oxides La1-xSrxMnO3
2.3 Sample characterization and electrochemical measurement
The precursors were characterized using thermo- gravimetric analysis(TG) and differential thermal analysis(DTA) with heating rate of 10 ℃/min in air from 20 ℃ to 1 100 ℃. The baked process of oxide was confirmed by the peak value of radiation and decalescence.
The phase analyses perovskite-type crystalline and precursors calcined at various temperatures were carried out on X-ray diffractometer(XRD) using Cu Kα radiation (l=0.154 184 nm). The conditions of spectral recording were in a 2q angle step scanning from 10 ℃ to 80 ℃ with a 2.4 s delay for each 0.05 step[10-11].
A transmission electron microscopy(TEM) was used to analyze the particle size, the morphology of particles and the dispersion of powders.
To prepare the active layer of the air electrode, a mixed catalyst, active carbon and PTFE suspension (60% in H2O, mass fraction) were ground in excess ethanol and then dried at 60 ℃ to give a dough-like paste, which was finally rolled into a thin layer of 200 μm in thickness. The air electrode was characterized with a three- electrode configuration with a nicked board counter- electrode and a Hg/HgO/OH (7 mol/L) reference electrode. The electrolyte was 6 mol/L KOH, and the area of electrode was 1 cm2. The polarization curves were acquired on an electrochemical work station (LK98) under a constant potential-sweep rate of 3 mV?s-1. The discharge curves were acquired on a testing instrument of batteries (DC-5) with a zincic board cathode, constant current discharge under 120 mA/cm2.
3 Results and discussion
3.1 Analysis of pH value and citric acid capacity
Citric acid is a ternary carboxylic acid, and there are different compounds with lanthanon when the pH and citric acid capacity are changed. Steady compound can be formed by adjusting pH of solution. In the acidic solution, compound can be formed by rare earth ion and citric acid anion (H2Cit)- or (HCit)2-, but metal and ion can not generate complex reaction completely if pH is too low. When pH is 6-8, neutral compound [LnCit] can be formed by the equal rare earth ion and citric acid, while [Ln2(Cit)3]3- and [Ln(Cit)2]3- will be formed when citric acid is superfluous; the neutral compound may solve when pH>7 because its hydroxyl can be neutralized and form Ln(Cit)-1[12]. Orthogonal test shows that depositions appear when pH is 2-3 and along with the increase of pH, and deposition can not be dissolved. This verifies that metal and ion cannot react completely when molar ratio of citric acid to nitrate is 1:3.
Table 2 shows the stability of La1-xSrxMnO3 precursor solutions and gels under different pH and contents of citric acid. This verifies that steady sols and gels can not be formed when pH is 2-4 and metalline ion separates out in deposition from solution. Steady sols and transparent gels can be formed when pH is 6-8 and molar ratio of citric acid to nitrate is 2?1, but muddy gels would appear when the ratio is 1?1. In order to avoid three-dimensional meshy structure of gels to be destroyed and powder particle size augment when they were baked, the pH cannot exceed 10.
Table 2 Stability of LaxSr1-xMnO3 precursor solutions and gels
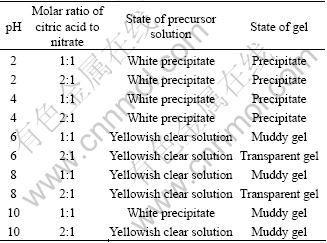
When the ratio is 1?1, white precipitate appears in the commix solution when pH is 3-3.5, then solution gets translucent when pH is increased from 6 to 8. But when the solution is evaporated, depositions will be separated out and form muddy gels. In order to obtain transparent gels, the fitting ratio of citric acid to nitrate is 2?1 and pH is 6-8.
3.2 Analysis of TG/DTA
The thermogravimetry of La0.8Sr0.2MnO3 precursor in Fig.1 shows that the mass the of drying gels begins as soon as the procedure is heated up. The process of mass- loss slows down at 160-220 ℃, which is caused by the volatilization of ethanol; while the process is fleet at about 230 ℃, which attributes to the decomposing of superfluous citric acid. There is a mass loss at 570 ℃, which is believed that the compounds of citric acid are decomposed and the perovskite phase is obtained.
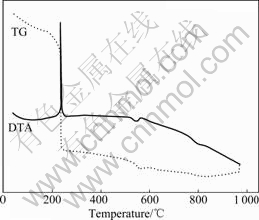
Fig.1 DTA-TG curves of oxide La0.8Sr0.2MnO3
3.3 Analysis of XRD and TEM
The baking condition has an important effect on the preparation of oxides. Perovskite crystal cannot be formed if the baking temperature is too low. The particle may reunite and surface areas may reduce if using exorbitant baking temperature. The heatedly decomposition were researched by TG-DTA before the dry gel were baked. The time and baking temperature, at which perovskite crystal structure maintains were confirmed by XRD finally.
Fig.2 shows the XRD pattern of gel precursors that is baked in air at 873 K for 2 h. It can be seen that the oxides have obvious characteristic diffraction peaks and less imparity, and a single-phase powder with the perovskite structure can be synthesized at 873 K.

Fig.2 XRD pattern of catalyst La0.8Sr0.2MnO3
Fig.3 shows the TEM image of powders that are prepared by absolute alcohol as solvent. Fig.4 shows the TEM image of powers that are prepared in the water. It can be seen from Fig.3 that powders’ dispersing is improved effectively. It is verified that absolute alcohol as solvent can reduce capillarity remarkably and prevent original particles from reuniting. It can be seen from Fig.4 that there are serious reuniting.

Fig.3 TEM image of powers with absolute alcohol as solvent

Fig.4 TEM images of powers with water as solvent
3.4 Analysis of catalytic activity
Two nanometer powders La0.8Sr0.2MnO3 were prepared by different methods. Air electrodes were prepared with the two oxides as catalyst. Fig.5 shows the polarization curves of different air electrodes and Fig.6 shows the discharge curves.
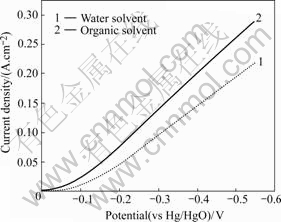
Fig.5 Polarization curves of air electrode with different solvents
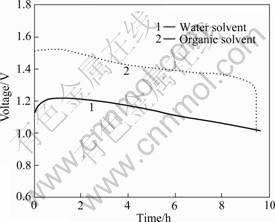
Fig.6 Discharge curves of air electrode with different solvents
It is verified that La0.8Sr0.2MnO3 is prepared by absolute alcohol as solvent, which can remarkably decrease cathode polarization of air electrode and improve discharge voltage. Its electrochemical capability has precedence over the powders, which are prepared by usual sol-gel method.
Fig.7 shows the polarization curves of air electrode with different substitutions of Sr. It can be seen that the polarization of electrode decreases with increasing Sr content (from 0 to 0.3). But the inflexion appears when x=0.4. La0.7Sr0.3MnO3 has the highest catalytic activity but LaMnO3 has the lowest one. The order for catalytic activity under same voltage with different contents of Sr is La0.7Sr0.3MnO3>La0.8Sr0.2MnO3>La0.6Sr0.4MnO3>La0.9Sr0.1MnO3>LaMnO3. The causation is that the content of Mn4+ is increased when La3+ ions are replaced by Sr2+, namely, LaMnO3+Sr2+→![]()
![]() thereby more electronics are transferred between Mn4+ and Mn3+[13]. Oxygenic reduction reaction is usually processed by two electronics transferred in alkaline solution.
thereby more electronics are transferred between Mn4+ and Mn3+[13]. Oxygenic reduction reaction is usually processed by two electronics transferred in alkaline solution.![]() is intermediate product and OH- is the final product[14].
is intermediate product and OH- is the final product[14].
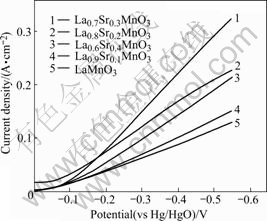
Fig.7 Polarization curves of air electrode with different contents for Sr
The course of reaction is
O2+2e+H2O→![]() +OH- (1)
+OH- (1)
![]() →O2↑+2OH- (2)
→O2↑+2OH- (2)
When partial La is replaced by Sr, the decomposing speed of ![]() is increased and the over voltage of oxygenic reduction reaction is debased, thereby the electro-catalytic activity of catalyst is heightened remarkably[15]. But the crystal structure of oxides will distort when the content of Sr is superfluous[16], thus the La0..4Sr0..6MnO3 has infra-electro-catalytic activity.
is increased and the over voltage of oxygenic reduction reaction is debased, thereby the electro-catalytic activity of catalyst is heightened remarkably[15]. But the crystal structure of oxides will distort when the content of Sr is superfluous[16], thus the La0..4Sr0..6MnO3 has infra-electro-catalytic activity.
4 Conclusions
1) Nanometer oxide La1-xSrxMnO3 powders were synthesized by absolute alcohol as solvent using citric acid as the chelating agents. It is verified that absolute alcohol as solvent can remarkably improve powders’ dispersing and prevent original particles from reuniting.
2) Stable La1-xSrxMnO3 precursor gels can be obtained with higher pH and superfluous citric acid. The results show that the optimal pH is 8, and the molar ratio of citric acid to nitrate is 2:1.
3) The oxide La1-xSrxMnO3 powders have better catalytic activity when they are synthesized by absolute alcohol as solvent. The cathodal polarization of oxygen electrode is notably debased and discharge voltage is notably heightened. There are the highest electric catalytic activity when x=0.3.
References
[1] WEI Zi-dong, HUANG Wen-zhang, ZHANG Sheng-tao. Carbon-based air electrodes carrying MnO2 in zinc-air batteries [J]. J Power Sources, 2000, 91: 83-85.
[2] WENG Rui, DING Hong-mei, XU Lu-hua. Effect of strontium and cerium on performance of nanometer LaMnO3+x rare-earth perovskite [J]. Journal the Chinese Rare Earth Society, 2001, 19(4): 338-342.
[3] CHI Yu-juan, YU Hai-tao, LI Huan-yu. Study on preparing perovskite-type complex oxides La1-xSrxFeO3 nanocrystal [J]. J Natural Science of Heilongjiang University, 2000: 17(1): 73-76.
[4] CHENG Guo-qin, WU Gao-hui, ZHU De-zhi, YANG Zi-qin, ZHAO Jun, XIE Zi-zhang. Microstructure and thermal and electric conductivities of high dense Mo/Cu composites [J]. Trans Nonferrous Met Soc China, 2005, 15(3): 110-114.
[5] JONESSL N M. Dehydration of hydrous zirconia with methanol [J]. J Amer Ceramic Soc, 1988, 71(4): 190-191.
[6] HIDEAKI M. Reduction of lead migration during drying of a gel [J]. J Non-Cryst Solids, 1990, 121: 61-65.
[7] YU Dong-sheng, ZHOU Zhen-tao. The study on synthesis and electro-catalytic properties of La1-xSrxMnO3 [J]. Rare Metal Materials and Engineering, 2005, 34(3): 463-467.
[8] YOUICHI S, KENCHI U, HARUYUKI M. Bi-function oxygen electrode using large surface area La1-xCaxCoO3 for rechargeable metal-air batteries [J]. J the Electrochemical Soc, 2000, 137: 3430- 3433.
[9] SONG Shi-dong, TANG Zhi-yuan, PAN Li-zhu. Study on the electrochemical properties of La1-xSrxNi1-yFeyO3 bifunctional oxygen electrodes [J]. Acta Chimica Sinica, 2005, 5: 363-371.
[10] XU Gang, ZHAO Gao-ling, REN Zhao-hui. PVA assisted synthesis of nanosized perovskite PZT powder by a two-stage precipitation route [J]. Materials Letters, 2006, 60: 685-688.
[11] TAGLIAZUCCHI M, SANCHEZ R D, TROIANI H E. Synthesis of lanthanum nickelate perovskite nanotubes by using a template-inorganic precursor [J]. Solid State Communications, 2006, 137: 212-215
[12] Y X W, HUANG C H, LIU Y J. Lanthanon scandium [J]. Inorganic Chemistry Series, 1992(7): 242-244.
[13] ENIYA L D, KENICHI O, HIROYUKI N, EISHUN T. Electrocatalysis for dioxygen reduction by a μ-oxo decavanadium complex in alkaline medium and its application to a cathode [J]. Journal of Power Sources, 2004, 130: 286-290.
[14] ZHANG H M, SHIMIZU TERAOKA Y. Oxygen sorption and catalytic properties of La1-xSrxCo1-yFeyO3 perovskite-type oxide [J]. Journal of Catalysis, l990, l2l: 432-440.
[15] MULLER S, STRIEBEL K, HAAS C. LaO0.6CaO0.4COO3: A stable and powerful catalyst for bifunctional air electrodes [J]. Electro-Chimica Acta, 1994, 39: 1661-1668.
[16] GU Jun, SUI Sheng, LI Guang-qiang. Catalytic activities of La1-XCaxFe1-yCoyO3 for oxygen reduction [J]. Journal of Inorganic Materials, 1999, 14(4): 618-622.
Corresponding author: SHAO Guang-jie; Tel: +86-335-8061569; E-mail: shaogj@ysu.edu.cn


